Abstract
Personal pronouns, such as ‘I’ and ‘you’, require a speaker/listener to continuously re-map their reciprocal relation to their referent, depending on who is saying the pronoun. This process, called ‘deictic shifting’, may underlie the incorrect production of these pronouns, or ‘pronoun reversals’, such as referring to oneself with the pronoun ‘you’, which has been reported in children with autism. The underlying neural basis of deictic shifting, however, is not understood, nor has the processing of pronouns been studied in adults with autism. The present study compared the brain activation pattern and functional connectivity (synchronization of activation across brain areas) of adults with high-functioning autism and control participants using functional magnetic resonance imaging in a linguistic perspective-taking task that required deictic shifting. The results revealed significantly diminished frontal (right anterior insula) to posterior (precuneus) functional connectivity during deictic shifting in the autism group, as well as reliably slower and less accurate behavioural responses. A comparison of two types of deictic shifting revealed that the functional connectivity between the right anterior insula and precuneus was lower in autism while answering a question that contained the pronoun ‘you’, querying something about the participant’s view, but not when answering a query about someone else’s view. In addition to the functional connectivity between the right anterior insula and precuneus being lower in autism, activation in each region was atypical, suggesting over reliance on individual regions as a potential compensation for the lower level of collaborative interregional processing. These findings indicate that deictic shifting constitutes a challenge for adults with high-functioning autism, particularly when reference to one’s self is involved, and that the functional collaboration of two critical nodes, right anterior insula and precuneus, may play a critical role for deictic shifting by supporting an attention shift between oneself and others.
Keywords: autism, functional connectivity, pronoun reversal, precuneus, insula
Introduction
‘Personal pronouns are repeated just as heard, with no change to suit the altered situation. The child, once told by his mother, “Now I will give you your milk”, expresses the desire for milk in exactly the same words. Consequently, he comes to speak of himself always as “you”, and of the person addressed as “I”’ Kanner (1943, p. 244).
Efficient communicators achieve an understanding of another’s knowledge, information and emotion, and use this understanding to make appropriate adjustments when delivering their messages. The human neural system conducts this intricate process in an online and relatively spontaneous manner. Dysfunction of such dynamic flexibility in reciprocal communication has been delineated as a characteristic of Autism Spectrum Disorder. For example, as described in Leo Kanner’s seminal documentation of autism above, children with autism sometimes incorrectly refer to themselves by using the second-person pronoun, ‘you’, instead of the first-person pronoun, ‘I’, by repeating the pronoun they heard someone else use when referring to them. Such atypical production of personal pronouns, called ‘pronoun reversals’, has long been recognized as a common impairment in autism.
Interestingly, it is also not uncommon to observe pronoun reversals among typically developing children (Dale and Crain-Thoreson, 1993). These authors posited that the substantial processing demand of updating the anchoring site of an utterance, and shifting the relationship between an utterance-generating speaker and a referred-to listener, a process called ‘deictic shifting’, challenges children and triggers pronoun reversals in early development. In autism, several studies have described the decrease or even cessation of the use of pronoun reversals in later childhood (Kanner, 1943, 1971; Cantwell et al., 1989). Both typically developing children and children with autism eventually master correct pronominal deixis, but it is undetermined whether a neural trace of such dexis difficulty in autism remains in adulthood. Thus, although pronoun reversals are often thought of as an issue in early development, adults with high-functioning autism may also experience difficulty in transforming a personal pronoun to an appropriate form in a linguistic task that requires deictic shifting. The present study aimed to examine this process of deictic shifting in a linguistic perspective-taking task among adults with high-functioning autism whose verbal IQ is within the normal range. We employed a computerized perspective-taking task that required deictic shifting of the personal pronouns ‘I’ and ‘you’, similar to a paradigm reported by Lee et al. (1994), and we collected both behavioural and neuroimaging measures of the process.
Another important component of deictic shifting, involving personal pronouns such as ‘I’ or ‘you’, is relating oneself to another person, depending on who is speaking. As indicated in the term ‘autism’, derived from the Greek word autos meaning ‘self’, ‘concept of self’ is a central element of this disorder, particularly the relation between the self and others. Individuals with autism exhibit atypical behaviour regarding themselves, namely extreme self-focus and lack of higher order understanding of self, and this phenomenon may have something in common with the deictic shifting problem (Frith and de Vignemont, 2005; Lombardo and Baron-Cohen, 2010).
Based on accumulating brain imaging studies with functional MRI in autism, which indicate frontal–posterior functional under connectivity theory (a lower level of synchronization of functional MRI-measured activation between brain areas) (Just et al., 2004, 2007; Koshino et al., 2005; Villalobos et al., 2005; Kana et al., 2006, 2007, 2009; Mason et al., 2008), the present study hypothesized that diminished functional communication between frontal and posterior brain regions may constrain the process of deictic shifting in autism. One of the brain areas believed to be involved in the representation of self, and hence in deictic shifts involving the self, is the precuneus (Ruby and Decety, 2001; Farrer and Frith, 2002; Vogeley et al., 2004; Frings et al., 2006; Zaehle et al., 2007), situated in the medial posterior region of the parietal cortex and adjoining the posterior cingulate cortex. Regions of the posterior parietal region are believed to contribute to the dorsal visual pathway by processing both egocentric (body-dependent) and allocentric (body-independent) spatial information (Culham and Kanwisher, 2001; Marshall and Fink, 2001), and by playing a role in selective attention (Behrmann et al., 2004). Cavanna and Trimble (2006) suggest that the integration of those functions allows the precuneus to play an essential role in shifting attention between targets of attention. For deictic shifting, involvement of the precuneus may be critical for shifting attention between two people: a speaker and a listener. Whitney and colleagues (2009) support this view by reporting the involvement of the precuneus in shifting of person, time, location or action in a narrative comprehension task.
Another region that may be involved in deictic shifting is the anterior insula, believed to be a neural substrate of self-awareness (Critchley, 2005; Craig, 2009). The anterior insula exhibits this role in various ways, such as right-lateralized activation for interoceptive signals (temperature: Craig et al., 2000; heartbeat: Critchley et al., 2004; pain: Wager et al., 2004), visual recognition of one’s own face (Uddin et al., 2005; Devue et al., 2007) and subjective feelings (Damasio et al., 2000; Jabbi et al., 2007). By contributing to the intrinsic understanding of one’s own position in the schematic space, the right anterior insula may provide a central axis for self- and other-representations. Thus, interregional communication between the right anterior insula and precuneus might underpin a functional network involved in deictic shifting, computing where one’s self stands in the reciprocal communication with another person.
