Abstract
Cyclin-dependent kinase 5 is activated by small subunits, of which p35 is the most abundant. The functions of cyclin-dependent kinase 5 signalling in cognition and cognitive disorders remains unclear. Here, we show that in schizophrenia, a disorder associated with impaired cognition, p35 expression is reduced in relevant brain regions. Additionally, the expression of septin 7 and OPA1, proteins downstream of truncated p35, is decreased in schizophrenia. Mimicking a reduction of p35 in heterozygous knockout mice is associated with cognitive endophenotypes. Furthermore, a reduction of p35 in mice results in protein changes similar to schizophrenia post-mortem brain. Hence, heterozygous p35 knockout mice model both cognitive endophenotypes and molecular changes reminiscent of schizophrenia. These changes correlate with reduced acetylation of the histone deacetylase 1 target site H3K18 in mice. This site has previously been shown to be affected by truncated p35. By restoring H3K18 acetylation with the clinically used specific histone deacetylase 1 inhibitor MS-275 both cognitive and molecular endophenotypes of schizophrenia can be rescued in p35 heterozygous knockout mice. In summary, we suggest that reduced p35 expression in schizophrenia has an impact on synaptic protein expression and cognition and that these deficits can be rescued, at least in part, by the inhibition of histone deacetylase 1.
Keywords: animal models, brain, cognition, schizophrenia, signalling
Introduction
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase that functions in synaptic plasticity and memory formation (Fischer et al., 2002; Hawasli et al., 2007, 2009; Sananbenesi et al., 2007; Lai and Ip, 2009). Cdk5 activity is governed by the expression of its activators p35 and p39 (Tsai et al., 1994; Humbert et al., 2000). p35 can be cleaved to p25 by calpain, which detaches the activator from the membrane and may increase Cdk5 activity (Patrick et al., 1999; Peterson et al., 2010). p35 has a variety of functions in brain development and synaptic plasticity (Angelo et al., 2006), yet the role of p35 in disorders with cognitive deficits has been mostly neglected.
Schizophrenia is a severe and chronic psychiatric disorder (Van Os & Kapur, 2009) that includes positive, negative and cognitive symptoms (Kellendonk et al., 2009; van Os and Kapur, 2009). Cognitive impairments occur in 75–85% of patients with schizophrenia and are often present before the onset of other symptoms. Their strong correlation with day-to-day functional disability of patients (Liddle, 2000; Reichenberg et al., 2006) highlights the need for effective treatments that are currently unavailable (Gray and Roth, 2007).
Schizophrenia is believed to result from abnormal glutamatergic and dopaminergic signalling (Gaspar et al., 2009). Cdk5 activity is regulated by both these pathways and Cdk5 itself can feedback on them (Bibb et al., 1999; Chergui et al., 2004; Wei et al., 2005; Hawasli et al., 2007). Furthermore, the functions of various candidate genes of schizophrenia are altered by Cdk5 signalling (Meyer et al., 2008; Wen et al., 2008; Singh et al., 2010). Despite these indirect implications, it remains unclear whether dysfunctional signalling of Cdk5 has a pathological role in schizophrenia.
Here, we provide evidence that p35 levels are reduced in post-mortem schizophrenia brain. Furthermore, septin 7 and OPA1, proteins downstream of p25 (Engmann et al., 2011), are downregulated in a tissue-specific manner. We show that heterozygous knockout mice with reduced expression of p35 display cognitive deficits and decreased expression of septin 7 and OPA1 similar to endophenotypes of schizophrenia. Pharmacological inhibition of histone deacetylase 1 (HDAC1) is sufficient to increase proteins downstream of p35. Finally, some of the deficits in p35 heterozygotes are rescued by an HDAC1-inhibitor.
Materials and methods
Post-mortem tissue
Hippocampal and prefrontal cortical samples from post-mortem brains of patients with schizophrenia and controls were obtained from the brain banks of the Institute of Psychiatry, King’s College London, UK and Magdeburg University, Germany. All tissue collection and processing was carried out under the regulations and license of the Human Tissue Authority and in accordance with the Human Tissue Act, 2004. The post-mortem delay was balanced between groups. The tissue was prepared for western blot by homogenizing at 4°C at 7000 rpm with 15 up and down strokes in 5 volumes of 0.25% sodium dodecyl sulphate-containing radioimmunoprecipitation assay buffer (Cell Signalling Technology) as described (Engmann et al., 2011). The antibodies used in this study are shown in Supplementary Table 1 and the case data are in Supplementary Table 2.
