Abstract
Introduction
While benign ampullary tumors are removed endoscopically, due to their potential to progress to malignant disease, the favored treatment for adenocarcinoma is pancreaticoduodenectomy. We reviewed our institution’s experience in order to identify which patients were at highest risk of disease progression following surgical resection, as well as evaluate whether localized T1 tumors are best treated by pancreaticoduodenectomy.
Methods
We retrospectively reviewed 157 patients who presented with an ampullary mass, from 2001 to 2010, and identified 51 with benign adenoma and 106 with adenocarcinoma.
Results
Patients with malignant tumors most often presented with larger tumors and jaundice, which alone was predictive of survival (OR 67). Forty-five percent of patients with pathologically confirmed T1 tumors had positive lymph nodes and median survival was modest at 60 months. Lymph node involvement was predictive of recurrence and decreased survival.
Conclusions
Patients with malignant tumors often present with jaundice and larger tumors. These findings should warrant suspicion for cancer and expedited preoperative workup. Based on our finding that nearly half the patients with T1 tumors had positive lymph nodes, we recommend pancreaticoduodenectomy for any patient with biopsy proven adenocarinoma who is a suitable candidate for surgery.
INTRODUCTION
With the exception of individuals with familial adenomatous polyposis (FAP), adenomas of the Ampulla of Vater arise sporadically and rarely. These tumors necessitate removal because of their known progression to carcinoma and for relief of symptoms associated with blockage of the ampulla1. Although traditionally treated surgically, advances in endoscopic technique has seen endoscopic papillectomy become an attractive alternative in the removal of benign ampullary adenomas2. Ampullary adenocarcinomas are widely treated by pancreaticoduodenectomy (PD)3 because of their propensity to spread to lymph nodes and high incidence of recurrence. In some series preoperative endoscopic biopsy has a high rate of false negative results4, prompting some researchers to recommended PD for all ampullary adenomas.
Fit patients with tumors exhibiting high-grade dysplasia or adenocarcinoma are generally treated by PD with low recurrence rates5. The perioperative mortality rate for PD has declined6, and is widely accepted at academic care centers where the procedure is routinely performed. For ampullary adenocarcinoma postoperative survival rates are rising. Recent series report 5-year postoperative survival rates of 53 - 68%7. Successful endoscopic removal of high-grade intraepithelial neoplasia, in situ tumors, and focal T1 cancers has been reported, but is not widely practiced8-10.
For patients with slow-growing benign tumors, endoscopic resection is an attractive alternative with decreased cost and morbidity11,12. Endoscopic papillectomy has a recurrence rate of 30% with 13.9% of endoscopically treated patients subsequently undergoing follow-up surgery. Success rates vary from 80% to 92% but have been high enough to prompt recommendation of endoscopic resection for benign ampullary tumors13-15. Endoscopic papillectomy’s relatively low complication rate and the elimination of standard risks associated with surgery are advantageous. The high recurrence rate of the procedure is mitigated by follow-up endoscopic surveillance2.
Can the success of the endoscopic resection be extended to small localized T1 adenocarcinomas? Selection criteria have been suggested and small series and isolated cases in high risk individuals have been reported. Our institutional bias has been to surgically resect all patients with adenocarcinoma unless they are not acceptable surgical candidates. We anticipate that the debate on the roles of surgery and endoscopy in the treatment of small cancerous tumors will likely increase as experience with endoscopic resection increases. This study aims to evaluate the Washington University Experience with tumors of the ampulla of Vater and present a strategy for optimal treatment of resectable tumors at the present time.
METHODS
Patient Selection
We retrospectively reviewed the charts of 157 patients at a tertiary teaching hospital in the Midwest who had undergone endoscopy or PD for an ampullary mass from November 2001 to June 2010. In order to achieve the most homogenous population of ampullary tumors, we excluded any patients where there was suspicion that the tumor might originate from another location and secondarily involve the ampulla (biliary, duodenal, or pancreatic). Final pathologic assessment was the gold standard for all resected tumors and radiologic, clinical and endoscopic data were reviewed in all cases for consistency with the diagnosis of an ampullary primary. All charts were reviewed to collect demographics, presenting signs and symptoms, workup and therapeutic procedures, pathology of specimens, and recurrence and survival outcomes.