In summary, we predicted that adults with high-functioning autism would exhibit poorer behavioural performance for deictic shifting in the perspective-taking task. We employed functional connectivity analysis by using functional MRI to examine the degree of blood oxygen level-dependent signal synchronization between the precuneus and right anterior insula, the postulated underlying neural basis for suboptimal performance of deictic shifting in autism, and expected to observe diminished synchronization. This frontal–posterior network may be supporting interpersonal attention shifting.
Materials and methods
Participants
Participants were 15 adults (14 males and 1 female) with high-functioning autism and 15 matched controls (all males), and all participants were native English speakers. All the analyses were repeated with the one female participant in the autism group excluded, and resulted in the same conclusions for all behavioural and functional MRI measures. Both groups were matched for age, full scale IQ, performance IQ and verbal IQ scores, which were determined by the Wechsler Adult Intelligence Scale-Revised (WAIS-R). There were no significant group differences in age or in any of the IQ measures (Table 1).
Table 1.
Participant characteristics
| Demographic Information | Autism | Control | t(28) | P |
|---|---|---|---|---|
| Age (years) | 24.7 ± 7.8 | 24.7 ± 7.7 | 0.00 | 1.00 |
| Full scale IQ | 106.3 ± 10.7 | 108.7 ± 5.1 | 0.81 | 0.43 |
| Performance IQ | 106.9 ± 16.1 | 107.4 ± 6.1 | 0.12 | 0.91 |
| Verbal IQ | 104.4 ± 12.7 | 108.0 ± 5.8 | 1.00 | 0.33 |
| Handedness (right:left) | 11:4 | 14:1 | ||
| Gender (male:female) | 14: 1 | 15:0 |
Values are represented as mean ± SD.
The diagnosis of autism was determined using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994), supplemented with confirmation by expert clinical opinion. Potential participants with autism were excluded if they had an identifiable cause of autism, such as fragile-X syndrome, tuberous sclerosis and foetal cytomegalovirus infection. Potential control and autism participants were also excluded if there was evidence of birth asphyxia, head injury or a seizure disorder. Exclusionary criteria were based on neurological history and examination, and chromosomal analysis or metabolic testing, if indicated.
The control participants were community volunteers recruited to match the autism participants on age, full scale IQ, race and socioeconomic status of family of origin, as measured by the Hollingshead method. Potential control participants were screened by questionnaire, telephone, face-to-face interview and observation during screening psychometric tests. Exclusionary criteria, evaluated through these procedures, included current or past psychiatric and neurological disorders, birth injury, developmental delay, school problems, acquired brain injury, learning disabilities, substance abuse and medical disorders with implications for the CNS or those requiring regular medication. Potential control participants were also screened to exclude those with a family history (in parents, siblings and offspring) of autism, developmental cognitive disorders, affective disorders, anxiety disorders, schizophrenia, obsessive compulsive disorder, substance abuse or other neurological or psychiatric disorders thought to have a genetic component. Handedness was determined with the Lateral Dominance Examination from the Halstead–Reitan Neuropsychological Test Battery (Reitan, 1985), revealing that four participants in the autism group and one in the control group were left handed. The brain activation data from these left handers were clearly similar to their respective groups, and therefore, the data were not separated by handedness.
This study was approved by the Institutional Review Boards of the University of Pittsburgh and Carnegie Mellon University. Participants were recruited from the participant pool of the Collaborative Program for the Autism Centres for Excellence at the University of Pittsburgh.
Experimental paradigm
For each participant, the stimulus texts were customized to use the participant’s first name, such as ‘John’, to refer to the participant in the stimulus sentences (Table 2 and Fig. 1). The other character depicted in the pictorial part of the stimuli was referred to as ‘Sarah’ when a proper name was used. All participants received written instructions from the experimenter and then participated in a practice session to become familiar with the task.
Table 2.
Summary of conditions
| Type of Task | Deixis |
|
|---|---|---|
| SHIFT (Pronoun) | FIXED (Name) | |
| Main Task (‘What’ question) | ||
| Target | ||
| SELF (Participant’s view) | ‘What can you see now?’ | ‘What can John see now?’ |
| Answer choice | Carrot House | Carrot House |
| OTHER (Depicted person’s view) | ‘What can I see now?’ | ‘What can Sarah see now?’ |
| Answer choice | Carrot House | Carrot House |
| Pronoun | Name | |
|---|---|---|
| Manipulation Check (‘Who’ question) | ||
| SELF (Participant’s view) | ‘Who can see the carrot now?’ | ‘Who can see the carrot now?’ |
| Answer choice | You can I can | Sarah can John can |
| OTHER (Depicted person’s view) | ‘Who can see the house now?’ | ‘Who can see the house now?’ |
| Answer choice | You can I can | Sarah can John can |
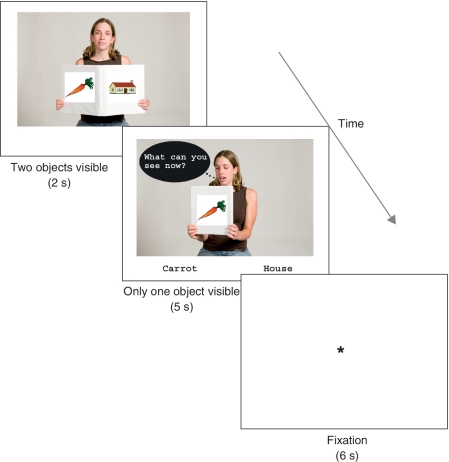
Examples of experimental conditions where the participant’s name was John. Both upper and lower panels describe the cases introduced: a picture of ‘house’ on the front cover (right side) and a picture of ‘carrot’ on the back cover (left side) are displayed during the 2 s of ‘open book,’ while the carrot is displayed on the back cover for 5 s when the book is closed (Fig. 1).
Figure 1.
Schematic diagram of the experimental stimuli.
In the perspective-taking task, the participants were asked to generate a response from either a first- (SELF) or second-person (OTHER) perspective. The task was composed of two different scenes (Fig. 1): an opened book and a closed book. First, the opened book scene was displayed for 2 s, with Sarah (the depicted person in the scene) holding a book with two objects visible, one on the front and one on the back cover. The depicted objects were vegetables and buildings, and one of four items from each category was shown on either side. The number of appearances for each object was equated. The position (front or back cover) of the object was pseudo-randomized, as well as the pairing of the two objects. This scene acquainted the participant with the two objects involved in the task.
Following the opened book scene, the closed book scene, displayed for 5 s, illustrated Sarah holding the closed book, with only one object visible and a question about the picture with two possible answer choices. The participant could only see one side of the book with one of the two objects, while Sarah was able to see the other side of the book showing the other object. A word balloon read, ‘What can [the participant (SELF) or Sarah (OTHER)] see now?’ The two possible answer choices were displayed on the bottom of the screen and the participants were instructed to answer by pressing one of the two buttons as quickly and accurately as possible.
