Abstract
Purpose
To describe clinical and functional features of a patient with Bietti crystalline dystrophy and atypical electroretinogram responses.
Methods
The patient underwent a thorough medical anamnesis, genetic counseling, peripheral blood draw for CYP4V2 gene analysis and electron microscopy, and a complete ophthalmological assessment including optical coherence tomography, indocyanine green angiography, microperimetry, full-field electroretinogram and multifocal electroretinogram.
Results
The most striking features of the retina were deposits of yellowish-white glistening crystals and focal lobular areas of choriocapillary atrophy at the posterior pole and midperiphery. The full-field electroretinogram was normal and the multifocal electroretinogram showed extinguished central recordings. Mutation analysis revealed a homozygous c. 332T>C p.I111T mutation in exon 3 of the CYP4V2 gene. Typical cytoplasmic inclusions containing crystalline-like structure and large degenerative lysosomes were seen on electron microscopy of peripheral leukocytes.
Conclusion
Here we describe a patient with Bietti crystalline dystrophy with a CYP4V2 gene mutation and typical leukocyte inclusions who showed the classical retinal lesions but had a normal electroretinogram. This suggests the existence of less severe forms of BCD related to relatively mild CYP4V2 mutations.
Keywords: bietti, electroretinogram, CYP4V2 gene
INTRODUCTION
Bietti crystalline dystrophy (BCD) is a rare autosomal recessive retinal dystrophy characterized by the presence of glistening intraretinal crystals associated with atrophy of Retinal Pigment Epithelium (RPE), pigment clumping, choroidal sclerosis, and in some cases with crystal deposits at the corneal limbus.1–3 The first symptoms typically appear in the third to fourth decade of life with night blindness, decreased visual acuity and progressive visual field constriction.
BCD is caused by mutations in the CYP4V2 gene, a novel family member of the cytochrome P450 genes on chromosome 4q35.4 This gene codes for a protein, whose structure suggests that it may be active in fatty acid metabolism; biochemical studies show that abnormal lipid metabolism is present in patients with BCD,5 and biochemical characterization of CYP4V2 shows that it is a fatty acid omega-hydroxylase.6
Previous studies have shown clinical heterogeneity in BCD patients, although there was good correlation between clinical stages of the disease and electroretinographic (ERG) changes.2,3,7 The RPE-choriocapillaris complex is the anatomical structure initially involved in the early stage of the disease, at which point phototransduction remains normal. The ERG responses and visual fields are affected progressively during the course of changes of RPE and choriocapillaris (CC).2,3,7
This study describes a case of BCD, with an identified CYP4V2 mutation and typical leukocyte inclusions. Clinically the patient shows the presence of glistening intraretinal crystals associated with extensive atrophy of the RPE and choriocapillaris. However, there are normal full-field ERG responses.
CASE REPORT
Materials and methods
The patient was consented at the Referral Centre for Hereditary Retinopathies of the Second University of Naples and this work is approved by the Second University of Naples and National Eye Institute IRBs. He underwent a thorough medical anamnesis, genetic counseling, peripheral blood draw to assess cholesterol and triglyceride levels and a complete ophthalmological exam including central visual acuity with EDTRS charts, Ishihara color vision tests, slit lamp biomicroscopy of the anterior segment, fundus exam, fundus photography, Goldman visual field, Optical coherence tomography (OCT), indocyanine green angiography (ICG), microperimetry (MP1), multifocal ERG (mfERG) and standard ERG according to the standard protocol of the International Society for Clinical Electrophysiology of Vision (ISCEV).8,9 Our normal ERG values are as follows: scotopic blue flash 379 (SD 104) μV, photopic white flash 210 (86) μV 30 Hz flicker 75 (35) μV. Eighteen normal control subjects (age range 30–55 years) provided normative data for electroretinogram measurements. Research was carried out in compliance with the Helsinki Declaration.
BCD diagnosis was made assessing typical ophthalmoscopic findings according to the Yuzawa classification10 and clinical symptoms.
DNA was isolated and CYP4V2 exon and flanking sequences were determined as previously described.4 Primer sequences amplifying each exon and approximately 100 bases of flanking introns and their reaction conditions are described in Table A1 in the supplementary material of Li et al.4 Briefly, DNA was sequenced with the ABI BigDye Terminator cycle sequencing kit v3.1, according to the manufacturer's recommendations (Applied Biosystems), through use of an ABI 3130 DNA sequencer. The sequence information was imported into Mutation Surveyor V3.25, (SoftGenetics LLC, State College, PA, USA) and sequences of affected and normal individuals and consensus sequence were aligned to identify variations. Electron microscopy was performed as previously described.4
RESULTS
Genetic assessment revealed that the patient was born from a non-consanguineous marriage, highlighting the sporadic nature of the disease. The medical history excluded systemic diseases such as cystinosis, oxalosis, gyrate atrophy and the use of drugs (tamoxifen) that can cause intraretinal crystalline clusters. Furthermore, peripheral blood exam evidenced hypercholesterolemia that was treated only by diet.