Surgical Technique
Patients with benign tumors underwent endoscopic therapy, which consisted of standard snare papillectomy followed by protective pancreatic stent placement16 Those with pathologically confirmed adenocarcinoma of the Ampulla of Vater were offered a PD if they were surgically fit. No surgical ampullectomies were performed.
Statistical Analysis
Mann-Whitney non-parametric test was used to detect the difference between medians for age, BMI, ASA score, and tumor size. For other demographic analyses, the difference between proportions was analyzed by Fisher’s exact test to determine signficance by a p - value less than 0.05. The incidence of presenting signs, symptoms, and laboratory values were analyzed by a multivariate contingency table and coorelations presented as the odds ratios (OR) with 95% confidence intervals (CI) and represented with a Forest plot. An OR greater than one and a 95% CI with a lower limit not less than one indicates a greater probability of malignancy. Kaplan-Meier survival estimates were used to compare time to death in subsets of patients with a Log-rank (Mantel-Cox) test to determine significance with a P value less than 0.05. Cox proportional hazards models were used to estimate Hazard Ratios (HR) with 95% confidence interval. SPSS 12.0 and GraphPad Prism 5.0 were utilized for statistical analysis and graphical generation.
RESULTS
Patient population
We identified 157 patients from our institution’s record who presented with a resectable ampullary mass, with a median follow-up of 52 months (Table I). Fifty-one had benign masses on pathology and 106 had pathologically confirmed adenocarcinoma of the ampulla. Of the patients with benign disease, two indicentally had intraductal papillary mucinous neoplasm (IPMN) and underwent PD for that disease. Eleven patients were identified with high grade dysplasia on endoscopic biopsy, four of which ultimately proved to harbor adenocarcinoma following PD (n = 6). Ninety-nine of the patients with adenocarcinoma received surgical resection by PD, while seven underwent endoscopic palliative care with sphincterotomy and stenting. Patients in benign and malignant groups were similar in demographics according to age, sex, BMI, and ASA. A trend towards male prediliction was observed for the malignant group, but did not reach statistical signficance by Fisher’s exact test, P = 0.08 (OR 1.8, 95% CI 0.94 – 3.68), and there were an increased number of black patients presenting with adenocarcinoma (8.4%) versus benign masses (3.5%), P = 0.33, Fisher’s exact test (OR 2.55, 95% CI 0.52 – 12.07). Our patient population included ten with familial adenomatous polyposis, one of which had adenocarcinoma.
Table I.
Demographics
| Characteristics | Benign (n = 51) | Malignant (n = 106) |
|---|---|---|
| Age (y)* | 43 (21 - 90) | 71 (39 - 87) |
| Sex (male : female)† | 24 : 26 | 67 : 39 |
| Race (white : black : other)‡ | 48 : 2 : 1 | 96 : 9 : 1 |
| BMI* | 27.3 (19.3 - 42.7) | 26.3 (15.9 - 53.5) |
| ASA* | 3 (1 - 4) | 3 (1 - 4) |
| Familial adenomatous polyposis | 9 | 1 |
| Pancreaticoduodenectomy | 1 | 99 |
median (range)
male gender p = 0.08, Fisher’s exact test for difference between proportions
black race p = 0.33, Fisher’s exact test for difference between proportions
Tumor size
Tumor size was compared between those with benign and malignant disease (Table II). After ampullary masses were confirmed benign pathologically, patients underwent endoscopic papillectomy where the median diameter of the specimen was 1.3 cm (range 0.4 - 3.5 cm). Patients with adenocarcinoma either had specimens measured on pathology following PD, or determined by endoscopic ultrasound for the seven who did not receive PD. The median diameter for these was 2.0 cm (range 0.3 - 7.5 cm), which was statistically larger by a two-tailed Mann-Whitney test, P < 0.001. A tumor size greater than one cm was also predictive of malignant disease by Fisher’s exact test, P < 0.001, (OR 3.6, 95% CI 1.68 - 7.78).