The questions were constructed using personal pronouns or proper names. Therefore, there were two within-subject independent variables: Target (SELF versus OTHER) and Deixis (SHIFT versus FIXED), resulting in four conditions: SHIFT-TO-SELF, SHIFT-TO-OTHER, FIXED-SELF and FIXED-OTHER (summarized in the upper panel of Table 2). For example, in the SHIFT-TO-SELF condition, the question was ‘What can you see now?’ The participant had to comprehend that the personal pronoun ‘you’ referred to him/her (SELF) and select the object that s/he could see. In the SHIFT-TO-OTHER condition, Sarah asked ‘What can I see now?’ The participant had to comprehend that the pronoun ‘I’ referred to Sarah (OTHER), and select the object that she was facing, but which the participant could not see. For the FIXED conditions, the questions used proper names, such as ‘What can John see now?’ for the SELF condition, and ‘What can Sarah see now?’ for the OTHER condition. These four conditions were pseudo-randomly presented 12 times in two separate blocks of 24 trials.
Manipulation check
In addition, participants answered 48 questions that used a ‘Who’ question form in order to confirm the manipulation for deictic shifting (the lower panel of the Table 2). These questions asked who could see a particular object, the participant or Sarah, e.g. ‘Who can see the carrot now?’ The participant indicated an answer using either pronouns or proper nouns: ‘I can’ or ‘You can,’ or ‘John can’ or ‘Sarah can.’ The aim of having two different question forms (‘What’ and ‘Who’) was to compare the conditions with and without deictic shifting (see details in the Results and Discussion sections). The ‘Who’ questions were only employed for a manipulation check and were presented separately from the ‘What’ questions. They were excluded from the main analyses. In sum, the participants completed a total of 96 trials throughout the experiment, which alternated between 24-trial blocks of ‘What’ and ‘Who’ questions. After each trial, the participants saw a fixation ‘X’ in the middle of the screen for 6 s, with instructions to fixate on the ‘X’ while relaxing their minds and waiting for the next question.
Data acquisition
The scanning was conducted on a 3.0T Siemens Allegra scanner at the Brain Imaging Research Centre (BIRC), jointly owned by Carnegie Mellon University and the University of Pittsburgh. Activation was measured using blood oxygen level-dependent contrast. The stimuli were rear projected onto a semi-translucent plastic screen and participants viewed the screen through a mirror attached to the head coil. The study was performed with a gradient echo, echo planar imaging sequence with repetition time = 1000 ms, echo time = 30 ms and a 60° flip angle. Seventeen oblique axial slices were acquired; each slice was 5 mm thick with a gap of 1 mm between slices. The acquisition matrix was 64 × 64 with 3.125 × 3.125 × 5 mm voxels.
Functional magnetic resonance imaging analyses
The data were analysed using SPM2. Images were corrected for slice acquisition timing and head motion, and were normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels and smoothed with an 8 mm Gaussian kernel to decrease spatial noise. The time-series data for each participant was high-pass filtered with a 128 s cut-off to remove low frequency drifts. Statistical analysis was performed on individual and group data by using the general linear model as implemented in SPM2 (Friston et al., 1995). Group analyses were performed using a random-effects model. Within-group and between-group t-maps at P < 0.001 (uncorrected) and an extent threshold of ten 8 mm3 voxels was used.
Functional region of interest definition
The central analyses focused on the two regions of the self-processing network that we hypothesized to be critically involved in deictic shifting: the precuneus and the right anterior insula. Both functional regions of interest (precuneus and right anterior insula) were defined to encompass the main clusters of activation in the group activation map for each group in the overall task versus fixation contrast. The defined centres of the regions of interest (in MNI coordinates) were (x = 0, y = −64, z = 50) for the precuneus, and (x = 32, y = 26, z = 6) for the right anterior insula, and the locations were verified with reference to the parcellation of the MNI single subject T1-weighted dataset carried out by Tzourio-Mazoyer and colleagues (2002). A sphere was defined for each cluster with a radius of 12 mm that best captured the cluster of activation in the map for each group.
Percentage change in signal intensity
The average per cent signal change across all voxels in the right anterior insula and precuneus regions of interest was computed for participants and each experimental condition relative to the fixation condition. The mean per cent signal change of the eight images acquired with an offset of 5 s from the stimulus onset (to account for the delay in haemodynamic response) is reported. These averaged data for each participant were submitted to separate mixed ANOVAs for each region of interest. For these region of interest-based analyses, effects were considered significant at P < 0.05 (uncorrected).
Functional connectivity
The functional connectivity was computed (separately for each participant) as a correlation between the average time-course of signal intensity of all the activated voxels of regions of interest. The activation time-course extracted for each participant over the activated voxels within each region of interest originated from the normalized and smoothed images, which were high-pass filtered and had the linear trend removed. One participant in the control group who did not have activation in a given functional region of interest was excluded from further analysis involving that region of interest. The functional connectivity correlation was computed on the images belonging only to the experimental conditions, so it reflects the synchronization of the activation between two areas while the participant is performing the task and not during the baseline condition. Fisher’s r to z transformation was applied to the correlation coefficients for each participant prior to averaging and statistical comparison of the two groups. The transformed values for each participant were submitted to mixed ANOVAs and effects were considered significant at P < 0.05.
In order to assess the robustness of any group differences in functional connectivity, two anatomical regions of interest were also defined, based on peak activations reported in previous relevant self-processing experiments (for the precuneus: Ruby and Decety, 2001; Farrer and Frith, 2002; Vogeley et al., 2004; Frings et al., 2006; Zaehle et al., 2007; Whitney et al., 2009 and for the right anterior insula: Craig et al., 2000; Damasio, et al., 2000; Critchley et al., 2004; Uddin et al., 2005; Devue et al., 2007). The criteria for these two anatomical regions of interest were that they encompass the targeted anatomical regions comprehensively (capturing all of the activation therein), yet exclusively (excluding neighbouring anatomical areas). In order to restrict the insula region of interest to the anterior segment, peak-activations located posterior to y = 0 in MNI space were excluded from the calculation to determine the centroid (Craig, 2010). The average reported peak-activations of these studies were (x = 0, y = −62, z = 54) for the precuneus, and (x = 36, y = 20, z = 8) for the anterior insula. A sphere was defined around each of the centroids, and then further refined by masking out adjacent cortical and subcortical regions using anatomical regions of interest defined by Tzourio-Mazoyer et al. (2002), in order to limit the spherical regions of interest exclusively to the precuneus and anterior insula. For the precuneus, this involved removal of anterior somatosensory association cortex [Brodmann area (BA) 5], and for the right anterior insula, this involved removal of areas identified as the caudate, putamen, lateral inferior frontal cortex and the posterior insula. In order to approximately equate the total volume of the two regions of interest after removal of adjacent cortical and subcortical areas, an initial sphere with a radius of 14 mm was used for the precuneus, and 18 mm was used for the right anterior insula (where the activation volume was larger).