Immunohistochemistry
Immunohistochemistry was carried out as previously published (Maekawa, 2009; Hortobagyi et al., 2011). In brief, sections of 7-µm thickness were cut from the paraffin-embedded frontal cortex tissue blocks, deparaffinized in xylene, endogenous peroxidase blocked by H2O2 in methanol and immunohistochemistry performed. To enhance antigen retrieval, sections were kept in citrate buffer for 10 min following microwave treatment. After blocking in normal serum (DAKO), primary antibody was applied overnight at 4°C [p35 (C-19) Santa Cruz 1:50]. Following washes, sections were incubated with biotinylated secondary antibody (DAKO), followed by avidin:biotinylated enzyme complex (Vectastain® Elite ABC kit). Finally, sections were incubated for 10–15 min with 0.5 mg/ml 3,3′-diaminobenzidine chromogen (Sigma-Aldrich Chemicals) in Tris-buffered saline containing 0.05% H2O2. Sections were counterstained with haematoxylin and immunostaining analysed using a Leica microscope. Staining intensity was rated in layers 2–6 of the cortex in the dendrites, nucleus and soma on a scale of 0–2.
p35 mouse line
p35 heterozygous knockout mice and wild-type littermates (all in the C57BL/6J background) were bred and genotyped as described previously (Chae et al., 1997). In brief, the p35 knockout allele had been introduced previously via 129-delivered J1 embryonic stem cells into the germline. Since 1997, the mutant allele was maintained by backcrossing in the C57BL/6J background for multiple generations. All mice were housed in groups of two to six and treated according to the UK Animals (Scientific Procedures) Act, 1986.
Behavioural and biochemical studies with p35+/− mice
At the beginning of the behavioural analysis, mice were 7–9 weeks old; experiments were performed over the course of 4 months. All tests were undertaken blind to the genotypes. After finishing the behavioural battery (see below), mice were allowed to rest for at least 2 weeks before being sacrificed. Hence, they were pseudo-naive at the point of biochemical analysis. The hippocampi and prefrontal cortex were dissected and total lysates were analysed by western blot as described (Plattner et al., 2006).
Accelerating rotarod
Mice were placed onto an elevated metal rod, which was rotating at an accelerating speed. The height of the rod was 30 cm. First, the rod rotated at 4 rpm for 10 s, and was then constantly accelerating to a rate of 20 rpm. Three trials were given per mouse and the average was used for analysis.
Horizontal bar test
The horizontal bar task is a test of muscle strength (Barclay et al., 1981). The mice were placed with both forepaws onto a thin and exposed horizontal metal bar at the height of 49 cm and the time was measured until the mice reached the end of the bar or fell off.
Black–white alley
The black–white alley paradigm allows quantification of the preference for the novel but anxiogenic stimulus of a white chamber over a dark and familiar chamber. The alley consisted of a white and black box, joined at the open ends. In the beginning, the mice were placed into the dark part of the alley, facing the wall. In a 2-min test trial, latency to enter the white alley (data not shown) and the time spent in each alley were recorded.
Hyponeophagia
In the hyponeophagia task, an anxiogenic environment counteracts the drive to ingest the food reward (Rudebeck et al., 2007). The outcome of this test (measured by the time taken until the mouse tastes and ingests the food) reflects both the motivation to find food and anxiety. Prior to the experiment, the mice had been food deprived overnight to 50% of their normal food supply. Fifteen minutes before the test, the mice were singly housed to prevent social transmission of food preferences. The apparatus consisted of an exposed, brown, wooden alley (20 × 6 cm). A sucrose pellet was placed in a metal well ∼2 cm from the end of the alley. Each mouse was placed at the end of the alley facing away from the pellet. The latency of contact with the sucrose pellet and latency to eat were measured, with a maximum length of 2 min per trial, and was stopped as soon as the mouse started to ingest the pellet. If after 2 min no eating event occurred, the trial was repeated up to twice more and the times were added together.
Open field
The apparatus consisted of a brightly illuminated white metal drum (60 cm diameter) without a possibility of escape, therefore creating an anxiogenic environment. The mice were left for 5 min to explore the open field, in which recordings were taken and analysed in Ethovision XT. The surface of the drum was cleaned with 20% ethanol between trials. The time spent in the centre of the field and the total distance travelled were recorded.
Rewarded alternation
In this appetitively motivated working memory task, mice had to explore the two arms of a T-maze in an alternating manner to find a food reward. The maze did not contain spatial cues and olfactory cues were avoided by regular redistribution of a thin layer of sawdust in the maze. Each arm of the maze was grey, 10 cm wide, 29 cm long and 30 cm high. In the two arms perpendicular to the start arm, a food reward was presented. Both arms could be blocked by guillotine doors. Prior to the experiment, mice were food deprived to 90% of their body weight and habituated to the maze for 2 days: food wells in each arm were filled with condensed milk and the mice were allowed to explore all arms until they had emptied the wells.
The experimental phase consisted of 10 trials, in which both food wells were filled with condensed milk. First, one arm was blocked. The mouse was placed in the start arm of the maze facing towards the wall and was then allowed to explore the accessible arm and drink the food reward. Next, the block was removed from the other arm and the mouse was placed in the start arm again and allowed a free choice of arms. The arm first entered by the mouse was recorded. Alternation was encouraged by the fact that only the previously unexplored arm contained a food reward. An entrance was counted when the mouse had all four paws in one arm. If a wrong choice was made (no alternation), the mouse was not allowed to explore the unvisited arm.