Table II.
Tumor Size
| Benign | Adenocarcinoma by Stage | ||||||
|---|---|---|---|---|---|---|---|
| All | I | II | III | IV | Unknown* | ||
| Number | 51 | 106 | 25 | 50 | 23 | 1 | 7 |
| Size (cm) | |||||||
| Median | 1.3 | 2.0 | 2.0 | 2.1 | 2.0 | 3.0 | 1.2 |
| Min | 0.4 | 0.3 | 0.3 | 0.3 | 1.0 | 3.0 | 1.0 |
| Max | 3.5 | 7.5 | 5.0 | 7.0 | 7.5 | 3.0 | 4.0 |
| >1.0 cm (n) | 46‡ | 94‡ | 21 | 45 | 21 | 1 | 5 |
| ≤1.0 cm (n) | 23 | 13 | 4 | 4 | 2 | 0 | 2 |
These patients did not receive surgery.
p < 0.001, Mann-Whitney two-tailed t-test
p < 0.001, Fisher’s exact test for difference between proportions.
Lymph Node Status
Patients were identified according to T classification and substratisfied according to lymph node status to determine whether this was an independent predictor of recurrence and survival (Table III). Sixty percent of all patients undergoing PD had lymph node metastases (LNM) at the time of surgery. In addition, we observed positive LNM in 45% of T1, 41% of T2, 70% of T3, and 86% of T4. For patients with positive LNM, recurrence was twice as high (n = 10 vs. 5) and the 5-year survival rate was decreased from 63% to 47%. A statistically significant trend was not observed by substratisfication for recurrence or survival.
Table III.
Lymph Node Metastases (LNM) Status
| All (n = 97) |
T1 (n = 11) |
T2 (n = 32) |
T3 (n = 40) |
T4 (n = 14) |
|
|---|---|---|---|---|---|
| LNM Negative | |||||
| Number | 39 | 6 | 19 | 12 | 2 |
| 3 yr survival | 71% | 100% | 73% | 44% | 100% |
| 5 yr survival | 63%* | 75% | 73% | 44% | 0% |
| Recurrence (n) | 5† | 0 | 2 | 2 | 1 |
| LNM Positive | |||||
| Number | 58 | 5 | 13 | 28 | 12 |
| 3 yr survival | 61% | 75% | 100% | 40% | 39% |
| 5 yr survival | 47%* | 25% | 88% | 34% | 0% |
| Recurrence (n) | 10† | 2 | 1 | 5 | 2 |
p = 0.19, Log-rank (Mantel-Cox) test.
p = 0.78, Fisher’s exact test for difference between proportions.
Presenting signs and symptoms predict malignancy
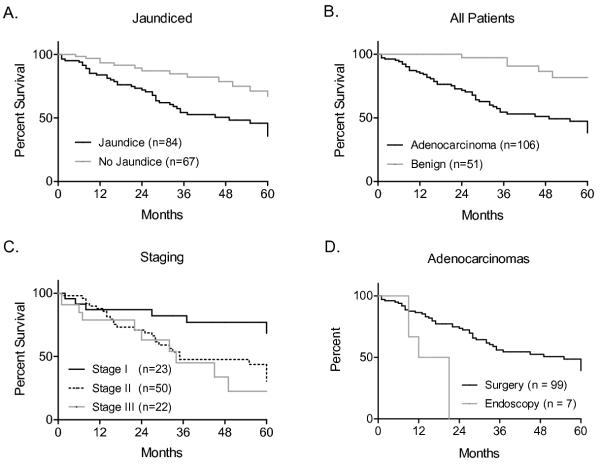
Common signs and symptoms of ampullary tumors on presentation included abdominal pain, steatorrhea, fever and chills, gastrointesinal bleed, nausea and vomiting, dark urine, pruritis, and jaundice (Fig 1). A total of 150 patients with benign or malignant disease were compared by each symptom and the odds ratio for malignancy calculated. Steatorrhea was moderately predictive of malignancy (OR 5.07, 95% CI 1.12 - 22.38), while dark urine (OR 14.18, 95% CI 1.86 - 108), pruritis (OR 16.36, 95% CI 2.15 - 124.3), and jaundice (OR 67.24, 95% CI 15.17 - 297.7) were highly predictive of malignancy. Gastrointestinal bleed, fever and chills, and nausea and vomiting were not predictive, while abdominal pain (OR 0.35, 95% CI 0.17 - 0.72) was more suggestive of benign tumor. Since jaundice had the highest odds ratio, and can be noted non-invasively on physical exam, we compared the survival of all patients who presented with or without jaundice in order to predict which patients would have a more favorable outcome (Figure 3A). Five-year survival was 43% in jaundiced patients (n = 84) and 71% for those without (n = 67), p < 0.001, Log-rank test (HR 2.52, 95% CI 1.48 – 4.31).
