Abstract
Importance of the field
Chemotherapy induced nausea and vomiting (CINV) is a common complication in the treatment of patients with cancer. The introduction of the first in class neurokinin-1 receptor antagonist aprepitant provided additive control on CINV in combination to existing antiemetics. Due to formulation issues, aprepitant is only available for oral administration. Fosaprepitant, a prodrug of aprepitant, was introduced to the market in 2008 as an intravenous bioequivalent to aprepitant.
Areas covered in this review
This review examines the chemical development of fosaprepitant, its pharmacokinetic properties, approved uses, and potential applications.
What the reader will gain
The reader will get up-to-date information on the pharmacology and clinical uses of fosaprepitant. Clinical studies have demonstrated pharmacokinetic bioequivalence of aprepitant 125-mg to fosaprepitant 115-mg, as well as comparable efficacy in prevention of acute and delayed emesis following the first day of chemotherapy regimens.
Take home message
Fosaprepitant is an IV pro-drug of aprepitant that offers a new alternative to patients with CINV. Currently, fosaprepitant can substitute oral aprepitant in the first day of a 3-day regimen. Current studies show that a single-day fosaprepitant regimen is also bioequivalent to the 3-day aprepitant regimen, this could significantly simplify the care for CINV patients in the future.
Keywords: fosaprepitant, aprepitant, emesis, chemotherapy induced nausea and vomiting, neurokinin-1 receptor antagonist
1. Introduction
Nausea and vomiting are common adverse events experienced following chemotherapy which can lead patients to delay or refuse potentially curative treatment. This condition, known as chemotherapy-induced nausea and vomiting (CINV), and is divided in two phases: acute onset, which occurs within 24 hours of administration of chemotherapy agent or delayed onset, which occurs more than 24 to 120 hours after treatment.1 CINV is further divided into anticipatory, which is considered a conditioned response occurring before a secondary cycle of chemotherapy in those who have had previous CINV; breakthrough, which occurs despite the use of prophylactic treatment and/or requires rescue antiemetic agents; and refractory, which occurs during subsequent treatment cycles when prophylaxis and/or rescue have failed in earlier cycles.2
Antiemetic therapy has greatly advanced in the past twenty years and currently CINV can be prevented in either more than or > 80% of patients if proper prophylactic treatment is employed.3 The ideal treatment of CINV is prevention through the use of prophylactic modalities, incorporating a combination of several agents which modulate neurotransmitter receptors in the central nervous system (CNS) and gastrointestinal tract. Traditional antiemetic agents for CINV have included serotonin 5-HT3 receptor antagonists, corticosteroids, and dopamine receptor antagonists. A more recent development has been the neurokinin-1 (NK1) receptor antagonists. The optimal selection of agents is driven by the acuity of CINV (acute or delayed) and emetogenic potential of chemotherapy. The Multinational Association of Supportive Care in Cancer (MASCC), the American Society of Clinical Oncology (ASCO), and the National Comprehensive Cancer Network (NCCN) publish guidelines which classify chemotherapeutic agents based on their emetogenic potential. All guidelines employ a classification of four emetic risk groups: high (>90%), moderate (30–90%), low (10–30%), and minimal (<10%), based on the percentage of patients having emetic episodes when no prophylactic antiemetic protection is provided.1, 4, 5
Patients also commonly encounter nausea and emesis in the peri-operative time period. Post operative nausea and vomiting (PONV) is prevented and treated with many of the same agents used in CINV6. The development of newer agents, including the second-generation 5-HT3 receptor antagonist palonosetron and the first-in-class NK1 receptor antagonist aprepitant offer additional benefit to patients at risk for emesis. Animal and human studies have shown that aprepitant augments the antiemetic affect of currently available 5-HT3 receptor antagonists and corticosteroids.7, 8 The approval of fosaprepitant dimeglumine, an intravenous pro-drug of aprepitant, has provided a new option for healthcare providers and patients (see drug summary box).
Drug Summary Box.
| Drug name | Fosaprepitant Dimeglumine |
| Phase | Launched |
| Indication | Radio/chemotherapy-induced nausea and vomiting |
| Pharmacology description | Neurokinin 1 receptor antagonist |
| Route of administration | Intravenous |
| Chemical structure |
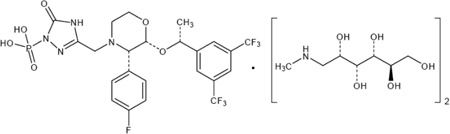
|
| Pivotal trial(s) | Lasseter, KC et al. J. Clin. Pharm. (2007) |
Pharmaprojects - copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
1.1 NK1 Receptor Antagonists: Aprepitant, Fosaprepitant, and Casopitant
The preferred ligand of the NK1 receptor is substance P, an 11-amino acid neuropeptide of the tachykinins family.9 The NK1 receptor, which is highly expressed in the CNS and other tissues, mediates the vomiting reflex. Substance P released from enterochromaffin cells binds to enteral and CNS NK1 receptors. The clinical efficacy of NK1 receptor antagonists is correlated to high levels (> 90%) of occupancy of NK1 receptors in the brain, demonstrating the primacy of CNS receptors in emetic pathways.10 The antiemetic effect of NK1 receptor antagonists was initially demonstrated in a ferret model of cisplatin-induced emesis by the non-peptide NK1 receptor antagonist CP-99,994.11 Human clinical trials leading to the approval of the first-in-class NK1 receptor antagonist aprepitant established the key role of NK1 receptor antagonism in obviating emetic symptoms. Aprepitant, known as Emend and previously identified as L-754030 and MK-0869, is FDA-approved for the prevention of CINV and PONV. Aprepitant efficacy in CINV was demonstrated in a series of Phase III trials in patients with highly emetogenic chemotherapy.7, 12, 13 The efficacious CINV regimen consists of intravenous ondansetron 32-mg, oral aprepitant 125-mg, and oral dexamethasone 8-mg on day 1, followed by oral aprepitant 80-mg on days 2–4, and oral dexamethasone 8-mg daily on days 2–4.14 The addition of aprepitant relative to standard dual therapy increases complete response rates significantly in all cases14. Aprepitant efficacy was also demonstrated in the prevention of CINV in breast cancer patients who received an anthracycline plus cyclophosphamide MEC regimen.15
Following FDA approval of aprepitant in 2003, revised guidelines for antiemetic treatment of CINV were published by ASCO, MASCC and NCCN.1, 4, 16,3 All three guidelines suggest a combination of 5-HT3 receptor antagonist, dexamethasone, and aprepitant within the first 24 hours for acute CINV with highly emetogenic chemotherapy. A combination of dexamethasone and aprepitant is suggested for delayed CINV with highly emetogenic therapy. There are differences in the guidelines when it comes to acute CINV with moderately emetogenic therapy. The ASCO and MASCC recommend aprepitant included in the triple regimen for patients receiving a regimen based on combination anthracycline and cyclophosphamide. In contrast, the NCCN guidelines suggest the use of aprepitant in selected patients receiving other moderately emetogenic therapies. Aprepitant is recommended for delayed CINV with moderately emetogenic chemotherapy as monotherapy if it has been used in the prevention of acute CINV. Guidance on the use of antiemetics for the prevention of CINV have also been developed by the British Columbia Cancer Agency and the European Society of Medical Oncology (ESMO)16, 17.