Morris water maze
The water maze study with the p35+/− mice was performed as previously described (Deacon et al., 2002). Mice were trained to find a submerged platform (20 cm diameter, ∼1 cm below water surface) in a defined spatial position in an open field water maze (2.0 m diameter, water temperature at 20°C, made opaque by adding milk; the water was changed every 3 days). Eight different start points were defined around the pool and used in a pseudorandom order, which was the same for every mouse. Each animal was assigned to one of two fixed platform positions, which were counterbalanced over groups. Four trials were given per day over the course of 12 days. In the beginning of each trial, mice were placed into the water on the rim of the pool, facing towards the wall. The maximum duration per trial was 90 s or until the platform was found. If the platform was not found, the mouse was gently guided to it. The mouse was allowed to rest on the platform for 30 s before being removed from the test environment. Probe trials were given on Days 7, 10, 13 and after reversal learning (data not shown for Days 7 and 13). During the probe trials, the platform was removed from the pool and the number of crossings through the platform quadrant and the percentage of time spent in this quadrant were measured. Probe trials lasted 60 s, except for the last probe trial, which lasted 90 s, as extinction effects are not to be taken into consideration. For reversal learning, the platform was moved to the opposite quadrant of the maze.
For analysis of H3K18 acetylation, water maze training was conducted as described (Angelo et al., 2003). Swim controls spent similar amounts of time in the pool as trained mice, but in the absence of a platform. Spatial cues were removed by a uniform, white curtain around the pool.
Social interactions
Two non-littermates of the same genotype and sex that were not known to each other were paired. Prior to the experiment, the mice had been habituated to the test chamber over 2 days: on the first day, all mice of one cage were allowed to explore the cage for 5 min, while on the next day each mouse was individually left in the cage for 5 min. During the experiment, the interactions were analysed in a group-wise manner over the course of 10 min. The measurements were taken from video recordings of the mice meeting in a clean rat holding cage (35 × 55 × 18 cm) in dim light (30 lux). The analysis was performed separately for minutes 1–5 and 6–10.
Prepulse inhibition
The experiment was essentially performed as described (Pletnikov et al., 2008). Seven identical startle chambers (S-R LAB, San Diego Instruments) contained a Plexiglas cylinder, in which the mice were placed. A loudspeaker of ∼10 cm from the mouse provided the acoustic signals and a broadband background noise of 65 dB. An accelerometer located underneath the mouse recorded the movement of the chamber, which is proportional to the startle response of the animal. Because the movement in the chamber is higher with increasing body weight of the mouse, all parameters were analysed with weight as covariate. The experiment was preceded by a 5 min acclimatization period to the background noise. Startling responses to the tones were measured with an accelerometer below the mouse. Prepulse inhibition was determined by comparing the average startling amplitudes to pulses that had or had not been preceded by a prepulse of a lower intensity as described (Pletnikov et al., 2008). Habituation to sounds was measured by comparing the response to a set of five 120 dB tones before and after the prepulse session with pulse interval times of 100 ms. Baseline startle was measured as response to the first set of five 120 dB pulses. Prepulse–pulse interval times were 50, 100, 200 and 300 ms. Prepulse intensities were 4, 8 or 12 dB above the background. Three presentations of each trial occurred in pseudorandom order. Prepulse inhibition was measured as: (mean startle amplitude for pulses alone / mean startle amplitude on prepulse–pulse trials) × 100 whereby the mean startle amplitude for the pulses alone was obtained from the average mean startle amplitude to three 120 dB pulses given in the middle of the trial series. For prepulse inhibition and subsequent biochemical analysis after MS-275 treatment, only animals with increased H3K18 acetylation (compared with the vehicle group of similar genotype) were considered.
Pharmacology
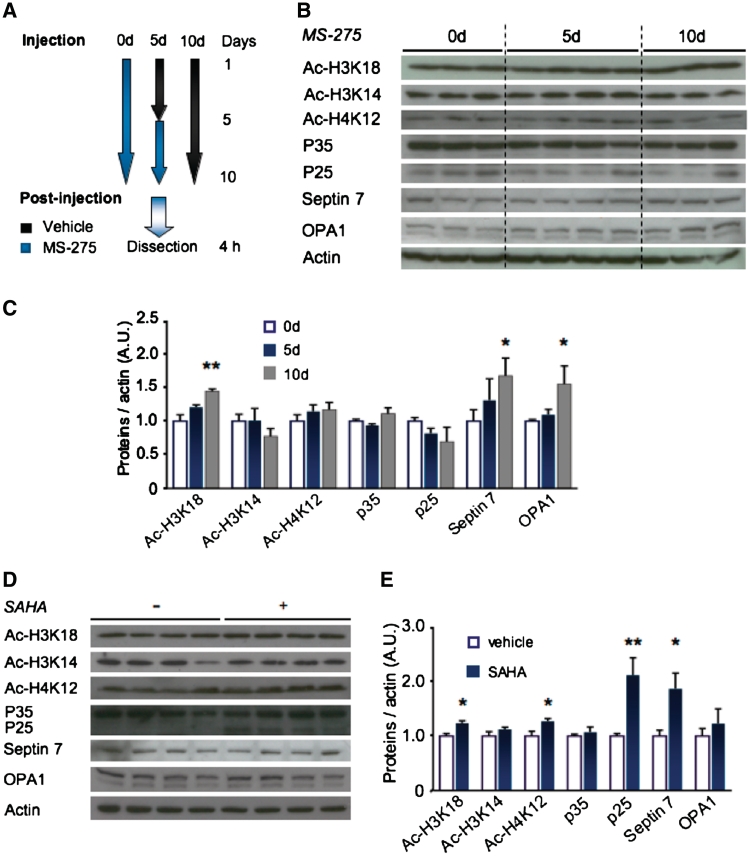
Clozapine administration was performed as described (Smalla et al., 2008). For inhibition of HDAC1, female 8-week-old C57BL/6 mice were intraperitoneally injected for 5 or 10 days with 12.5 mg/kg MS-275, 25 mg/kg suberoylanilide hydroxamic acid (SAHA; both Selleckchem) or vehicle (37.5% dimethylsulphoxide in saline; for dosing, compare with Fischer et al., 2007 and Kim et al., 2008). Hippocampi were dissected 4 h after the last injection. Total lysates were prepared as described above and analysed by western blot.