Figure 1.
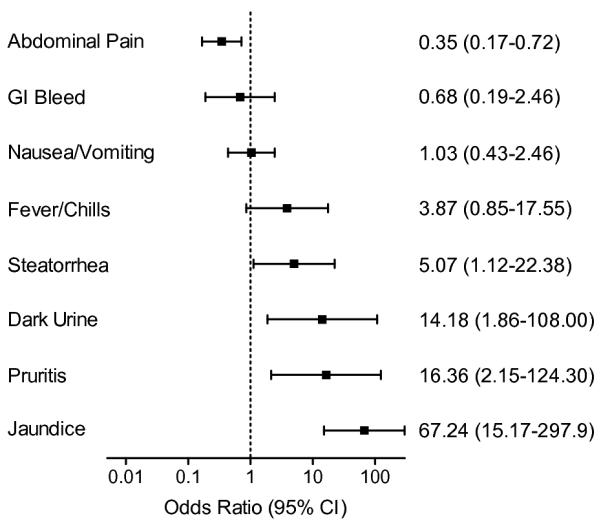
Presenting signs and symptoms for patients with ampullary tumors are predictive of malignancy (n = 150). An odds ratio greater than one is predictive of malignancy, while the the lower limit of the 95% confidence interval above one is statistically significant.
Figure 3.
Kaplan-Meier survival estimates for patients presenting with ampullary tumors. A. Patients with jaundice had a 5-year survival rate of 43% compared to 71% for those without, p < 0.001, Log-rank test. B. Patients with adenocarcinoma had a 5-year survival rate of 45% versus 82% for those with benign disease, p < 0.0001, Log-rank test. C. Patients with Stage I disease had a 5-year survival rate of 68% compared to 31% and 23% for Stages II and III respectively, p < 0.01 for Stage I vs Stage II or III, p < 0.01, Log-rank test. D. The five-year survival rate for all patients with adenocarcinoma undergoing surgery was 39% versus 0% for those without surgery, p < 0.01, Log-rank test.
To determine whether common laboratory tests correlate with signs and symptoms on presentation, we determined the odds ratio for values of the complete blood count (n = 145), serum liver enzymes (n = 138), and serum pancreatic enzymes (n = 39 − 45) (Fig 2). A white blood count greater than 9.8 mm3 was not predictive (OR 0.65, 95% CI 0.29 - 1.48), but a hematocrit less than 36.1%, while not statistically significant, trended towards malignancy (OR 2.04, 95% CI 0.94 - 4.44). Liver enzymes were most predictive with an albumin less than 3.6 g/dL (OR 2.95, 95% CI 1.22 - 7.14), total bilirubin greater than 1.1 g/dL (OR 7.97, 95% CI 2.62 - 24.29), alkaline phosphatase greater than 126 U/L (OR 9.73, 95% CI 3.81 - 24.29), ALT greater than 47 U/L (OR 2.43, 95% CI 1.08 - 5.45), and AST greater than 55 U/L (OR 2.22, 95% CI 0.99 - 4.94). Pancreatic enzymes were not predictive of malignancy, with an amylase greater than 100 IU/L (n = 39) (OR 1.69, 95% CI 0.44 - 6.47), lipase greater than 99 IU/L (n = 45) (OR 1.48, 95% CI 0.42 - 5.16), and CA 19-9 greater than 36 U/mL (n = 43) (OR 1.73, 95% CI 0.10 - 29.8). These findings suggest that increased liver enzymes are the most predictive of malignancy of these laboratory tests, and that they correlate with the signs and symptoms of biliary obstruction.