This 57-year-old patient presented to our Clinic referring a progressively decreasing visual acuity and night blindness that started at the age of 35. Central visual acuity was 20/600 in the right eye and 20/50 in the left eye with a myopic refraction of –1; color vision results were normal and slit lamp biomicroscopy showed no corneal crystals but presence of nuclear sclerosis of the lens.
A fundus exam showed diffuse glistening yellow crystalline deposits in the posterior pole and the mid-periphery; RPE and choriocapillaris atrophy, more evident in the right eye; and normal optic discs and retinal vessels (Figure 1a and c). The ophthalmoscopic conditions can be classified as Yuzawa stage 2.10 The Goldman visual field examination showed the presence of bilateral paracentral scotomas with a slight narrowing of the peripheral isopters. The OCT exam showed an abnormal level of diffuse hyper-reflectivity in the neuroretinal layers, RPE atrophy and a noticeable reduction of macular thickness.
FIGURE 1.
(a, c) Retinography shows diffuse glistening yellow crystal deposits in the posterior pole and in midperiphery, RPE and choriocapillaris atrophy; (b, d) indocyanine green angiography shows large atrophy of retinal pigment epithelium, choriocapillaris and choroidal sclerosis.
ICG revealed focal lobular areas of choriocapillary atrophy at the equator and at the posterior pole (Figure 1b and d). MP1 showed bilateral central scotomas with unstable fixation in the right eye and stable fixation in the left eye. The standard ERG showed normal scotopic (RE: 255.4 μV; LE: 259.2 μV) and photopic (RE: 115.6 μV; LE: 117.8 μV) recordings (Figure 2). The multifocal ERG showed extinguished central recordings and microvolted peripheral recordings.
FIGURE 2.
Full-field electroretinograms of the patient and a normal subject.
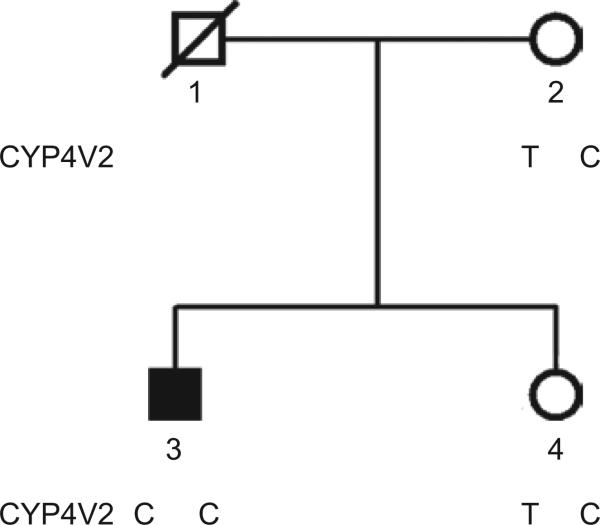
Molecular analysis of the CYP4V2 gene revealed a homozygous c.332T>C, p. I111T mutation in exon 3. The pedigree was consistent with recessive inheritance of the mutant CYP4V2 allele as the mother and only sibling inherited are heterozygous for the I111T mutant allele (Figure 3).
FIGURE 3.
Pedigree of family 33019 showing the 332T (wild type) or 332C (I111T mutant) genotypes.
The ultrastructural examination revealed abnormal cytoplasmic lysosomes containing complex crystals and granular osmiophilic material in a few peripheral blood leukocytes (Figure 4A). Many large degenerative cytoplasmic inclusions are found in the leukocytes (Figure 4B).
FIGURE 4.
(a) EM image showing few cytoplasmic lysosomes containing typical crystalline slits (arrows), limited by membrane, in some lymphocytes. (b) A large cytoplasmic inclusion (asterisk) packed with numerous small degenerative lysosomes in a cytoplasm of leukocyte. Scale bar: 500 nm for A and B, 250 nm for inset.
DISCUSSION
The examinations performed demonstrate that our patient meets all clinical criteria for BCD. The diagnosis was made on the basis of a history of night blindness, decreased visual acuity, progressive visual field constriction, and diffuse clumping of typical yellowish-white glistening crystals and choriocapillary atrophy appearing towards the third to fourth decade of life. Our patient showed no crystal deposits at the limbus, but the literature previously has reported cases of BCD in which crystals are present only in the retina.11
We unexpectedly observed that ERG amplitude and latency in our patient remained within normal limits even 20 years after the onset of symptoms, while previous studies have reported that the ERG in BCD correlates with disease progression.2,3,7 However in this case the multifocal ERG detected markedly depressed central responses that correlated with the macular lesion observed by OCT.