Statistics
Data were analysed in Statistical Package for the Social Sciences. Depending on the number of factors, Student’s t-test, one-, two- or three-way ANOVA, were performed. For post hoc analysis, the test of choice was the Kruskal–Wallis test for one-way ANOVA, Student Newman–Keuls test for two-way ANOVA or Dunn’s test, in case the samples were not equally distributed. For two-way ANOVA with repeated measures, simple main effects were determined for post hoc analysis, using least significant difference.
Results
p35 is reduced in post-mortem schizophrenia brain
p35 is involved in cognition and neuronal development (Angelo et al., 2006). We hypothesized that p35 signalling may be disturbed in schizophrenia, a neurodevelopmental disorder associated with cognitive deficits. To this end, post-mortem samples from prefrontal cortex and hippocampus of patients with schizophrenia and controls (Supplementary Table 2) were analysed by western blot and immunohistochemistry. As p35 is known to be degraded post-mortem (Taniguchi et al., 2001), we balanced post-mortem delays between control and patient groups and made an effort to obtain group sizes similar to exemplary post-mortem studies (Patrick et al., 1999; Emamian et al., 2004).
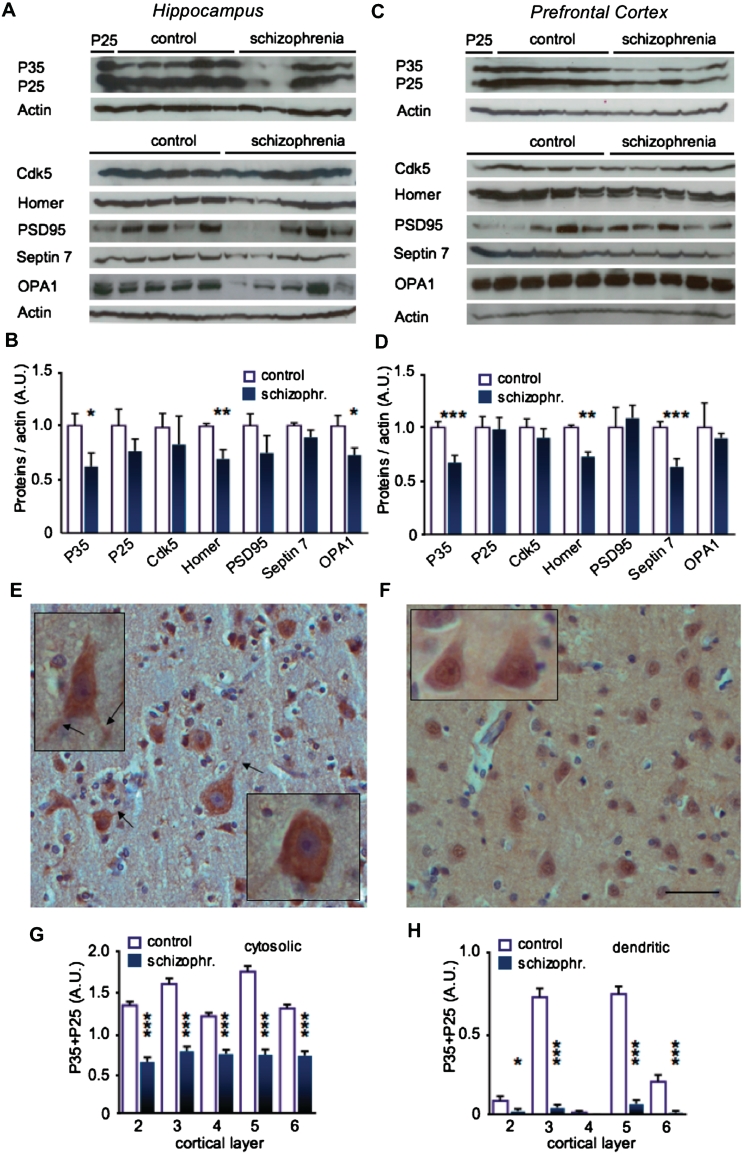
p35 levels were significantly downregulated both in prefrontal cortex (P < 0.001) and in hippocampus of schizophrenia brains (P < 0.05; Fig. 1A–D). There was no change in p25 levels, which may be due to the fact that p25 is a small fraction of p35 and the long post-mortem delay may have saturated p25 formation. Furthermore, p35 and p25 levels are regulated by separate mechanisms (Engmann et al., 2011). A downregulation of p35 expression was confirmed by immunohistochemistry with an antibody that recognizes primarily the abundant p35. Because of the limited availability of samples, paraffin-embedded sections could only be obtained for prefrontal cortex of one patient and a control (post-mortem delay = 7–8 h). In this case, a highly significant decrease in cytosolic staining of p35 and p25 was detected in all cortical layers (P < 0.001; Fig. 1E–H). Furthermore, the dendritic signal for p35 and p25 decreased in all layers except layer 4, which showed low staining in control tissue as well (P < 0.001; Fig. 1E, F and H). Interestingly, while no nuclear staining occurred in the control sample, there was a clear signal for p35 and p25 in nuclei of all layers in schizophrenia tissue. This suggests that despite an overall decrease of the signal, the remaining p35 and p25 may translocate into the nucleus.