Figure 2.
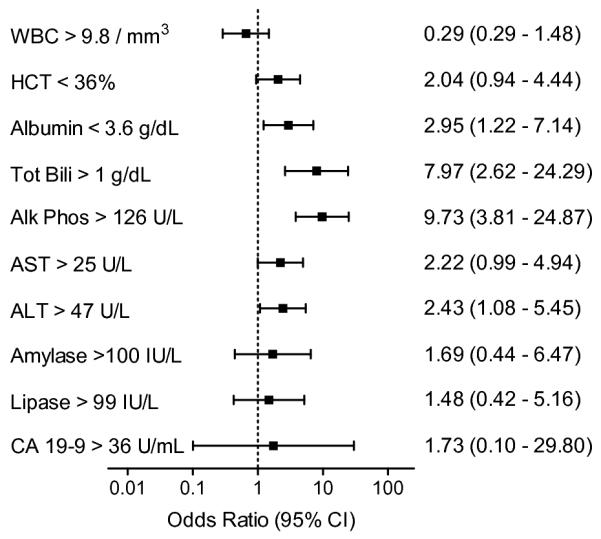
Presenting laboratory values for patients with ampullary tumors are predictive of malignancy. Complete blood count n= 145, liver enzymes n = 138, and pancreatic enzymes n = 39 − 45. An odds ratio greater than one is predictive of malignancy, while the the lower limit of the 95% confidence interval above one is statistically significant.
Disease progession predicts survival
All patients had tissue specimens evaluated by pathologists and the survival of those presenting with benign or malignant disease was compared by the Kaplan-Meier survival estimate (Figure 3B). Patients with benign disease (n = 51) had a 5-year survival rate of 82% compared to 45% for those with malignant disease (n = 106), P < 0.0001, Log-rank test. Those with malignant disease were stratisfied by Stage, and those with Stage I disease (n = 23) had a 5-year survival rate of 68% (P < 0.05), while those with Stage II (n = 50) and Stage III disease (n = 22) 31% and 23% respectively, P > 0.05, Log-rank test.
Outcomes for patients treated palliatively by endoscopy
We identified seven patients who had adenocarinoma but did not undergo PD. These patients were slightly older, with a mean age 79 ± 6 years, and a slightly higher ASA score of 3. Male sex and black race were increased to 71% and 43% respectively. Reasons to forego PD included poor nutritional status, cardiovascular disease, advanced age with poor performance status, poor medical compliance, or refusal. As expected, survival of those patients with endoscopic palliation, consisting of debulking, sphincterotomy, and stenting, was decreased compared to those undergoing surgical resection of adenocarcinoma, 5-year survival of 0% and 39% respectively , P < 0.01, Log-rank test (Figure 3D).
T1 tumor size is not predictive of LNM
Eleven patients who underwent PD were found to have T1 tumors, 5 of whom had positive LNM (Table IV). Since other studies have established that the presence of LNM is associated with recurrence and decreased survival3,17, we compared tumor size and histologic differentiation amongst T1 tumors with LNM status. The median tumor diameter for negative LNM was 2.0 cm (range 0.3 – 2.5) versus 1.25 cm (range 0.5 – 1.7 cm) for positive LNM, the difference of which was not statistically significant, P > 0.05, Mann-Whitney test. Specimens were graded by differentiation on histology and we observed three well-differentiated, five moderately-differentiated, and three poorly-differentiated, which did not correlate with either recurrence or LNM, P > 0.05, Fisher’s exact test.
Table IV.