The intravenous pro-drug of aprepitant, fosaprepitant, has been approved by the FDA and EMEA as a substitute for oral aprepitant in day one of a 3-day regimen for the prevention of acute and delayed nausea and vomiting associated with highly and moderately emetogenic chemotherapy in combination with a 5-HT3 antagonist and dexamethasone.3, 8
The NK1 receptor antagonist casopitant mesylate has been investigated for use in CINV and PONV. Casopitant was originally filed for approval with the FDA in May 2008. FDA requests for additional safety data led to the sponsor to withdraw both EMEA and FDA approval applications for the drug in September 2009.18, 19 The sponsor has no currently active investigational protocols with this compound.20
2. Chemical Development of Fosaprepitant and Pre-Clinical Studies
An intravenous formulation of an antiemetic has advantages over oral in many circumstances. Attempts to develop an intravenous preparation of aprepitant were limited by a very low water solubility (0.2 µg/mL in isotonic saline), which precluded development in an aqueous form. Initially, sulfonic acid salts of weakly basic aprepitant were generated, however these salts dissociated in aqueous media and resulted in precipitation of aprepitant.21 In order to overcome these issues, a prodrug approach was used. The strategy targeted the development of a N-phosphoryl derivative of aprepitant with the potential to be metabolized in vivo back to the original compound.
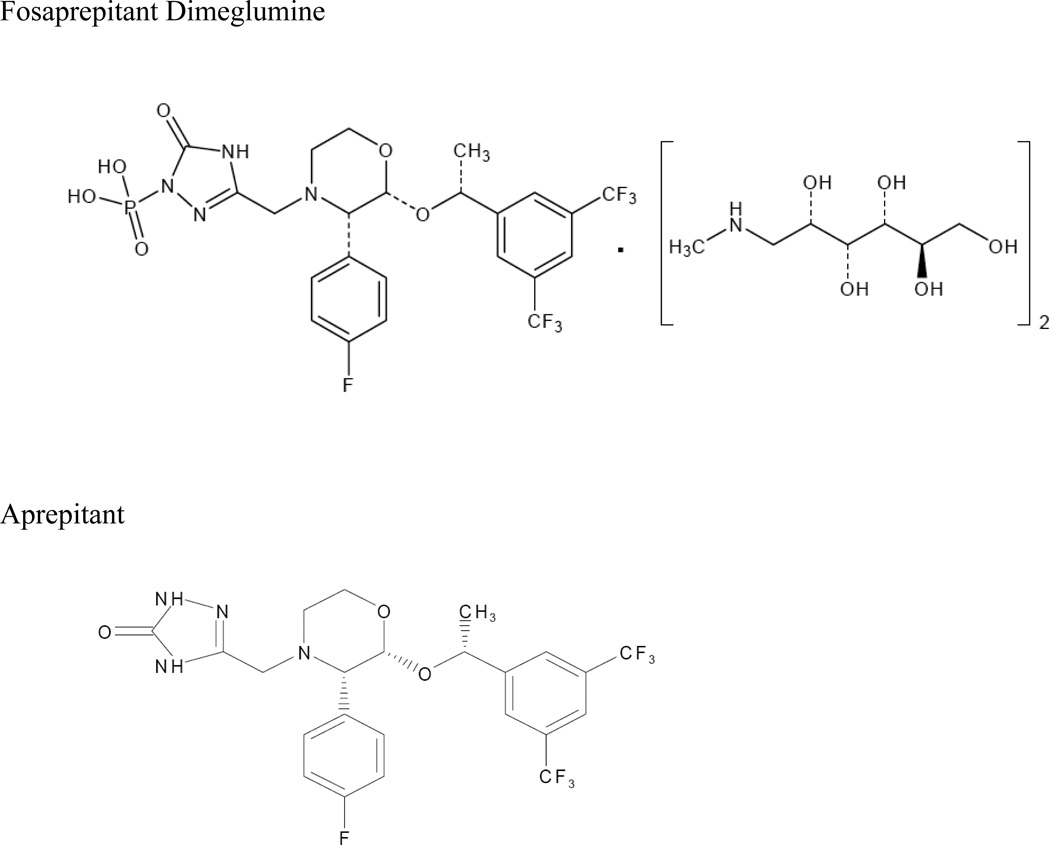
A series of synthetic routes were investigated which yielded the generation of a stable salt with greatly enhanced solubility (12mg/mL in isotonic saline).21 The resulting compound is chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]phosphonate (2:1) (salt). Its empirical formula is C23H22F7N4O6P • 2(C7H17NO5).8 Fosaprepitant dimeglumine, identified previously as MK-0517 or L-785298, is a white to off-white amorphous powder, its molecular weight is 1004.83, and is freely soluble in water. The chemical structures of aprepitant and fosaprepitant are shown in figure 1.