Figure 1.
p35 and p25-regulated proteins are downregulated in schizophrenia post-mortem brain. Total lysates of post-mortem samples from the hippocampus (A and B) and the prefrontal cortex (C and D) were western blotted against p35/p25, Cdk5, homer, PSD95 and the p25-regulated proteins septin 7 and OPA1. Protein levels were normalized against β-actin. Hippocampal total lysate from a p25 transgenic mouse (P25) was loaded as a control for specificity of staining on the western blots against p35/p25. (C) p35 and homer were decreased in hippocampus. However, in this tissue OPA1, but not septin 7, was reduced. Cdk5 levels remained unchanged. (D) There was a significant downregulation of p35, homer and septin 7 in prefrontal cortex of patients with schizophrenia. No changes were detected for OPA1, PSD95 or Cdk5 levels. (E and F) The downregulation of p35 was confirmed by immunohistochemistry on the prefrontal cortex (layer 3). (E) In control subjects, p35 antibody revealed evenly distributed labelling of moderate intensity in the neuronal cytoplasm and cell processes (arrows) with no significant nuclear staining. (F) In patients with schizophrenia, weak or absent cytoplasmic and cell process labelling was noted with moderate nuclear labelling. (G–H) Quantification of immunostaining against p35+25. (G) Somatic p35+p25 was strongly reduced in all prefrontal cortical layers in patients with schizophrenia. (H) Dendritic p35+p25 was reduced in layers 3, 5 and 6 of prefrontal cortex from patients with schizophrenia. (A and B): n = 13–15 per group; (C and D): n = 8–9 per group; (G and H): n = 95–105 cells per group. *P < 0.05; **P < 0.01; ***P < 0.001; average ± SEM is shown; (E and F): scale bar = 50 micrometer; 25 µm (insets). A.U = arbitrary units.
Septin 7 and OPA1 are reduced in post-mortem schizophrenia brain
Recent studies on p25 transgenic mice have led to the identification of several downstream proteins of p25, among them septin 7 and OPA1 (Engmann et al., 2011). Septin 7 is a guanine triphosphate binding protein localized in the neck of spines, where it controls shape and formation of spines (Hall et al., 2005; Tada et al., 2007; Xie et al., 2007). OPA1 is a mitochondrial protein responsible for mitochondrial fusion and spine formation (Cipolat et al., 2004; Chen et al., 2007). As p25 is a cleavage product of p35 that is fully contained in the p35 sequence, we hypothesized that septin 7 and OPA1 may also be regulated by p35. This was confirmed (see below). Hence, we tested whether the protein expression of septin 7 and OPA1 is altered in schizophrenia post-mortem tissue as well. Indeed, septin 7 and OPA1 levels were significantly decreased: OPA1 expression was reduced in the hippocampus (P < 0.05; Fig. 1A and B), while septin 7 was downregulated in the prefrontal cortex (P < 0.001; Fig. 1C and D).
To date there are no pathological markers for schizophrenia, which could be used to confirm the quality of post-mortem schizophrenia samples. However, polymorphisms in the homer 1 gene have been linked with schizophrenia (Spellmann et al., 2010). Therefore, we analysed homer 1 protein levels in our post-mortem samples. Homer expression was decreased both in the hippocampus and in the prefrontal cortex of the post-mortem schizophrenia brains (both P < 0.01; Fig. 1).
As the studied proteins are all localized postsynaptically, we analysed the postsynaptic marker PSD95 to assess whether the detected changes were due to substantial synaptic loss. Since PSD95 levels were not altered, this explanation is unlikely to account for the observed changes (Fig. 1). Furthermore, Cdk5 levels were not altered, demonstrating that the observed changes in other proteins are specific and not due to global protein degradation post-mortem.
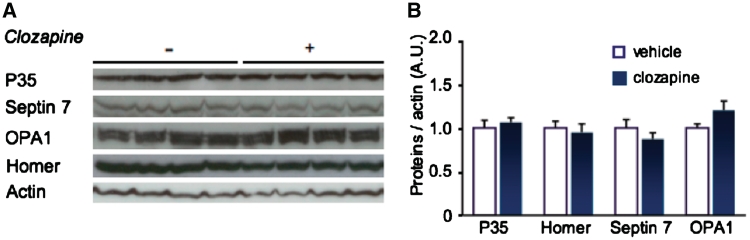
To exclude that our proteins of interest were artefacts of drug treatment, we chronically administered the antipsychotic clozapine to rats (Smalla et al., 2008). Clozapine treatment did not change expression of p35, septin 7 or OPA1 (Fig. 2). This suggests that the detected post-mortem protein changes were unlikely to be caused by medication of the patients. However, it cannot be fully excluded that life-long exposure to a variety of medications may have had an impact on the expression of our proteins of interest in the patients. In summary, there are tissue-specific protein changes in p35 and the downstream proteins septin 7 and OPA1 in the schizophrenic post-mortem brain.