T1 Tumor Size
| Size (cm) |
Lymph Nodes | Histology (Differentiation) |
Recurrence | Survival (Months) |
Alive | |
|---|---|---|---|---|---|---|
| Examined | Positive | |||||
| 0.3 | 23 | 0 | Well | no | 61 | Yes |
| 0.3 | 7 | 6 | Moderate | yes | 15 | No |
| 0.5 | 16 | 2 | Moderate | no | 7 | Yes |
| 1.0 | 11 | 1 | Poor | no | 49 | No |
| 1.5 | 7 | 1 | Poor | no | 60 | No |
| 1.0 | 15 | 0 | Moderate | no | 44 | Yes |
| 1.6 | 5 | 5 | Well | no | 37 | No |
| 1.7 | 9 | 1 | Moderate | yes | 91 | Yes |
| 2.0 | 15 | 0 | Poor | no | 54 | Yes |
| 2.3 | 13 | 0 | Well | no | 14 | Yes |
| 2.5 | 18 | 0 | Moderate | no | 10 | Yes |
DISCUSSION
Treatment of benign ampullary tumors by endoscopic papillectomy has become the preferred approach over local surgical resection due to the decreased morbidity and cost. A local recurrence rate of up to 30%2 has been reported. Upper gastrointestinal endoscopy has been useful in the detection and evaluation of incidental masses, as well as routine survellience for patients with FAP and those who have undergone previous papillectomy. Due to the apparent benefits of endoscopy in the management of ampullary masses, some have suggested that focal T1 adenocarcinomas may be approachable by this method8,10,18. Evidence of oncologic equivalence for this approach is lacking, so we have evaluated our own experience to better understand the disease and guide future clinical therapy.
We offered PD for all patients with histologically proven adenocarcinoma or high grade dysplasia if they were acceptable sugical candidates. Four of eleven patients with high grade dysplasia ultimately had adenocarcinoma, and in patients with T1 disease, we found almost half had regional nodal disease. Patients who were LNM negative (n = 6) experienced excellent survival and no recurrences have been detected , while those who were LNM positive (n = 5) experienced two recurrences and diminished survival. This is one of a few studies specifically reporting a comparison of LNM negative versus positive disease in T1 tumors and supports the growing literature showing early dissemination of ampullary cancers. In a recent study, Winter et al discovered that 28% of their 25 patients with T1 disease had positive LNM17. In another, Yoon et al reported that only 9% had positive LNM in 66 patients with localized tumors, however they experienced 12 recurrences (18%)8. Additionally, one study found the incidence of microlymphovascular invasion to be 57% in 30 patients with T1 disease19.
Our experience confirms a high level of lymph node metastases and highlights the importance of radical surgical intervention in those patients with localized T1 adenocarcinomas.. Currently there is insufficient data to determine if radical resection aids only in staging or if it contributes to increased cure rates. Certainly staging is important in directing patients towards appropriate adjuvant therpies. Much larger cohorts will be needed to fully address whether endoscopic resection should play any role in this disease. Selection of patients with T1 lesions for endoscopic resection may have to wait for advances in molecular diagnostics because we could not identify a group safe for local resection based on convential staging criteria (size, depth, differention).
While PD is the best therapeutic option for the treament of ampullary adenocarincoma, we found that seven of our patients were not candidates for surgical intervention. These patients were treated by endoscopic palliation with sphinctertomy and stenting, and endoscopic debulking and thermoablation (one patient). We observed rapid disease progression and low survival for those patients who did not undergo radical resection , with none surviving past 22 months. Several of these patients died of their comorbid diseases but it was apparent from the records that having an underlying, untreated malignacy contributed significantly to decisions regarding how aggressive to be with other medical problems.
Patients with ampullary tumors often present with signs and symptoms of biliary obstruction or gastrointestinal bleed that warrant workup. While ERCP, EUS, and CT can be confirmatory of a suspicion of malignancy, our review has shown that simple observation of signs and symptoms are similarly predictive. For all ampullary masess we found clinical jaundice was the strongest independent predictor of a cancer diagnosis and reduced long-term survival (OR 67, 95% CI 15 – 298), supporting similar observations20 by other investigators. Those presenting with jaundice had a five-year survival rate of 46% compared with 71% for those presenting without juandice. Not surprisingly laboratory tests that support signs and symptoms of biliary obstruction were also predictive of malignancy. Based upon these findings, we suggest that presentation with painless jaundice and/or elevated liver enzymes and an ampullary lesion should be considered a cancer until proven otherwise. Benign ampullary adenomas very rarely presented with biliary obstruction.