Figure 1. Chemical structures.
Pharmaprojects -- copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
Fosaprepitant was determined to have 10-fold less affinity for the human NK1 receptor than aprepitant (IC50 1.2 vs 0.09 nM, respectively).21 The conversion of the prodrug to the active form has been investigated ex vivo in rat, dog, and human blood.21, 22 Conversion to the active form is rapid in rat (t½ ~ 30 mins) and slower in dog (t½ > 300 mins). Fosaprepitant was stable in human blood with less than ~15% conversion observed in a two-hour incubation period. In contrast, conversion of fosaprepitant to the active compound in human liver microsomes is rapid, with only 3% of the prodrug remaining after 15 minutes. Intravenous administration of fosaprepitant in rat and dogs shows a near proportional increase in aprepitant at lower doses (1 and 8 mg/kg in the rat, 0.2 mg/kg in the dog). Nonlinear kinetics for the increase in AUC values of aprepitant have been observed for both models at higher doses (25 mg/kg in the rat, 2 and 32 mg/kg in the dog) possibly reflecting the saturation of elimination of aprepitant at these doses.21, 22
Fosaprepitant has antiemetic activity in the ferret and the guinea pig. Indeed, fosaprepitant is equipotent to aprepitant in suppressing an emetic response due to cisplatin in the ferret and in inhibiting peripheral inflammation after resiniferatoxin challenge in the guinea pig.21 Fosaprepitant is not predicted to cross the blood brain barrier due to its charge and size, so it is hypothesized that these activities are mediated by the CNS-penetrant aprepitant.
3. Pharmacokinetics and Metabolism of Fosaprepitant
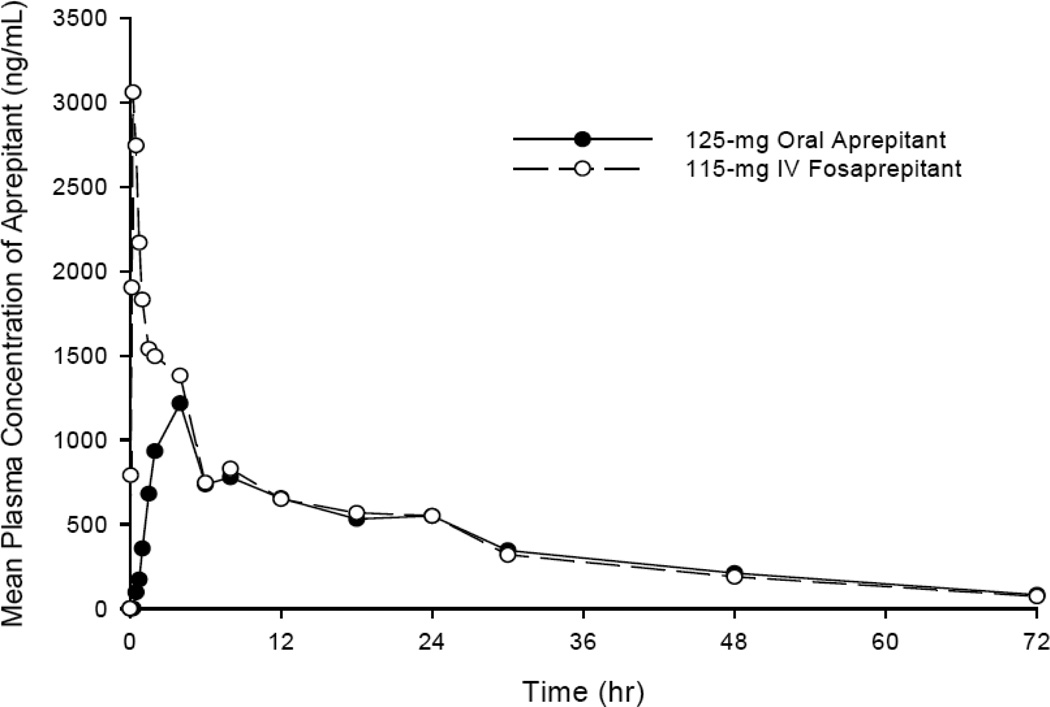
The plasma elimination half-life after a fifteen minutes IV infusion of fosaprepitant averaged ~2.3 minutes and the volume of distribution is ~5L in humans (table 1).23 Plasma levels of fosaprepitant are below the level of detection (10 ng/mL) within 30 minutes after the infusion. In vitro investigation demonstrates rapid conversion to aprepitant by preparations of human liver, kidney, lung, and ileum, indicating widely distributed metabolism. These results demonstrate that fosaprepitant is rapidly converted to aprepitant without significant tissue distribution of the prodrug parent compound. Conversion of 115-mg fosaprepitant to aprepitant releases 18.3-mg of phosphate and 73-mg of meglumine. At the 115-mg dose of IV fosaprepitant the aprepitant mean AUC0-∞ is 31.7 (±14.3) mcg•hr/mL and the Cmax is 3.27 (±1.16) mcg/mL in healthy volunteers. At 24 hours post-dose 115-mg IV fosaprepitant results in a mean plasma aprepitant concentration similar to that of 125-mg oral aprepitant (figure 2, Table 2).8
Table 1.
Pharmacokinetic Properties
| Pharmacokinetic properties | ||
|---|---|---|
| Aprepitant (125mg) | Fosaprepitant (115mg) | |
| formulation | Oral | IV |
| t1/2 | 9–13 h | 2.3 min |
| VD | 70 L | 4.8 L |
| Excretion | urine 50% feces 50% |
urine 57% feces 45% |
Figure 2. Mean plasma concentration of Aprepitant following 125-mg of oral Aprepitant and 115-mg IV Fosaprepitant.
Reproduced from the EMEND_IV product information sheet with permission from Merck Sharp & Dohme Corp.
Table 2.