Figure 2.
Cdk5 activators and downstream proteins are not affected by the antipsychotic drug clozapine. (A) Purified synaptic fractions from prefrontal cortex of rats that had been treated with clozapine or vehicle were western blotted against p35, the p25-regulated proteins septin 7 and OPA1 as well as against homer. (B) None of the analysed proteins were affected by clozapine treatment. (n = 4–7; average ± SEM is shown).
p35+/− mice show sex-specific cognitive endophenotypes
The observations of a reduction of p35, septin 7 and OPA1 made on post-mortem tissue were descriptive. Therefore, we next asked whether a reduction of p35 is an epiphenomenon of schizophrenia or whether it is sufficient to induce cognitive deficits related to schizophrenia.
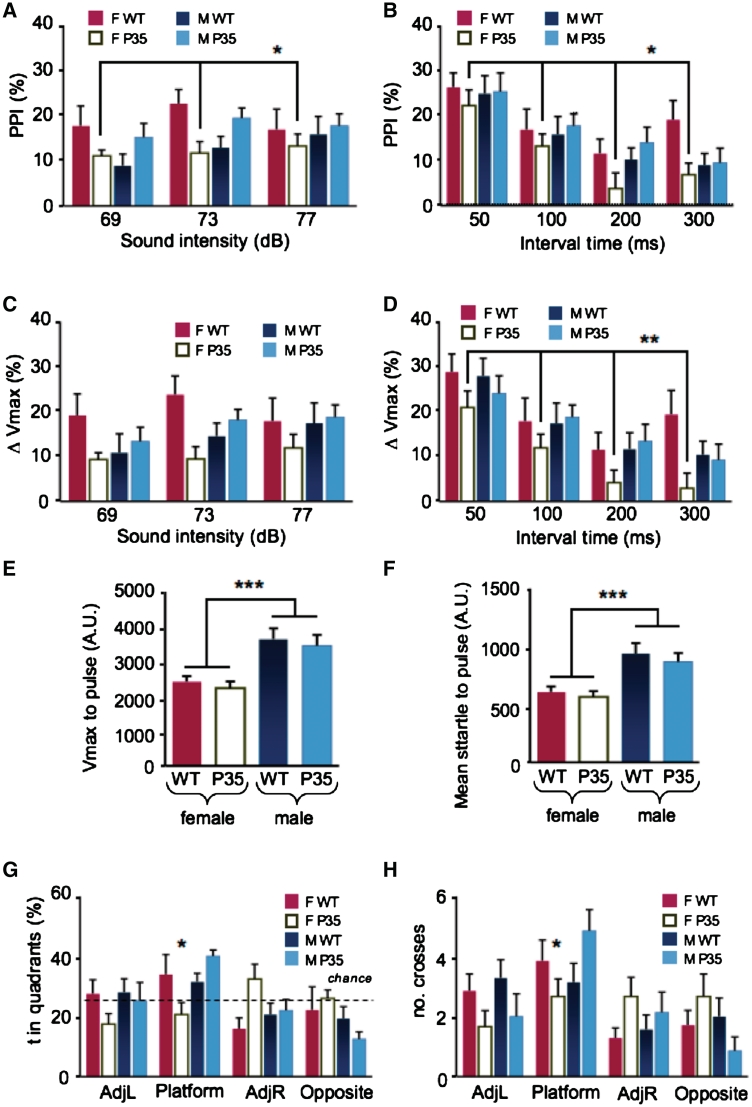
Homozygous p35 knockout mice show gross anatomical and behavioural abnormalities (Ohshima et al., 2005). However, under most physiological conditions, a total elimination of a protein is unlikely. In particular, we detected a reduction of p35 to ∼50% in schizophrenia brain. Therefore, we investigated heterozygous p35 knockout mice expressing 50% of the normal level of p35 (p35+/− mice; Supplementary Fig. 1). We studied these mutants in cognitive tests that are commonly used for mouse models of cognitive endophenotypes, such as prepulse inhibition and memory tasks. Additionally, we tested for the specificity of behavioural deficits by applying a battery of anxiety, social and locomotor tests. Because transgenic mice over-expressing the p35 cleavage product p25 have sex-specific differences (Ris et al., 2005) and since a variety of mouse models for schizophrenia endophenotypes show sex-specific deficits (e.g. Rojas et al., 2007; Pletnikov et al., 2008), we decided to analyse both sexes. We found that female, but not male, p35+/− mice had impaired prepulse inhibition (P < 0.01; Fig. 3A–F). Importantly, this female-specific impairment in prepulse inhibition was observed at different sound intensities and time intervals, and it was not caused by an altered response to the pulse itself. We also studied formation of spatial reference memory and its reversal in the water maze. Spatial memory formation was only subtly impaired in female, but not in male, p35+/− mice (Supplementary Fig. 2). However, reversal learning was severely impaired in the female mutants (Fig. 3G and H). Although the reason for the sex difference is still unclear, it is in agreement with the finding that female, not male p25 transgenic mice, have improved spatial memory formation (Ris et al., 2005). Furthermore, male p35+/− mice were impaired in cognitive tasks, in which the female mutants were not affected. In the rewarded alternation paradigm, which tests for spatial working memory (Chen et al., 2008), and in a social interaction paradigm male, but not female, p35+/− mice were impaired (Supplementary Fig. 2).