Results
Behavioural and neural activation measures provided converging evidence of the autism group’s greater difficulty in conditions involving deictic shifting. First, participants with high-functioning autism showed reliably slower and less accurate responses than the control group for the items requiring a deictic shift (SHIFT) compared with items using a fixed label (FIXED). Second, these slower and less accurate responses of the autism group were accompanied by lower functional connectivity between the right anterior insula and precuneus only for the SHIFT condition. Functional connectivity was reliably greater for the SHIFT than FIXED condition among controls, suggesting that the autism group failed to show a typical adaptive change of insula–precuneus communication. In addition, activation in the right anterior insula was significantly greater for the SHIFT than FIXED conditions in the autism group only. Further analyses indicated that underconnectivity between the right anterior insula and precuneus was observed when transforming ‘you’ to ‘I’ (SHIFT-TO-SELF), but not when transforming ‘I’ to ‘you’ (SHIFT-TO-OTHER), and that activation in the precuneus did not change between these two conditions in the autism group, but was significantly lower for the SHIFT-TO-SELF than the SHIFT-TO-OTHER conditions in the control group. Finally, for the SHIFT-TO-SELF condition, only the autism group showed a reliable positive correlation between right anterior insula–precuneus functional connectivity and verbal IQ, and a negative correlation between right anterior insula–precuneus functional connectivity and reaction time. All the analyses of functional connectivity above were repeated using the anatomical regions of interest and the results remained the same.
Behavioural results
Response time
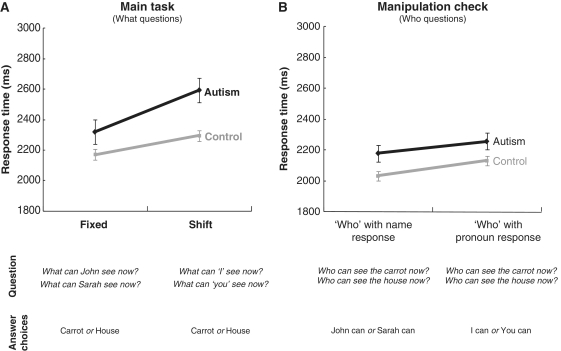
The results supported the hypothesis that deictic shifting should slow the response time of autism participants more than control participants. A 2 × 2 × 2 (Group: Autism, Control × Deixis: SHIFT, FIXED × Target: SELF, OTHER) mixed ANOVA showed an interaction for Group and Deixis [F (1,28) = 6.46, P = 0.02], as expected (Fig. 2A), along with the main effects of Deixis (taking longer than the FIXED condition), [F(1,28) = 47.99, P < 0.01] and Target (slower for SHIFT-TO-OTHER relative to SHIFT-TO-SELF) [F(1,28) = 33.76, P < 0.01]. Additional tests of the simple effect of Group within level of Deixis yielded a marginally slower response for the Autism group (2593 ms) relative to the Control group (2295 ms) in the SHIFT condition [F(1,28) = 3.28, P = 0.08], but no group difference in the FIXED condition (P = 0.35). Thus, the Group × Deixis interaction resulted from a relatively greater disadvantage among participants with autism when the task required processing a deictic shift, but not when the task used proper names.
Figure 2.
Mean reaction time. (A) A reliable interaction between the Group (Autism, Control) and Deixis (SHIFT, FIXED) (P = 0.02) for ‘What can X see now?’. (B) No reliable Deixis and Group interaction for ‘Who can see the Y now?’. The error bars represent the 95% confidence interval for the within-subject effect in each condition (Loftus and Masson, 1994).
Accuracy
The accuracy scores showed a very similar pattern to the response times. Both groups responded less accurately for SHIFT relative to FIXED and for OTHER relative to SELF. A 3-way ANOVA yielded a Group by Deixis interaction [F(1,28) = 11.05, P < 0.01], a main effect of Deixis [F(1,28) = 6.32, P = 0.02] and a main effect of Target [F(1,28) = 22.48, P < 0.01]. Tests for the simple main effect of Group within level of Deixis indicated that accuracy was reliably lower for the autism group relative to the control group in the SHIFT [F(1,28) = 5.12, P = 0.03] but not in the FIXED condition, and tests for the simple main effect of Deixis within each group showed that accuracy was reliably lower for SHIFT (0.91) than FIXED (0.97) in the Autism group [F(1,14) = 12.84, P < 0.01]. The accuracy results again indicate that the autism group had more difficulty with deictic shifting than controls.
Manipulation check for deictic shifting
Increased processing requirements associated with deictic shifting are postulated to be the cause of pronoun reversals (Dale and Crain-Thoreson, 1993). To compare the groups’ performance in the absence of a deictic shift, a control condition used a ‘Who’ question form, with pronouns and names (the lower panel of the Table 2), to probe the understanding of the depicted situation (who can see what) without requiring any deictic shift. In this manipulation check, the use of pronouns per se should impose no greater burden on the autism group than on the control group, so that no Group (Autism, Control) by Label (Pronoun, Name) interaction would be expected. Supporting this, the two-way ANOVAs for the ‘Who’ questions conducted for both reaction time and accuracy revealed no reliable interaction between these factors (Fig. 2B). For completeness, we report that the three-way ANOVAs [Group (Autism, Control) × Question (What, Who) × Label (Pronoun, Name)] show significant three-way interactions for reaction time [F(1,28) = 6.58, P = 0.02] and accuracy [F(1,28) = 9.89, P < 0.01], resulting from the difference between the ‘What’ and ‘Who’ conditions. Figure 2 displays the reaction time data for both conditions. Thus, the observed increase in reaction time and decreased accuracy for ‘What’ questions in the main conditions with pronouns (SHIFT), compared with those with names (FIXED), is consistent with the contention that the poorer performance in pronoun use in the autism group was due to deictic shifting.
Functional magnetic resonance imaging results
Activation distribution
The two groups showed similar cortical activation locations (Table 3). Both groups showed a large activation cluster in posterior cortical regions, extending from the occipital cortex to the posterior parietal lobule, and including the precuneus, inferior parietal lobule, posterior middle temporal gyrus, inferior temporal gyrus and cerebellum. Although frontal activation in the right hemisphere was similar for both groups, the autism group exhibited more activation in the left frontal cortex. A direct group comparison indicated that the autism group exhibited significantly greater activation in the right frontal and parietal areas; however, there was no area that showed greater activation for the control group than the autism group. In addition to these whole brain analyses with uncorrected P < 0.001, in multiple comparisons based on Gaussian random field theory across all voxels P = 0.01, only one cluster in each group survives a family-wise error correction [Autism: cerebellum (x = − 32, y = −56, z = −32); Control: left hippocampus (x = −24, y = −32, z = −2)], and none of the clusters survived, even with a more liberal threshold (P = 0.05, family-wise error) for the group comparison. Additionally, no contrasts between experimental conditions showed significant differences in either group at this threshold.