Aprepitant Concentration Following Aprepitant and Fosaprepitant Administration
| Pharmacokinetic parameters of aprepitant following oral Aprepitant or IV Fosaprepitant | |||
|---|---|---|---|
| Aprepitant (125mg) |
Fosaprepitant (115 mg) |
||
| Geometric Mean | Geometric Mean Ratio1 (fosaprepitant/aprepitant) |
||
| AUC0-∞, ng.h/mL | 27,759 | 29,611 | 1.13 (90% CI: 1.06, 1.20) |
| Cmax, ng/mL | 1,354 | 3,095 | 2.47 (95% CI:2.25, 2.71) |
| C24, ng/mL | 494 | 504 | |
| tmax, h | 4 | 0.25 | |
| t1/2, h | 14 | 13.6 | |
The boundaries of the 90% CI for the AUC geometric mean ratio used to prove the bioequivalence hypothesis were between 0.8 and 1.25.
In animal models the active drug aprepitant readily crosses the blood brain barrier in humans, and the placenta.8 The apparent volume of distribution at steady state is approximately 70 liters in humans and greater than 95% is bound to plasma proteins. Aprepitant exhibits nonlinear pharmacokinetics with a 26% greater-than-dose-proportional plasma AUC0-∞ between the 125-mg or the 80-mg doses.24 Aprepitant’s major route of metabolism is through CYP3A4 with minor metabolism by CYP1A2 and CYP2C19.8 Seven weakly active metabolites of aprepitant have been detected in human plasma. In addition to being a substrate of CYP3A4, aprepitant is a weak-to-moderate (dose-dependent) inhibitor and inducer of this enzyme. Due to this characteristic caution should be taken when administering fosaprepitant/aprepitant with drugs that are primarily metabolized by CYP3A4 since this may result in increases in the AUC of these drugs and of aprepitant itself. Precaution should also be taken with concomitant use of other CYP3A4 inducers, since this may result in decreased efficacy of aprepitant. In addition to effects on CYP3A4, aprepitant also induces the metabolism of drugs metabolized by CYP2C9. Close monitoring is recommended for drugs metabolized by this mechanism such as warfarin.25 Specific recommendations for dose adjustments are described in tables 4 and 5.
Table 4.
Concomitant Medication Dose Adjustments
| Dose Adjustments | |||
|---|---|---|---|
| Drug | Adjustments to other drugs administered concomitantly with fosaprepitant or aprepitant |
Mechanism | Pharmacokinetic effect on other drugs administered concomitantly with fosaprepitant/aprepitant |
| Dexamethasone | reduce by 50% | CYP3A4 substrate | AUC increase 2.2 fold on Days 1 and 5 |
| Methylprednisolone IV | reduce by 25% | CYP3A4 substrate | AUC increase 1.34 fold Day 1 and 2.5 fold on Day 3 |
| Methylprednisolone Oral | reduce by 50% | CYP3A4 substrate | |
| Warfarin | No (Close monitoring suggested) | CYP2C9 substrate | 34% decrease in S(−) warfarin trough concentration, 14% decrease in prothrombin time 5 Days after aprepitant dosing |
| Tolbutamide | No | CYP2C9 substrate | AUC decrease by 23% on Day 4, 28% on Day 8, and 15% on Day 15 |
| Midazolam | No | CYP3A4 substrate | Fosaprepitant ->AUC increase by 1.6 fold Oral Aprepitant -> AUC increase by 2.3 fold on Day 1 and 3.3 fold on Day 5 |
| Oral Contraceptives | May reduce efficacy, alternative methods recommended during and 1 month after last dose | AUC of ethinyl estradiol decrease by 19% on Day 10, trough levels decreased up to 64% on Days 9–21. No effect on AUC of norethindrone but up to 60% decrease on trough levels. | |
| Docetaxel | No | no influence | |
| Vinorelbine | No | no influence | |
| 5-HT3 antagonist | No | no influence | |
Table 5.
Fosaprepitant Indications
| Fosaprepitant Indications | |
|---|---|
| Labeled Indications | Regimen |
| Prevention of acute and delayed N/V associated with initial and repeat courses of highly emetogenic cancer chemotherapy | IV Fosaprepitant (115mg) in addition to a 5-HT3 antagonist on day 1, oral aprepitant (80mg) on days 2–3, and a corticosteroid on days 1–4. |
| Prevention of N/V associated with initial and repeat courses of moderately emetogenic cancer chemotherapy | IV Fosaprepitant (115mg) in addition to a 5-HT3 antagonist and a corticosteroid on day 1, oral aprepitant (80mg) on days 2–3. |
| Not evaluated indications | |
| PONV Pediatrics Refractory CINV Breakthrough CINV | |
4. Pharmacodynamics of Fosaprepitant
Clinical efficacy of fosaprepitant is attributed entirely to that of aprepitant. Fosaprepitant is approved by the FDA and EMEA for use on day 1 of moderately or highly emetogenic chemotherapy regimens in the prevention of acute and delayed CINV (Table 5). In place of the 125-mg oral aprepitant dose, fosaprepitant 115-mg can be substituted administered intravenously over 15 minutes. With limited CNS penetration, the antiemetic effects of fosaprepitant are attributed to aprepitant.8 Fosaprepitant does not have a labeled indication for days 2 or 3 of CINV prophylaxis or in the prevention of PONV.
5. Clinical Efficacy
5.1 Phase I Clinical Trials
The safety and tolerability of fosaprepitant was evaluated in several studies with approximately 700 subjects/patients.23 Single doses ranging from 0.2- to 200-mg reconstituted in saline or polysorbate 80 vehicle to a concentration of 1 mg/mL and infused over 15 to 30 minutes were administered. Fosaprepitant has been administered for 4 consecutive days as single (25 – 100 mg) doses. The results of these studies established that infusions at 1 mg/mL over 15 – 30 minutes were well tolerated whereas a concentration of 25 mg/mL was associated with venous irritation at 50- and 100-mg doses.