Figure 3.
p35+/− mice display sex-specific cognitive deficits. (A) Prepulse inhibition (PPI) was measured as the mean startle response to a set of prepulses of sound intensities of 69, 73 and 77 dB within a background noise of 65 dB. They were followed after 100 ms by a sound of 120 dB. (B) Another set of prepulses of 77 dB were given at interval times of 50, 100, 200 and 300 ms before a 120 dB sound. Female p35+/− mutants showed reduced prepulse inhibition for all tested parameters. (C and D) Female p35+/− mutants also showed a reduced maximum startle amplitude (ΔVmax) for various interval times at 77 dB. (E and F) The baseline startle response of female p35+/− mice was not altered. (G and H) p35+/− mice and wild-type (WT) littermates of both sexes were trained in the hidden platform version of the Morris water maze. (G) After reversal learning, female p35+/− mutants spent significantly less time in the platform quadrant and made fewer crosses through the platform area (H). (A–F): n = 12–18 per group, asterisks represent sex by genotype interaction and post hoc data; (G and H): n = 7–9 per group, asterisks represent post hoc data. *P < 0.05; average ± SEM is shown. F = female; M = male. AdJL = quadrants left of the platform quadrant; R: quadrants right of the platform quadrant; **P < 0.01, ***P < 0.001.
p35+/− mice of both sexes appeared normal, exhibiting unchanged locomotor activity in the open field and had no motor deficits. They behaved normally in the black–white alley and the hyponeophagia tests for anxiety (Supplementary Fig. 2). Therefore, the observed cognitive impairments were specific.
A reduction of p35 is sufficient to reduce septin 7 and OPA1 expression
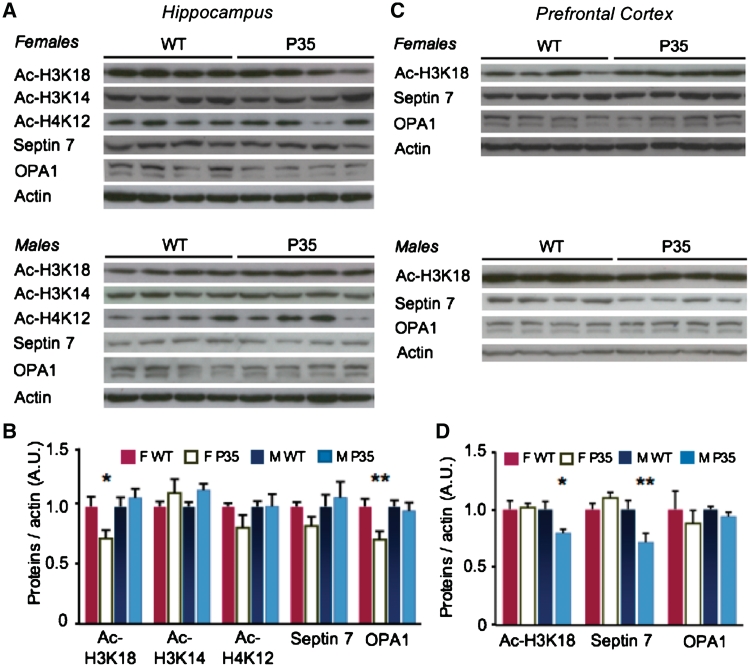
After completing the behavioural battery, we investigated whether septin 7 and OPA1 expression is affected by the reduced p35 expression in the p35+/− mice. Interestingly, OPA1 expression was reduced in the hippocampus of female, but not male, p35+/− mice (P < 0.05; Fig. 4A and B), while septin 7 levels were reduced in prefrontal cortex of male, but not female, p35+/− mice (P < 0.01; Fig. 4C and D). The causes for the sex differences are unclear (but see Mizuno and Giese, 2010). These findings indicate that septin 7 and OPA1 expression are reduced in response to changes of p35 expression and they suggest that this link in expression might apply to schizophrenia (Fig. 1A–D).
Figure 4.
Female p35+/− mice have reduced OPA1 expression and acetylation of H3K18 in hippocampus, while male p35+/− mice show decreased H3K18 acetylation and septin 7 expression in prefrontal cortex. (A) Hippocampal total lysates from p35+/− mice (P35) and wild-type (WT) littermates were probed by western blot for the acetylated forms of H3K18, H3K14 and H4K12 as well as septin 7 and OPA1. (B) In the hippocampus, acetylation of H3K18 and OPA1 expression were specifically reduced in female p35+/− mice. (C) Total lysates from prefrontal cortex of p35+/− mice and wild-type littermates were western blotted against acetyl-H3K18, septin 7 and OPA1. (D) Septin 7 expression and H3K18 acetylation were specifically reduced in the prefrontal cortex of male p35+/− mice. The downregulation in hippocampal OPA1 levels in response to reduced p35 is in contrast to an upregulation of septin 7 in response to p25 over-expression in our two mouse models. This might be due to differences in expression of transgene in neurons under the control of the alphaCaMKII promoter versus knockout in all p35-containing neurons (n = 10 per group; *P < 0.05; **P < 0.01; asterisks represent sex by genotype interaction and post hoc data; average ± SEM is shown). F = female; M = male.