Thus, despite the thinning and impaired retinal lamination seen in our proband at sites of complete RPE/CC atrophy, ERG responses suggest that the neural retina is still contributing to the ERG signals and is therefore still viable. In fact an electrophysiological study of BCD suggested that decreased numbers of photoreceptors and/or outer segment shortening may be present while the mechanism by which absorbed photons are converted into an electrical response remains normal and phototransduction becomes severely affected only when damage to the retina progresses.7 We find the apparent ability of the neural retina to survive remarkable and of therapeutic and prognostic relevance, suggesting that gene or conventional therapy might remain a viable option even in the late stages of the disease.
Moreover our patient showed hypercholesterolemia as has previously been described in some BCD patients. The presence of this lipid alteration might be related to the function of the BCD gene, CYP4V2, which is involved in cholesterol and fatty acid metabolism. In fact molecular analysis revealed a c.332T>C, p. I111T mutation in the CYP4V2 gene in our patient. While this mutation would not be predicted to be as severe as a termination or large deletion, it has been reported before in a number of BCD patients.1 Ultrastructure of peripheral blood leukocytes also demonstrated typical crystalline lysosomes in the cytoplasm.12
In conclusion, we have described a patient with retinal degeneration accompanied by retinal crystals typical of BCD, typical leukocyte inclusions, and a previously described CYP4V2 mutation, but with distinct ERG findings. These findings suggest the existence of less severe clinical variants of BCD that could be due to the c.332T>C, p.I111T in the CYP4V2 gene being relatively mild. Future studies should be able to provide a genotype phenotype correlation to explain mild BCD cases.
ACKNOWLEDGMENTS
We are grateful to Carmela Acerra for text editing.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
An earlier version of this paper, appearing online ahead of print, listed three author names incorrectly. These erroneous names (Li Anren, Zhang Jun, and Hejtmancik J. Fielding) have been corrected in this version of the paper (as Anren Li, Jun Zhang, and J. Fielding Hejtmancik).
REFERENCES
- 1.Mataftsi A, Zografos L, Millá E, Secrétan M, Munier FL. Bietti's crystalline corneoretinal dystrophy: a cross-sectional study. Retina. 2004;24:416–426. doi: 10.1097/00006982-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lee KY, Koh AH, Aung T, Yong VH, Yeung K, Ang CL, Vithana EN. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations. Invest Ophthalmol Vis Sci. 2005;46:3812–3816. doi: 10.1167/iovs.05-0378. [DOI] [PubMed] [Google Scholar]
- 3.Mansour AM, Uwaydat SH, Chan CC. Long-term follow-up in Bietti crystalline dystrophy. Eur J Ophthalmol. 2007;17:680–682. doi: 10.1177/112067210701700434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li A, Jiao X, Munier FL, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2. Am J Hum Genet. 2004;74:817–826. doi: 10.1086/383228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J, Jiao X, Hejtmancik JF, Kaiser-Kupfer M, Gahl WA, Markello TC, et al. The metabolism of fatty acids in human Bietti crystalline dystrophy. Invest Ophthalmol Vis Sci. 2001;42:1707–1714. [PubMed] [Google Scholar]
- 6.Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid {omega}-hydroxylase. Drug Metab Dispos. 2009;37:2119–2122. doi: 10.1124/dmd.109.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usui T, Tanimoto N, Takagi M, Hasegawa S, Abe H. Rod and cone a-waves in three cases of Bietti crystalline chorioretinal dystrophy. Am J Ophthalmol. 2001;132:395–402. doi: 10.1016/s0002-9394(01)00963-1. [DOI] [PubMed] [Google Scholar]
- 8.Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update). Doc Ophthalmol. 2004;108:107–114. doi: 10.1023/b:doop.0000036793.44912.45. [DOI] [PubMed] [Google Scholar]
- 9.Brigell M, Bach M, Barber C, Moskowitz A, Robson J. Guidelines for calibration of stimulus and recording parameters used in clinical electrophysiology of vision. Doc Ophthalmol. 2003;107:185–193. doi: 10.1023/a:1026244901657. [DOI] [PubMed] [Google Scholar]
- 10.Yuzawa M, Mae Y, Matsui M. Bietti's crystalline retinopathy. Ophthalmic Paediatr Genet. 1986;7:9–20. doi: 10.3109/13816818609058037. [DOI] [PubMed] [Google Scholar]
- 11.Grizzard WS, Deutman AF, Nijhuis F, de Kerk AA. Crystalline retinopathy. Am J Ophthalmol. 1978;86:81–88. doi: 10.1016/0002-9394(78)90019-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser-Kupfer MI, Chan CC, Markello TC, et al. Clinical biochemical and pathological correlations in Bietti's crystalline dystrophy. Am J Ophthalmol. 1994;118:569–582. doi: 10.1016/s0002-9394(14)76572-9. [DOI] [PubMed] [Google Scholar]