5.2 Phase II Clinical Trials
During the development of aprepitant, two Phase II studies were conducted with the use fosaprepitant. Cocquyt et al. conducted a double-blind, randomized, active-agent (ondansetron)-controlled study in cisplatin naïve male and female cancer patients.26 Fifty-three patients were randomized to received either a single dose of IV ondansetron (32-mg) or a single dose of fosaprepitant (60- or 100-mg) both infused over 30 min 1 hour before cisplatin therapy. The first nine patients that were randomized to fosaprepitant received the 60-mg dose, but based on an interim analysis by an unblinded statistician, the dose was increased to 100-mg for the rest of the patients while maintaining blinding of the investigators. Results showed a similar effect of fosaprepitant and ondansetron during the acute phase, however fosaprepitant was significantly better than ondansetron at controlling emesis in the delayed phase. In general, fosaprepitant was well-tolerated in this study with the only significant adverse event being increased diarrhea in patients receiving the NK1 antagonist.
In a subsequent trial Van Belle et al. randomized patients to three groups receiving either fosaprepitant/aprepitant, fosaprepitant/placebo or ondansetron/placebo regimens (See Table 6).27 The percent of patients with no emesis on day 1 who received the NK1 receptor antagonist was 47 to 49%, whereas those who received ondansetron was 84%. Moreover, on days 2–5 61 to 65% of patients who received fosaprepitant had no emesis compared to 41% in patients receiving placebo.
Table 6.
Treatment regimen in Prevention of cisplatin-induced acute and delayed emesis by selective neurokinin-1 antagonists trial
| Treatment Regimen Van Belle et al. 2002 | |
|---|---|
| Group | Regimen |
| I | Day 1 |
| 100-mg IV Fosaprepitant | |
| 20-mg IV Dexamethasone | |
| Cisplatin ≥ 70mg/m2 | |
| Day 2–5 | |
| 300-mg PO Aprepitant | |
| II | Day 1 |
| 100-mg IV Fosaprepitant | |
| 20-mg IV Dexamethasone | |
| Cisplatin > 70mg/m2 | |
| Day 2–5 | |
| Placebo | |
| III | Day 1 |
| 32-mg IV Ondansetron | |
| 20-mg IV Dexamethasone | |
| Cisplatin > 70mg/m2 | |
| Day 2–5 | |
| Placebo | |
5.3 Phase III Clinical Trials
The FDA approval of fosaprepitant was based to the premise that biotransformed parent prodrug has similar safety and pharmacokinetic bioequivalence to oral aprepitant. Bioequivalence is defined as the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study.28 In order to accept a dose as bioequivalent the 90% confidence interval (CI) for the AUC geometric mean ratio between products had to lie within 0.8 and 1.25. The study to determine which dose of fosaprepitant was bioequivalent to the approved aprepitant dose (125-mg PO) was conducted by Lasseter et al.23 The study was divided in three parts. Parts I and II investigated doses of fosaprepitant from 90- to 150-mg, based on the results from these parts 100- and 115-mg doses were selected for the Part III definitive proof of bioequivalence. Periods 1 and 2 on Part I using IV fosaprepitant or placebo were double blind while the rest of the study was conducted as open label.
In Part I the 150-mg dose of fosaprepitant did not meet the bioequivalence criteria. Although the 100-mg dose met the 90% CI criteria, it was out of the boundaries when using a 95% CI. Similarly, the 90 mg dose in Part II did not meet the criteria for AUC bioequivalence. In Part III the 100- and 115-mg doses were evaluated. Subjects received IV fosaprepitant 100- or 115-mg and oral aprepitant 125-mg in a crossover fashion. Fosaprepitant was rapidly converted to aprepitant (t½ = 2.3 minutes). Plasma concentrations of aprepitant were slightly higher 4 hours post-dose IV fosaprepitant than oral aprepitant. After the 4 hour time point aprepitant concentrations were similar for all treatments. The 100-mg dose of fosaprepitant showed slightly lower aprepitant concentrations than oral aprepitant 12 hours post-dose. The C24h of 115-mg dose of fosaprepitant and 125-mg aprepitant was similar, while the C24h of 100-mg fosaprepitant was lower than that 125-mg aprepitant. Plasma Cmax of aprepitant after the 115-mg fosaprepitant treatment was ~ 2.5 fold higher than 125-mg aprepitant. The AUC geometric ratio 115-mg fosaprepitant was 1.13 (90 % CI: 1.06, 1.20) and 0.87 (90% CI: 0.82, 0.93) for 100-mg fosaprepitant. However based on data unadjusted for actual dose received, the 100-mg dose fell outside of the prespecified range of accepted AUC mean ratio. Therefore, the 115-mg dose of fosaprepitant was determined to be bioequivalent to 125-mg aprepitant.
A Phase III non-inferiority trial to determine if single dose 150-mg IV fosaprepitant is equivalent to the 3-day oral regimen approved for aprepitant for the prevention of CINV associated with cisplatin chemotherapy was also completed (NCT Registry Number NCT00619359).29, 30 The primary outcome of this trial was a complete response (no vomiting/no use of rescue medicine) overall (during the 120 hours following initiation of cisplatin). There were two arms in this study, (See Table 7). Fosaprepitant 150-mg single dose IV was found to be non-inferior to the aprepitant regimen. In the fosaprepitant arm 795 out of 1106 patients (71.8%) showed complete response compared to 820 out of 1134 patients in the aprepitant arm (72%). Single dose fosaprepitant was also found to be non-inferior to the aprepitant regimen when analyzing a complete response in the delayed phase (25 to 120 hours following cisplatin) or no vomiting overall.
Table 7.