Our survival results are in accordance with those reported by the national SEER database for survival of ampullary adenocarinoma21. Our patient population revealed other features confirmed to be prognostic of survival, including male sex, black race, and tumor diameter. We observed fewer cases of malignancy in FAP patients (1 : 10), but they were under increased surveillance and may have received earlier intervention. Multiple groups have examined their own results with ampullary adenocarcinomas and found that increased stage, lymph node metastatses, and perineural invasion to be prognostic of overall survival17,22-25. In our cohort, we expected stage and LNM to be prognostic, however, only stage was signficantly associated with decreased survival. We attribute our inability to identify LNM as an independent predictor of survival as a deficiency in power. Despite this, we suggest that resection of T1 lesions with PD might aid in staging, direction of therapy, and potentially cure.
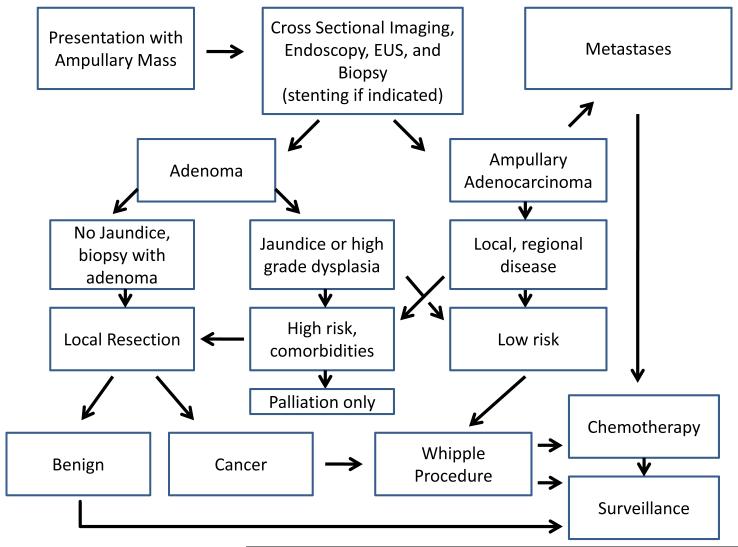
In this retrospective review of 157 patients with a mass of the Ampulla of Vater, we observed that many patients can be predicted to have malignant disease based on the presence of jaundice alone, and offer a schematic for optimal management (Figure 4). Based on our finding that nearly half of patients with a localized T1 tumor are LNM positive, we recommend PD for any patient with biopsy proven adenocarinoma. Until the advent of more precise staging criteria we plan to reserve endoscopic resection of T1 ampullary cancers to those patients who are not suitable surgical candidates.
Figure 4.
Acknowledgments
The authors would like to thank Kim Trinkaus, Division of Biostatistics, Siteman Cancer Center, for consultation of statistical analysis.