Table 3.
Areas of activation for the contrasts of all task conditions minus fixation
| Region | Cluster size | t(14) | MNI coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Control | |||||
| Occipital/inferior parietal/superior parietal/inferior temporal/middle temporal/cerebellum | 45 755 | 16.91 | −26 | −62 | 42 |
| Right middle frontal (BA9) | 121 | 6.08 | 50 | 34 | 36 |
| Anterior cingulate (BA32) | 162 | 5.97 | 12 | 22 | 38 |
| Right insula (BA13) | 565 | 5.91 | 20 | 30 | 6 |
| Right superior frontal (BA10) | 34 | 5.38 | 40 | 60 | −2 |
| Right middle frontal/precentral (BA6) | 109 | 4.98 | 36 | 4 | 62 |
| Right amygdala | 57 | 4.62 | 30 | 0 | −12 |
| Right middle frontal (BA10) | 13 | 4.62 | 34 | 58 | 26 |
| Right middle frontal (BA6) | 19 | 4.44 | 24 | −12 | 40 |
| Autism | |||||
| Occipital/inferior parietal/superior parietal/inferior temporal/middle temporal/cerebellum | 43 906 | 16.53 | −34 | −56 | −32 |
| Left middle frontal/precentral (BA6/9) | 2536 | 9.26 | −34 | 6 | 64 |
| Right middle frontal/precentral (BA6/9) | 1282 | 7.01 | 34 | 4 | 66 |
| Posterior cingulate | 357 | 6.14 | −2 | −36 | 24 |
| Right superior frontal (BA10) | 42 | 5.51 | 40 | 60 | 2 |
| Right inferior frontal (BA45) | 72 | 5.19 | 56 | 32 | 28 |
| Left superior frontal (BA10) | 18 | 4.75 | −20 | 60 | 0 |
| Cerebellum | 18 | 4.37 | 4 | −54 | −26 |
| Right insula (BA13) | 26 | 4.33 | 36 | 26 | −4 |
| Right middle frontal (BA46) | 22 | 4.32 | 32 | 34 | 24 |
| Right caudate | 13 | 4.30 | 14 | 16 | 20 |
| Right middle frontal (BA10) | 13 | 4.18 | 34 | 50 | 16 |
| Left caudate | 26 | 4.10 | −10 | 12 | 24 |
| Left postcentral | 10 | 3.92 | −54 | −18 | 22 |
| Autism > Control | |||||
| Right middle frontal/precentral/postcentral (BA6/4/3) | 467 | 4.67 | 44 | −18 | 60 |
| Right postcentral (BA5) | 45 | 4.41 | 44 | −44 | 60 |
| Right precuneus (BA19) | 12 | 4.04 | 22 | −86 | 38 |
| Post cingulate | 28 | 3.97 | −6 | −36 | 28 |
| Right posterior middle temporal (BA21) | 11 | 3.92 | 58 | −48 | −8 |
| Right middle frontal (BA6) | 64 | 3.92 | 38 | 10 | 36 |
The threshold for significant activation was P < 0.001 for a spatial extent of at least 10 voxels, uncorrected for multiple comparisons. Region labels apply to the entire extent of the cluster. T-values and MNI coordinates are displayed for the peak activated voxel in each cluster. For group comparison, there was no area that showed greater activation for the control group than the autism group.
Signal change in precuneus
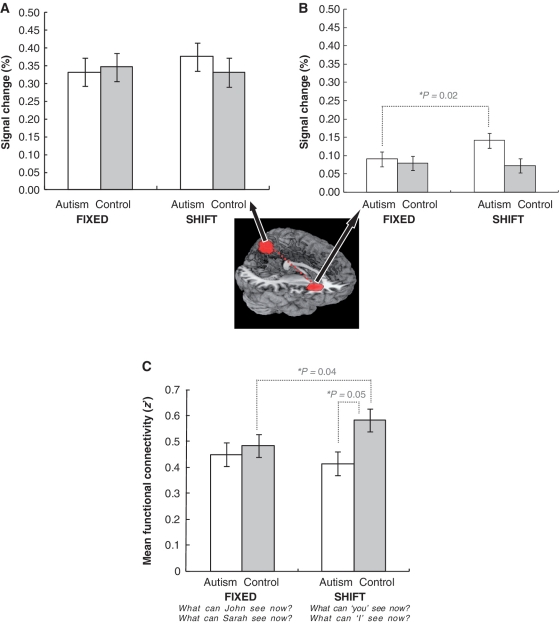
A three-way (Group × Deixis × Target) mixed ANOVA yielded a marginally significant main effect for Target [F(1,28) = 3.78, P = 0.06], such that the mean per cent signal change in the precuneus was marginally greater for OTHER (0.38) than for SELF (0.32), and there was a reliable three-way interaction [F(1,28) = 5.21, P = 0.03]. However, no significant interaction between Group and Deixis was found [F(1,28) = 1.21, P = 0.28] (Fig. 3A), indicating that both groups exhibited similar precuneus activation overall for the SHIFT condition relative to the FIXED conditions.
Figure 3.
FIXED versus SHIFT. Per cent signal change in the precuneus (A) and right anterior insula (B). Functional connectivity between the right anterior insula and precuneus (C). The error bars represent the 95% confidence interval for the within-subject effect in each condition. The image (centre) indicates the location of the region of interest (radius = 12 mm) for the precuneus [0, −64, 50] and right anterior insula [32, 26, 6] on the MNI coordinates.
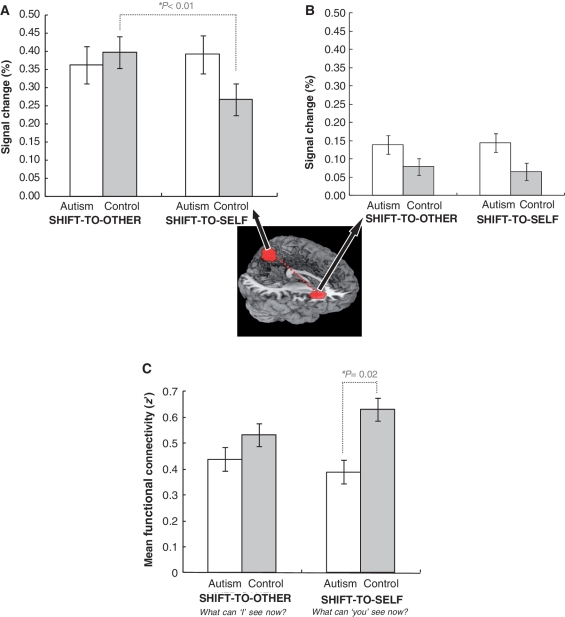
The three-way interaction was explored by examining the simple Group × Target interactions within each Deixis condition (SHIFT and FIXED), and a significant interaction was found only for the SHIFT condition [F(1,28) = 6.54, P = 0.02] (Fig. 4A). Tests of the main effect of Target within each group showed that the mean per cent signal change in the precuneus reliably increased when the shift was to the other person (SHIFT-TO-OTHER) (0.39), relative to the shift to the self (SHIFT-TO-SELF) (0.26), only in the control group [F(1,14) = 10.57, P < 0.01], as shown in Fig. 4A. Whereas the controls showed significantly lower per cent signal change in precuneus for SHIFT-TO-SELF relative to SHIFT-TO-OTHER, there was little difference between SHIFT-TO-SELF and SHIFT-TO-OTHER for the autism group.