Treatment regimen in a Phase III Non-inferiority trial
| Treatment Regimen Phase-III non-inferiority trial | |
|---|---|
| Arm 1 | Day 1 |
| 150-mg IV Fosaprepitant | |
| 32-mg IV Ondansetron | |
| 12-mg PO Dexamethasone | |
| Day 2 | |
| 8-mg PO Dexamethasone | |
| Day 3–4 | |
| 16-mg PO Dexamethasone | |
| Arm 2 | Day 1 |
| 125-mg PO Aprepitant | |
| 32-mg IV Ondansetron | |
| 12-mg PO Dexamethasone | |
| Cisplatin | |
| Day 2–3 | |
| 80-mg PO Aprepitant | |
| 8-mg PO Dexamethasone | |
| Cisplatin | |
| Day 4 | |
| 8-mg PO Dexamethasone | |
Another study assessing the bioequivalence of fosaprepitant and aprepitant and the effect of food on aprepitant bioavailability following single doses of 150-mg fosaprepitant or 165-mg and 185-mg aprepitant has also been completed, however no publically available results have been posted to date.30 Aprepitant was developed as a nano preparation to reduce the possible impact of food.31, 32In addition to investigating the efficacy of single dose fosaprepitant, other studies are currently recruiting to evaluate the utility of fosaprepitant as a rescue medication for breakthrough CINV, as well as the efficacy of fosaprepitant in the prevention of CINV in patients receiving radiotherapy in combination with cisplatin. Another Phase I study is also recruiting to determine the pharmacokinetic parameters of aprepitant and the safety and tolerability of aprepitant and fosaprepitant in pediatric patients.30
5.4 Post-Marketing Surveillance
In March 23, 2010 the FDA announced a safety label revision for fosaprepitant. The change added a warning related to hypersensitivity reactions reported in the post-marketing period. Isolated reports of immediate hypersensitivity reactions including flushing, erythema, and dyspnea have occurred during infusion of fosaprepitant. In general these reactions have responded to cessation of infusion and administration of appropriate therapy. Based on these new findings it is not recommended to reinitiate infusion in patients who experience these types of reactions.
6. Safety and Tolerability
Fosaprepitant is generally well tolerated. Studies employing 100-mg and 115-mg doses in healthy volunteers revealed no serious adverse events or withdraws due to tolerability.23 Single doses of up to 200-mg IV fosaprepitant were well tolerated in healthy subjects. The most commonly reported adverse events included headache and infusion site symptoms.8, 23 No clinically meaningful effect on QTc interval change was observed at any time point after a 200-mg dose of fosaprepitant in healthy subjects.33 It is not expected that fosaprepitant will cause any significant QTc prolongation at the current clinical dose, which is lower than the tested in this study.
Fosaprepitant was also generally well tolerated in patients receiving chemotherapy. The overall incidence of reported adverse events in clinical trials was similar among patients receiving fosaprepitant or standard therapy. The most common adverse events in patients included headache, asthenia, constipation, diarrhea, abdominal pain, anorexia, dizziness, and hiccups.26, 27, 30 No serious adverse events reported were linked to treatment with fosaprepitant.
7. Regulatory Affairs
Fosaprepitant was given approval by the FDA and EMEA on January 2008 for the prevention of acute and delayed nausea and vomiting associated with initial and repeated courses of highly emetogenic cancer chemotherapy, including cisplatin, and moderately emetogenic cancer chemotherapy. The recommended use of fosaprepitant is as a substitute in day 1 of a 3-day regimen of aprepitant. In day 1 of moderately or highly emetogenic chemotherapy, fosaprepitant 115-mg should be infused over 15 minutes, 30 minutes before the beginning of chemotherapy with a 5-HT3 antagonist and dexamethasone. This should be followed on days 2 and 3 by 80-mg oral aprepitant. The single-day fosaprepitant 150-mg regimen has been submitted to regulatory agencies for approval as a substitute of the current 3-day regimen. Fosaprepitant is not approved for use in the prevention of PONV.
8. Conclusion
The introduction of the NK1 antagonists represents a significant advancement in the prevention of CINV. Aprepitant, the initial approved agent in class, has been incorporated in treatment guidelines as a standard of care for acute and delayed CINV with highly and some moderately emetogenic chemotherapy. Fosaprepitant, a prodrug of aprepitant, was developed to address an unmet clinical need for chemotherapy patients who would benefit by parenteral administration. Bioequivalence studies showed that the 125-mg dose of aprepitant can be substituted with the 115-mg dose of fosaprepitant on day 1 of chemotherapy. However, fosaprepitant only substitutes oral aprepitant on day 1 and therefore an 80-mg dose of aprepitant is still recommended on days 2–3. Both aprepitant and fosaprepitant have a significant effect on emesis, but not a significant improvement on the incidence of nausea7, 12, 26, 27.
Currently fosaprepitant is under study as a single-day 150-mg dose for use in combination with a 5-HT3 antagonist and dexamethasone. The efficacy of such an approach has been supported by a clinical trial in which 150-mg single-day regimen is non-inferior to the 125/80/80-mg regimen of oral aprepitant in patients receiving cisplatin-based chemotherapy. If approved, this single-day regimen will likely simplify CINV treatment and increase compliance by removing the need for patients to fill an outpatient prescription for days 2 and 3 of treatment.
Future studies are expected to investigate the effectiveness of fosaprepitant and aprepitant in patients receiving radiation therapy in addition to chemotherapy, in pediatric populations, as well as in other types of CINV such as refractory and breakthrough. The results of these studies will be key in determining if the use of this new class of anti-emetics will expand.
9. Expert Opinion
The efficacy demonstrated in clinical trials by the NK1 receptor antagonists has confirmed the role of substance P in emesis. More importantly, the ability of these drugs to have an additive effect with 5-HT3 antagonists and corticosteroids results in net increased benefit to the patients. In addition, the favorable safety profile of aprepitant and fosaprepitant suggests their use can probably be extended to other populations in need, such as pediatrics. Under the Pediatric Research Equity Act (PREA) the FDA listed as post marketing study commitments in the fosaprepitant approval letter a “Study in adolescents and younger pediatric patients receiving chemotherapy to evaluate fosaprepitant PK, safety, and tolerability”. Such study must be completed by March 31, 2011. After its approval aprepitant was incorporated as standard therapy in the guidelines of the ASCO, MASCC, and NCCN, among others, for highly and AC-based moderately emetogenic chemotherapy. However, the lack of an intravenous formulation precluded the use of aprepitant in some clinics that only permit the use of IV drugs.