Footnotes
The authors have no conflicts of interest to disclose, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649–669. doi: 10.1016/s1052-5157(03)00101-6. [DOI] [PubMed] [Google Scholar]
- (2).Adler DG, Qureshi W, Davila R, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- (3).Brown KM, Tompkins AJ, Yong S, Aranha GV, Shoup M. Pancreaticoduodenectomy is curative in the majority of patients with node-negative ampullary cancer. Arch Surg. 2005;140:529–532. doi: 10.1001/archsurg.140.6.529. [DOI] [PubMed] [Google Scholar]
- (4).Jordan PH, Jr., Ayala G, Rosenberg WR, Kinner BM. Treatment of ampullary villous adenomas that may harbor carcinoma. J Gastrointest Surg. 2002;6:770–775. doi: 10.1016/s1091-255x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- (5).Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov. 2004;11:255–263. doi: 10.1177/155335060401100409. [DOI] [PubMed] [Google Scholar]
- (6).Strasberg SM, Drebin JA, Mokadam NA, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. 2002;194:746–758. doi: 10.1016/s1072-7515(02)01202-4. [DOI] [PubMed] [Google Scholar]
- (7).Schmidt CM, Powell ES, Yiannoutsos CT, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004;139:718–725. doi: 10.1001/archsurg.139.7.718. [DOI] [PubMed] [Google Scholar]
- (8).Yoon YS, Kim SW, Park SJ, et al. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann Surg. 2005;242:92–100. doi: 10.1097/01.sla.0000167853.04171.bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Yoon SM, Kim MH, Kim MJ, et al. Focal early stage cancer in ampullary adenoma: surgery or endoscopic papillectomy? Gastrointest Endosc. 2007;66:701–707. doi: 10.1016/j.gie.2007.02.049. [DOI] [PubMed] [Google Scholar]
- (10).Woo SM, Ryu JK, Lee SH, et al. Feasibility of endoscopic papillectomy in early stage ampulla of Vater cancer. J Gastroenterol Hepatol. 2009;24:120–124. doi: 10.1111/j.1440-1746.2008.05578.x. [DOI] [PubMed] [Google Scholar]
- (11).Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- (12).Katsinelos P, Paroutoglou G, Kountouras J, et al. Safety and long-term follow-up of endoscopic snare excision of ampullary adenomas. Surg Endosc. 2006;20:608–613. doi: 10.1007/s00464-004-2278-0. [DOI] [PubMed] [Google Scholar]
- (13).Saurin JC, Chavaillon A, Napoleon B, et al. Long-term follow-up of patients with endoscopic treatment of sporadic adenomas of the papilla of vater. Endoscopy. 2003;35:402–406. doi: 10.1055/s-2003-38767. [DOI] [PubMed] [Google Scholar]
- (14).Catalano MF, Linder JD, Chak A, et al. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- (15).Desilets DJ, Dy RM, Ku PM, et al. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- (16).Cote GA, Edmundowicz SA. The role of endoscopic ultrasonography (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) in the evaluation and management of ampullary adenomas. Tech.in Gastrointest.Endosc. 2009;11:49–57. [Google Scholar]
- (17).Winter JM, Cameron JL, Olino K, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010;14:379–387. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- (18).Jung MK, Cho CM, Park SY, et al. Endoscopic resection of ampullary neoplasms: a single-center experience. Surg Endosc. 2009;23:2568–2574. doi: 10.1007/s00464-009-0464-9. [DOI] [PubMed] [Google Scholar]
- (19).Lee SY, Jang KT, Lee KT, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–788. doi: 10.1016/j.gie.2005.09.015. [DOI] [PubMed] [Google Scholar]
- (20).Yokoyama N, Shirai Y, Wakai T, Nagakura S, Akazawa K, Hatakeyama K. Jaundice at presentation heralds advanced disease and poor prognosis in patients with ampullary carcinoma. World J Surg. 2005;29:519–523. doi: 10.1007/s00268-004-7709-5. [DOI] [PubMed] [Google Scholar]
- (21).O’Connell JB, Maggard MA, Manunga J, Jr., et al. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820–1827. doi: 10.1245/s10434-008-9886-1. [DOI] [PubMed] [Google Scholar]
- (22).de Paiva Haddad LB, Patzina RA, Penteado S, et al. Lymph node involvement and not the histophatologic subtype is correlated with outcome after resection of adenocarcinoma of the ampulla of vater. J Gastrointest Surg. 2010;14:719–728. doi: 10.1007/s11605-010-1156-4. [DOI] [PubMed] [Google Scholar]
- (23).Lowe MC, Coban I, Adsay NV, et al. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am Surg. 2009;75:754–760. [PubMed] [Google Scholar]
- (24).Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Qiao QL, Zhao YG, Ye ML, et al. Carcinoma of the ampulla of Vater: factors influencing long-term survival of 127 patients with resection. World J Surg. 2007;31:137–143. doi: 10.1007/s00268-006-0213-3. [DOI] [PubMed] [Google Scholar]