Figure 4.
SHIFT-TO-SELF versus SHIFT-TO-OTHER. Per cent signal change in the precuneus (A) and right anterior insula (B). Functional connectivity between the right anterior insula and precuneus (C). The error bars represent the 95% confidence interval for the within-subject effect in each condition.
Signal change in right anterior insula
A similar three-way mixed ANOVA was conducted for the per cent signal change of the right anterior insula. This analysis yielded a reliable Group × Deixis interaction [F(1, 28) = 4.80, P = 0.04], and tests of the simple main effect of Deixis condition within each group revealed significantly greater mean per cent signal change in the right anterior insula for SHIFT compared with FIXED only in the autism group, as seen in Fig. 3B [Autism: SHIFT (0.14) > FIXED (0.09), F(1,14) = 7.27, P = 0.02; Control: F(1,14) = 0.15, P = 0.71]. The simple main effect of Group within each Deixis condition yielded a trend of greater per cent signal change in the autism group than the control group only for the SHIFT condition [F(1,28) = 3.09, P = 0.09].
Additional tests of the simple Group × Target interaction for each Deixis condition were not significant, and there was only a marginal simple main effect of the Group for the SHIFT condition [F(1,28) = 3.24, P = 0.09] (Fig. 4B). Therefore, these results indicate that the activation in the right anterior insula tended to be greater in autism than in controls for both targets of the SHIFT condition (SHIFT-TO-SELF and SHIFT-TO-OTHER).
Functional connectivity
As predicted, underconnectivity between the right anterior insula and precuneus in autism was observed for deictic shifting, particularly when answering about oneself. A 2 × 2 × 2 (Group × Deixis × Target) mixed ANOVA was conducted on these functional connectivity measures, and the results showed a significant Group by Deixis interaction [F(1,27) = 4.92, P = 0.04]. Tests of the simple main effect of Group within each Deixis condition indicated significantly reduced functional connectivity in autism (0.41), relative to controls (0.58), for the SHIFT condition [F(1,27) = 4.27, P = 0.05]. In addition, the simple effect of Deixis was reliable only in the control group, with increased functional connectivity for the SHIFT compared with the FIXED condition [F(1,13) = 5.44, P = 0.04] (Fig. 3C).
There was also a reliable three-way interaction for the functional connectivity between the right anterior insula and precuneus [F(1,27) = 8.18, P < 0.01]. Tests of the simple effect of target conditions, when a deictic shift was required, showed reliably lower functional connectivity in the autism group (0.39) than in the control group (0.63) only for questions in the SHIFT-TO-SELF conditions (i.e. with the pronoun ‘you’) [SHIFT-TO-SELF: F(1,27) = 6.68, P = 0.02], and not in the SHIFT-TO-OTHER condition (i.e. with the pronoun ‘I’) [F(1,27) = 1.16, P = 0.29]. Tests of the simple effect of target for trials requiring a shift within each group, indicated a trend of increased functional connectivity for the SHIFT-TO-SELF condition compared with the SHIFT-TO-OTHER condition in the control group [F(1,13) = 3.35, P = 0.09], and no difference between those conditions in the autism group [F(1,14) = 0.80, P = 0.39]. These results, displayed in Fig. 4C, suggest that the autism group failed to exhibit an adaptive increase of functional connectivity between the right anterior insula and precuneus in the SHIFT-TO-SELF condition relative to the SHIFT-TO-OTHER condition.
Correlation between functional connectivity and verbal IQ
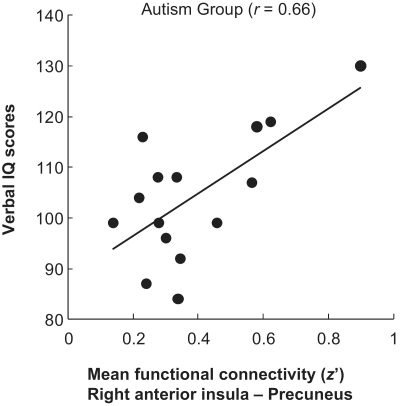
Among the participants with autism, the functional connectivity between the right anterior insula and precuneus during the SHIFT-TO-SELF (What can you see now?) condition was positively correlated with verbal IQ [r = 0.66, t(14) = 3.17, P < 0.01], as shown in Fig. 5, whereas the correlation was not significant in the control group [r = 0.31, t(13) = 1.18, P = 0.26]. Although both groups exhibited a positive correlation that did not differ statistically (z = 1.13, P = 0.26), inspection of the data indicated that the positive relationship among controls was due to one participant with low verbal IQ and a negative functional connectivity score. When this outlier was removed from the control group, the moderate positive correlation disappeared [r = −0.04, t(12) = −0.13, P = 0.45], and there was a significant difference between correlations of the two groups (z = 1.95, P = 0.05). Thus, the significant correlation in the autism group indicates that the participants with autism who exhibit greater functional connectivity between the right anterior insula and precuneus, when the task required deictic shifting to refer to oneself, tended to have higher language skill (indicated by high verbal IQ scores). Other psychometric measures (full scale IQ, performance IQ, and Autism Diagnostic Observation Schedule scores) did not show a significant correlation with the functional connectivity for either group.
Figure 5.
Scatterplot showing that in the autism group, verbal IQ increased as the functional connectivity between the right anterior insula and precuneus increased for the SHIFT-TO-SELF condition (What can you see now?).