The introduction to the market of fosaprepitant is expected to enhance the use of this drug in chemotherapy centers where an IV formulation is preferred as well as for patients who have difficulty swallowing on day 1 of their chemotherapy regimen. The availability of this formulation is expected to increase the likelihood of physicians to prescribe the fosaprepitant/aprepitant regimen. While fosaprepitant has not been comprehensively evaluated in clinical efficacy trials for CINV prevention, its safety profile paired with convincing pharmacokinetic equivalence strongly support the labeled dosage as pharmacodynamically equivalent to oral aprepitant.
It follows that fosaprepitant would have efficacy in the prevention of PONV, though this has not been systematically investigated in the same fashion as the CINV indication. At the current time, there is not an established dose that would be bioequivalent to the labeled 40-mg dose of aprepitant used for this indication. It does not appear that the sponsor is pursuing this indication.
Other studies are evaluating the use of fosaprepitant single dose as a rescue medication for breakthrough CINV. Although not a labeled indication, the EMSO guidelines suggest the use of fosaprepitant/aprepitant for refractory nausea if not already used34. This is one of two options suggested when refractory CINV occurs. However, it remains hard to consider adding aprepitant/fosaprepitant to the antiemetic regimen for prevention of refractory nausea because the NK1 antagonist has a significant effect on emesis but not a significant effect on nausea. So far no other guideline suggests the use of fosaprepitant/aprepitant for this indication. Recently, Muňoz et al. reported the effectiveness of aprepitant as a broad spectrum antitumor drug.35 In this study aprepitant at 5–70µM concentrations inhibited cell growth in a concentration dependent manner through NK1 receptor antagonism. This report warrants future studies on the possible use of aprepitant as an anti-tumor drug. As more information is gathered about the efficacy of fosaprepitant/aprepitant in different indications the use of this NK1 antagonist is likely to increase if safety issues are not uncovered.
Another important consideration for patients as well as health insurance providers is cost effectiveness of fosaprepitant/aprepitant use. A report by Moore et al. concluded that aprepitant provides modest incremental benefits when compared with conventional management of CINV.36 The use of aprepitant is more cost-effective when the risk of delayed CINV and the cost of rescue medications is high.
Table 3.
Dose Adjustments for Patient Characteristics
| Dose Adjustments | |||
|---|---|---|---|
| Characteristic | Adjustments to Fosaprepitant or Aprepitant dose |
Pharmacokinetic Effect on Aprepitant | |
| Gender | No | Females Cmax 16% higher, t1/2 25%lower than males | |
| Race | No | Hispanics AUC0–24hr 25% and 29% and Cmax 22% and 31% higher than whites and blacks | |
| Hepatic Insufficiency | *Relative to healthy subjects | ||
| mild | No | AUC0–24hr 11% lower on Day 1 and 36% lower on Day 3 | |
| moderate | No | AUC0–-24hr 10% higher on Day 1 and 18% higher on Day 3 | |
| severe | Not evaluated | ||
| Renal Insufficiency | *Relative to healthy subjects | ||
| severe | No | AUC0-∞ of total aprepitant decreased 21%, Cmax decreased 32% | |
| hemodialysis | No | AUC0-∞ of total aprepitant decreased 42%, Cmax decreased 32% | |
| Pediatric | Not evaluated | ||
| Geriatric | No | AUC0–24hr 21% higher on Day 1 and 36% higher on Day 5 (≥ 65 years), Cmax 10% higher on Day 1 and 24% higher on Day 5, relative to younger adults | |
Abbreviations List
- CINV
chemotherapy-induced nausea and vomiting
- CNS
central nervous system
- MASCC
Multinational Association of Supportive Care in Cancer
- ASCO
American Society of Clinical Oncology
- NCCN
National Comprehensive Cancer Network
- PONV
Post operative nausea and vomiting
- NK1
neurokinin-1
- ESMO
European Society of Medical Oncology
Footnotes
Declaration of Interest
Francheska Colon-Gonzalez was supported by NIH Postdoctoral training grant T32 GM08562. Walter K. Kraft has served as paid consultant to Merck.
References
- 1.Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, et al. American Society of Clinical Oncology Guideline for Antiemetics in Oncology: Update 2006. J Clin Oncol. 2006 June 20;24(18):2932–2947. doi: 10.1200/JCO.2006.06.9591. 2006. [DOI] [PubMed] [Google Scholar]
- 2.Curran MP, Robinson DM. Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs. 2009;69(13):1853–1878. doi: 10.2165/11203680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. The oncologist. 2007 Sep;12(9):1143–1150. doi: 10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology: Antiemesis V.2.2010. 2010 [cited 2010 July 10, 2010]; Available from: http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.
- 5.Roila F, Hesketh PJ, Herrstedt J. Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol. 2006 Jan;17(1):20–28. doi: 10.1093/annonc/mdj078. [DOI] [PubMed] [Google Scholar]
- 6.Le TP, Gan TJ. Update on the Management of Postoperative Nausea and Vomiting and Postdischarge Nausea and Vomiting in Ambulatory Surgery. Anesthesiology Clinics. 28(2):225–249. doi: 10.1016/j.anclin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003 Jun 15;97(12):3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 8.Merck & Co I. Emend (fosaprepitant dimeglumine) for injection White House, Station. NJ, USA: 2008. [Google Scholar]
- 9.Prommer E. Aprepitant (EMEND): the role of substance P in nausea and vomiting. Journal of pain & palliative care pharmacotherapy. 2005;19(3):31–39. [PubMed] [Google Scholar]
- 10.Hargreaves R. Imaging substance P receptors (NK1) in the living human brain using positron emission tomography. The Journal of clinical psychiatry. 2002;63 Suppl 11:18–24. [PubMed] [Google Scholar]
- 11.Tattersall FD, Rycroft W, Hargreaves RJ, Hill RG. The tachykinin NK1 receptor antagonist CP-99,994 attenuates cisplatin induced emesis in the ferret. European journal of pharmacology. 1993 Nov 30;250(1):R5–R6. doi: 10.1016/0014-2999(93)90649-3. [DOI] [PubMed] [Google Scholar]
- 12.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, deWit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003 Nov 15;21(22):4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006 Apr;14(4):354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 14.Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006 Jun;17(6):1000–1006. doi: 10.1093/annonc/mdl019. [DOI] [PubMed] [Google Scholar]
- 15.Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005 Apr 20;23(12):2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 16.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Annals of Oncology. 2010 May;21 suppl 5:v232–v243. doi: 10.1093/annonc/mdq194. 2010. [DOI] [PubMed] [Google Scholar]
- 17.BC Cancer Agency. SCNAUSEA Protocol. British Columbia Cancer Agency; 2009. May, 2009. [Google Scholar]
- 18.EMEA. Questions and answers on the withdrawal of the marketing authorisation application for Zunrisa: casopitant. 2009 [Google Scholar]
- 19.GSK. Regulatory update for REZONIC™ (casopitant mesylate) 2009 [Google Scholar]
- 20.2010 [cited 2010 May 29]; Available from: http://clinicaltrials.gov/ct2/results?term=casopitant.
- 21.Hale JJ, Mills SG, MacCoss M, Dorn CP, Finke PE, Budhu RJ, et al. Phosphorylated Morpholine Acetal Human Neurokinin-1 Receptor Antagonists as Water-Soluble Prodrugs. Journal of Medicinal Chemistry. 2000 Feb 25;43(6):1234–1241. doi: 10.1021/jm990617v. [DOI] [PubMed] [Google Scholar]
- 22.Huskey S-EW, Luffer-Atlas D, Dean BJ, McGowan EM, Feeney WP, Chiu S-HL. Substance P Receptor Antagonist I: Conversion of Phosphoramidate Prodrug after i.v. Administration to Rats and Dogs. Drug Metabolism and Disposition. 1999 November 1;27(11):1367–1373. 1999. [PubMed] [Google Scholar]
- 23.Lasseter KC, Gambale J, Jin B, Bergman A, Constanzer M, Dru J, et al. Tolerability of Fosaprepitant and Bioequivalency to Aprepitant in Healthy Subjects. J Clin Pharmacol. 2007 July 1;47(7):834–840. doi: 10.1177/0091270007301800. 2007. [DOI] [PubMed] [Google Scholar]
- 24.Majumdar AK, Howard L, Goldberg MR, Hickey L, Constanzer M, Rothenberg PL, et al. Pharmacokinetics of aprepitant after single and multiple oral doses in healthy volunteers. J Clin Pharmacol. 2006 Mar;46(3):291–300. doi: 10.1177/0091270005283467. [DOI] [PubMed] [Google Scholar]
- 25.Depre M, Van Hecken A, Oeyen M, De Lepeleire I, Laethem T, Rothenberg P, et al. Effect of aprepitant on the pharmacokinetics and pharmacodynamics of warfarin. European journal of clinical pharmacology. 2005 Jul;61(5–6):341–346. doi: 10.1007/s00228-005-0907-8. [DOI] [PubMed] [Google Scholar]
- 26.Cocquyt V, Van Belle S, Reinhardt RR, Decramer ML, O'Brien M, Schellens JH, et al. Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer. 2001 May;37(7):835–842. doi: 10.1016/s0959-8049(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 27.Simon VB, Michael RL, Rudolph MN, August MG, Marc LAD, Alain R, et al. Prevention of cisplatin-induced acute and delayed emesis by the selective neurokinin-1 antagonists, L-758,298 and MK-869. Cancer. 2002;94(11):3032–3041. doi: 10.1002/cncr.10516. [DOI] [PubMed] [Google Scholar]
- 28.FDA. Code of Federal Regulations Title 21. 2009 21CFR320.1. [Google Scholar]
- 29.Grunberg DTC SM, Maru A, DeVandry S, Boice JA, Hardwick J, Taylor A, Carides A, Roila F, Herrstedt J. Phase III randomized double-blind study of single-dose fosaprepitant for prevention of cisplatin-induced nausea and vomiting. Journal of Clinical Oncology. 2010;28 doi: 10.1200/JCO.2010.31.7859. (15s (suppl; abstr 9021^)) [DOI] [PubMed] [Google Scholar]
- 30.ClinicalTrials.gov. 2010 April 18; 2010 [cited; Available from: http://clinicaltrials.gov/ct2/results?term=fosaprepitant.
- 31.Kesisoglou F, Panmai S, Wu Y. Nanosizing -- Oral formulation development and biopharmaceutical evaluation. Advanced Drug Delivery Reviews. 2007;59(7):631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Junghanns JU, Muller RH. Nanocrystal technology, drug delivery and clinical applications. International journal of nanomedicine. 2008;3(3):295–309. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marbury TC, Jin B, Panebianco D, Murphy MG, Sun H, Evans JK, et al. Lack of effect of aprepitant or its prodrug fosaprepitant on QTc intervals in healthy subjects. Anesthesia and analgesia. 2009 Aug;109(2):418–425. doi: 10.1213/ane.0b013e3181ac1066. [DOI] [PubMed] [Google Scholar]
- 34.Herrstedt J, Roila F. Chemotherapy-induced nausea and vomiting: ESMO Clinical Recommendations for prophylaxis. Annals of Oncology. 2009 May;20 suppl 4:iv156–iv158. doi: 10.1093/annonc/mdp160. 2009. [DOI] [PubMed] [Google Scholar]
- 35.Munoz M, Rosso M. The NK-1 receptor antagonist aprepitant as a broad spectrum antitumor drug. Investigational new drugs. Apr;28(2):187–193. doi: 10.1007/s10637-009-9218-8. [DOI] [PubMed] [Google Scholar]
- 36.Moore S, Tumeh J, Wojtanowski S, Flowers C. Cost-effectiveness of aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with highly emetogenic chemotherapy. Value Health. 2007 Jan–Feb;10(1):23–31. doi: 10.1111/j.1524-4733.2006.00141.x. [DOI] [PubMed] [Google Scholar]