Correlation between functional connectivity and reaction time
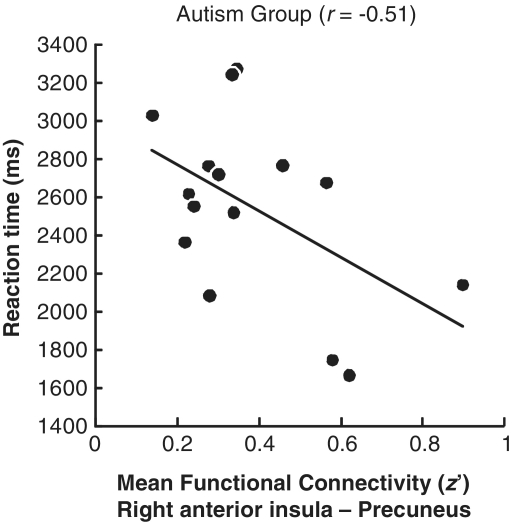
Among the participants with autism, the functional connectivity between the right anterior insula and precuneus during the SHIFT-TO-SELF (What can you see now?) condition showed a significant correlation with reaction time [r = −0.51, t(14) = −2.14, P = 0.03], as shown in Fig. 6, whereas the correlation was not significant in the control group [r = 0.18, t(13) = 0.63, P = 0.27], and there was a significant difference between correlations of the two groups (z = 1.78, P = 0.04). Thus, the correlation in the autism group indicates that the participants with autism who had lower functional connectivity between the right anterior insula and precuneus tended to show a slower response when the task required deictic shifting to refer to oneself.
Figure 6.
Scatterplot showing that in the autism group, reaction time for the SHIFT-TO-SELF condition (What can you see now?) decreased as the functional connectivity between the right anterior insula and precuneus increased.
Discussion
The aim of the study was to evaluate behavioural performance and compare neural activity between adults with high-functioning autism and neurotypical adults during deictic shifting (updating the relationship of generated and referred agents) in a perspective-taking task. The primary finding was diminished functional connectivity between the right anterior insula and precuneus in autism when the task required deictic shifting. Although the functional connectivity between two neural nodes was lower during a deictic shift in autism, the activation of the frontal node of the network, the right anterior insula, was increased relative to when the task used fixed labels of people (i.e. proper names). Another contribution of the present study was that the functional connectivity of the same neural network was particularly low when recognizing ‘you’ as referring to the self in autism. Activations of the posterior neural node, the precuneus, in autism, were similar for both directions of deictic shifting (transforming ‘you’ to ‘I’ for referencing one’s self, and ‘I’ to ‘you’ for referencing the other person), although the control participants’ activations were greater for the referencing to self relative to the other person. These findings extend the previously postulated frontal–posterior underconnectivity theory in autism (Just et al., 2007) by providing evidence for the near-absence of the relevant functional network, with an atypical reliance on a local neural resource in autism, possibly indicating compensatory processing.
Frontal–posterior network for deictic shifting
The present study focused on a cortical network involving the right anterior insula and precuneus for several reasons. First, the posterior node, the precuneus, comprises part of the dorsal visual stream and plays a critical role in spatial information processing (Haxby et al., 1991), and Culham and colleagues (1998) reported activation in the precuneus for mental navigation of a moving object, indicating an involvement of the area for tracking/shifting spatial attention. Further investigations have revealed that the attention shift function of the precuneus is not limited to spatial information (Nagahama et al., 1999) and is modality independent (Cavanna and Trimble, 2006; Shomstein and Yantis, 2006). The precuneus may contribute to deictic shifting processes by updating the anchor of a speech act from self-to-other and from other-to-self.
Second, emerging evidence suggests that the right anterior insula is involved in self-awareness and self-consciousness (Craig, 2009). The anterior insula is also believed to mediate integration of internally oriented information and externally derived processing (Sridharan et al., 2008). Uddin and Menon (2009) proposed that there is dysfunctional connectivity of the anterior insula in autism, lowering its ability to play the role of a network hub. For deictic shifting in the perspective-taking task, the right anterior insula may provide an axis on which to position oneself on a representational map of self-and-other relationships by being involved in internally oriented information processing. The observed lower functional connectivity between those two neural nodes in the autism group, therefore, may result in disturbed perspective-taking processes in shifting a centre of reference between self and other (Tager-Flusberg, 1990, 1994), based on an understanding of where one stands in a given moment or situation. Elevated activation of the right anterior insula during deictic shifting in the autism group (Fig. 3) suggests that this process was more effortful (showing more activation) in autism.
On the other hand, when deictic shifting was not required (i.e. when both the depicted person and the participant were directly referred to by proper name), underconnectivity of the network in autism was not observed. Atypical overreliance on a particular noun expression, as reflected by diminished employment of personal pronouns, has been reported in autism (Lee et al., 1994). The present study employed a paradigm similar to that of Lee et al. (1994), and in addition to providing congruent behavioural results, it also suggests that diminished frontal–posterior network bandwidth may restrict deictic shifting processes, resulting in an inappropriate preference of proper names (a fixed label referring to a person regardless of who generated the speech).
Egocentrism and autism: mapping self onto the pronoun ‘I’
Another important finding of the present study was the atypical neural activity for transforming the pronoun ‘you’ to ‘I’ (SHIFT-TO-SELF: ‘What can you see now?’) in autism, relative to ‘I’ to ‘you’ (SHIFT-TO-OTHER: ‘What can I see now?’). For the transformation of ‘you’ to ‘I’, the autism group showed atypically elevated activation of the precuneus (Fig. 4A) and diminished functional collaboration with the right anterior insula (Fig. 4C). On the other hand, there was no group difference in precuneus activation and its functional connectivity with the right anterior insula when reversing in the other direction (i.e. ‘I’ to ‘you’, as in ‘What can I see now?’). In order to answer this question, the participant could determine that the pronoun ‘I’ referred to the experimenter independently from one’s own representational position. These findings indicate that the critical disturbance in the successful operation of deictic shifting in autism may be dysfunctional processing when recognizing the self as a referent of ‘you’, and shifting to map self onto the pronoun ‘I’. An observed positive correlation between functional connectivity and verbal IQ (Fig. 5), and a negative correlation between functional connectivity and reaction time in autism (Fig. 6), may also indicate that diminished interregional synchronization between the right anterior insula and precuneus restricts the neural communication underlying the shift of a deictic centre from another person to oneself. Furthermore, the present study did not require participants to say their responses, but pronoun reversals in autism may entail the same underlying basis and reflect a consequence of unsuccessful reversal, resulting in producing an overt statement of ‘you’ (e.g. saying ‘You can see the carrot’ when expressing that the participant him/herself is able to see the carrot).
The recent functional MRI studies of self- and other-representation in autism provide complementary evidence: a greater group difference in brain activity has been found for self-related processing relative to other-related (Chiu et al., 2008; Lombardo et al., 2010). Therefore, as suggested by Frith and de Vignemont (2005), idiosyncratic egocentrism in autism may be characterized as dysfunction of representing the external world on the basis of understanding its relation to oneself. Pronoun reversals in autism may reflect a disturbed processing of understanding of self and other in the reciprocal relationship, rather than a semantic error to adjust pronominal forms.
Limitations
The reported under-connectivity in autism in the present study was derived from measures of synchronization of activation between the right anterior insula and precuneus, but did not include measures of white matter tissues that provide anatomical connectivity (that can be measured by diffusion tensor imaging). Diffusion tensor imaging studies in autism have previously reported reduced white matter integrity (Barnea-Goraly et al., 2004; Keller et al., 2007). Further investigation of the white matter tracts connecting the right anterior insula and precuneus may enhance our understanding of the perspective-taking issues in autism.
Conclusion and future directions
Over six decades ago, Kanner (1946) documented unique referential expressions with personal pronouns, referred to as pronoun reversals, among young individuals with autism. The current state-of-the-art tool, functional MRI, allowed us to assess the underlying neural basis of deictic shifting as a critical component of pronoun reversals, and found an elevated level of neural activity with lower coordination of relevant brain centres. Pronoun reversals are described as idiosyncratic language impairment in autism, but the findings suggest that they may also characterize an atypical understanding of the social world because deictic shifting is embedded in understanding the self- and other-relationship, which requires the recognition of the self-stance relative to the other’s existence. MacWhinney (2005) advocates this view by emphasizing the significance of the ability to flexibly shift the viewpoint in social communication in stating that ‘perspective-taking is at the very core of language structure and higher-cognition’ (p. 198). If the system of perceiving an external world were rooted in the understanding of self-stance, dysfunction of the system would affect not only interpersonal interactions but also intrapersonal cognitive states, such as memory, temporal and spatial mental navigation, and Theory of Mind, all possibly sharing a common neural basis (Spreng et al., 2009). Although our findings may be limited to the disturbed fundamental understanding of self and other among individuals in autism, such disturbances are apparent in many different tasks, such as difficulty with motor mirroring between self and other that may result from mirror neuron system dysfunction (Dapretto et al., 2005; Williams et al., 2006), as well as problems with more abstract cognitive self- and other-representations, possibly resulting from atypical cingulate activation that is associated with an altered default-mode system (Chiu et al., 2008; Lombardo et al., 2010). In particular, the frontal portion of the system, including medial prefrontal and anterior cingulate cortex, has shown activation for both self- and other-processing in the neurotypical population across different domains (Gillihan and Farah, 2005). An investigation of a potential differential involvement and an interaction of the medial frontal regions and insula for self-related processing and social cognition in autism could enhance our understanding of this neurodevelopmental disorder. Furthermore, it may be useful for future investigations to examine the neural basis of various coordinate systems within which the various aspects of the world are mentally represented in autism and control groups. These coordinate systems include space (here/there), time (now/then), memory (semantic/episodic), referential frame (egocentrism/allocentrism) and meta-representation or Theory of Mind (self/other).
Funding
This research was supported by the Autism Centers of Excellence Grant HD055748 from the National Institute of Child Health and Human Development, and the Pre-Doctoral Fellowship 4868 from the Autism Speaks Foundation.
Acknowledgements
We would like to express our sincere appreciation to the individuals and families who generously gave their time and courage to participate in this research. We also appreciate the assistance of the members of the Centre of Cognitive Brain Imaging, particularly Kara Cohen and Jennifer Moore for editorial comments on the article.
References
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–7. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Cantwell DP, Baker L, Rutter M, Mawhood L. Infantile autism and developmental receptive dysphasia: a comparative follow-up into middle childhood. J Autism Dev Disord. 1989;19:19–31. doi: 10.1007/BF02212715. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–73. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–77. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3:184–90. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–70. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol. 2001;11:157–63. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dale PS, Crain-Thoreson C. Pronoun reversals: who, when, and why? J Child Lang. 1993;20:573–89. doi: 10.1017/s0305000900008485. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2005;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, et al. Here I am: the cortical correlates of visual self-recognition. Brain Res. 2007;1143:169–82. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Frings L, Wagner K, Quiske A, Schwarzwald R, Spreer J, Halsband U, et al. Precuneus is involved in allocentric spatial location encoding and recognition. Exp Brain Res. 2006;173:661–72. doi: 10.1007/s00221-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Spatial registration and normalization of images. Hum Brain Map. 1995;2:165–89. [Google Scholar]
- Frith U, de Vignemont F. Egocentrism, allocentrism, and Asperger syndrome. Conscious Cogn. 2005;14:719–38. doi: 10.1016/j.concog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psy Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991;88:1621–5. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Soc Neurosci. 2009;4:135–52. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: Decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Kanner L. Follow-up study of eleven autistic children originally reported in 1943. J Autism Child Schizophr. 1971;1:119–45. doi: 10.1007/BF01537953. [DOI] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–7. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–21. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lee A, Hobson RP, Chiat S. I, you, me, and autism: an experimental study. J Autism Dev Disord. 1994;24:155–76. doi: 10.1007/BF02172094. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychon Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Baron-Cohen S. Unraveling the paradox of the autistic self. Wiley Interdiscip Rev Cogn Sci. 2010:393–403. doi: 10.1002/wcs.45. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. 2010;133:611–24. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MacWhinney B. The emergence of grammar from perspective. In: Pecher D, Zwaan RA, editors. Grounding cognition: the role of perception and action in memory, language and thinking. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Marshall JC, Fink GR. Spatial cognition: where we were and where we are. Neuroimage. 2001;14:S2–7. doi: 10.1006/nimg.2001.0834. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–80. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–9. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Halstead-Reitan neuropsychological test battery. Tucson, AZ: Reitan Neuropsychological Laboratories, University of Arizona; 1985. [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci. 2006;26:435–9. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Dissociations in form and function in the acquisition of language by autistic children. In: Tager-Flusberg H, editor. Constraints on language acquisition: studies of atypical children. Hillsdale, NJ: Erlbaum; 1994. pp. 175–194. [Google Scholar]
- Tager-Flusberg H, Calkins S, Nolin T, Baumberger T, Anderson M, Chadwick-Dias A. A longitudinal study of language acquisition in autistic and Down syndrome children. J Autism Dev Disord. 1990;20:1–21. doi: 10.1007/BF02206853. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakacs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal ‘mirror’ network in the right hemisphere: an event-related fMRI study. NeuroImage. 2005;25:926–35. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33:1198–203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. NeuroImage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16:817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Whitney C, Huber W, Klann J, Weis S, Krach S, Kircher T. Neural correlates of narrative shifts during auditory story comprehension. Neuroimage. 2009;47:360–6. doi: 10.1016/j.neuroimage.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Jordan K, Wustenberg T, Baudewig J, Dechent P, Mast FW. The neural basis of the egocentric and allocentric spatial frame of reference. Brain Res. 2007;1137:92–103. doi: 10.1016/j.brainres.2006.12.044. [DOI] [PubMed] [Google Scholar]