Histone acetylation at lysine 18 of histone 3 (H3K18) is reduced in p35+/− mice
Next, we were interested in the cause of altered gene expression in the p35+/− mice. Previous studies by us and others have shown that p25 levels correlate with acetylation of the HDAC1 target-site H3K18, but not with target sites of other HDACs, H4K12 and H3K14 (Kim et al., 2008; Engmann et al., 2011). Hence, we tested whether the same is the case in p35+/− mice as the p25 sequence, which inhibits HDAC1, is contained in p35 (Kim et al., 2008). Indeed, H3K18 acetylation was reduced in the p35+/− mice (Fig. 4). Interestingly, reduced H3K18 acetylation was found in the hippocampus of female, but not male mutants, while prefrontal H3K18 acetylation was reduced in male, but not female p35+/− mice (both P < 0.05). Hence, H3K18 acetylation correlates with septin 7 and OPA1 expression in mice.
Inhibition of HDAC1 by MS-275 and SAHA increases the expression of p35-regulated proteins
To test whether the inhibition of HDAC1 was sufficient to alter expression of p35-regulated proteins, we administered the selective HDAC1 inhibitor MS-275 (Simonini et al., 2006) to wild-type mice (Fig. 5A–C). Indeed, inhibition of HDAC1 was sufficient to increase expression of septin 7 and OPA1 (both P < 0.05), suggesting a causal link between HDAC1 activity and expression of these two proteins. Acetylation of H3K18, but not of H4K12 or H3K14, was increased after MS-275 administration, confirming the specificity of the drug (P < 0.01; Fig. 5C).
Figure 5.
Expression of septin 7 and OPA1 are affected by administration of HDAC inhibitors. (A) Scheme of drug administration: cohorts were administered 10 days of vehicle (0d), 5 days of vehicle followed by 5 days of MS-275 (5d) or 10 days of MS-275 or SAHA (10d). (B and C) Total hippocampal lysates from mice that had obtained MS-275 injections or vehicle were western blotted against acetylated forms of H3K18, H3K14 and H4K12, against p35/p25, septin 7 and OPA1, and normalized against β-actin. (C) Acetylation of H3K18 was increased after MS-275 administration, indicating successful drug treatment. Acetylation of H3K14 and H4K12 were not changed, confirming the specificity of the drug. The expression of septin 7 and OPA1 was significantly increased after 10 days of MS-275 administration, while p35/p25 levels were unaltered. (D and E) After 10 days of SAHA treatment, p25 and septin 7 levels were significantly upregulated. SAHA increased acetylation of H3K18 as well as H4K12 in agreement with its unspecific inhibition of class I HDACs. Expression of p35, H3K14 and OPA1 were not affected by SAHA treatment (n = 6–8 per group; *P < 0.05; **P < 0.01; average ± SEM shown).
The unspecific class I HDAC-inhibitor SAHA has been shown to improve hippocampus-dependent contextual fear memory (Fischer et al., 2007) and increases acetylation of H3K18 and H4K12 (Guan et al., 2009; Fig. 5D and E). Therefore, we tested whether H3K18 acetylation and increase in septin 7 and OPA1 also correlate after the injection of SAHA. Indeed SAHA administration increased septin 7 expression and H3K18 acetylation (both P < 0.05; Fig. 5D and E). However, SAHA did not significantly increase OPA1 expression (P > 0.05). As SAHA inhibits a variety of HDACs including HDAC1, it is likely that other pathways are activated, which lead to an inhibition of OPA1 expression.
Interestingly, SAHA also induced the formation of p25 without a detectable reduction in p35 expression (P < 0.01; Fig. 5D and E) at a dose that does not induce neurodegeneration (Guan et al., 2009). On the other hand, selective inhibition of HDAC1 by MS-275 (Simonini et al., 2006) did not induce p25 formation (P > 0.05; Fig. 5A–C). This may be due to regulation of the p35 gene by acetylation of H4K12, an HDAC2 target site (Guan et al., 2009). We have recently demonstrated that p25 formation is a physiological event during spatial memory formation (Engmann et al., 2011). As SAHA is associated with memory improvements (Fischer et al., 2007), p25 formation under these conditions is in agreement with our previous data (Engmann et al., 2011).
The HDAC1 substrate H3K18 is hyperacetylated during spatial memory formation
Inhibition of HDAC1 has so far been associated with neurotoxicity. As we established a link between formation of p25, the cleavage product of p35 and septin 7 expression during spatial memory formation in the water maze paradigm (Engmann et al., 2011), we were interested whether changes in H3K18 acetylation also occur physiologically during memory formation. Indeed, H3K18 acetylation was increased in mice that had been trained in the water maze but not in swim controls (P < 0.05; Supplementary Fig. 3). Hence, H3K18 acetylation correlates with memory formation and occurs under physiological conditions of spatial memory formation. This is, to our knowledge, the first proof that H3K18 acetylation occurs during spatial reference memory in the hippocampus.
Interestingly, in swim controls, H3K18 acetylation is downregulated in a highly significant manner. This may be due to stress as the absence of an escape resembles certain paradigms of learned helplessness, and a reduction in histone acetylation may be associated with chronic stress (Uchida et al., 2011).
Treatment with MS-275 rescues molecular changes and prepulse inhibition deficits in female p35+/− mice
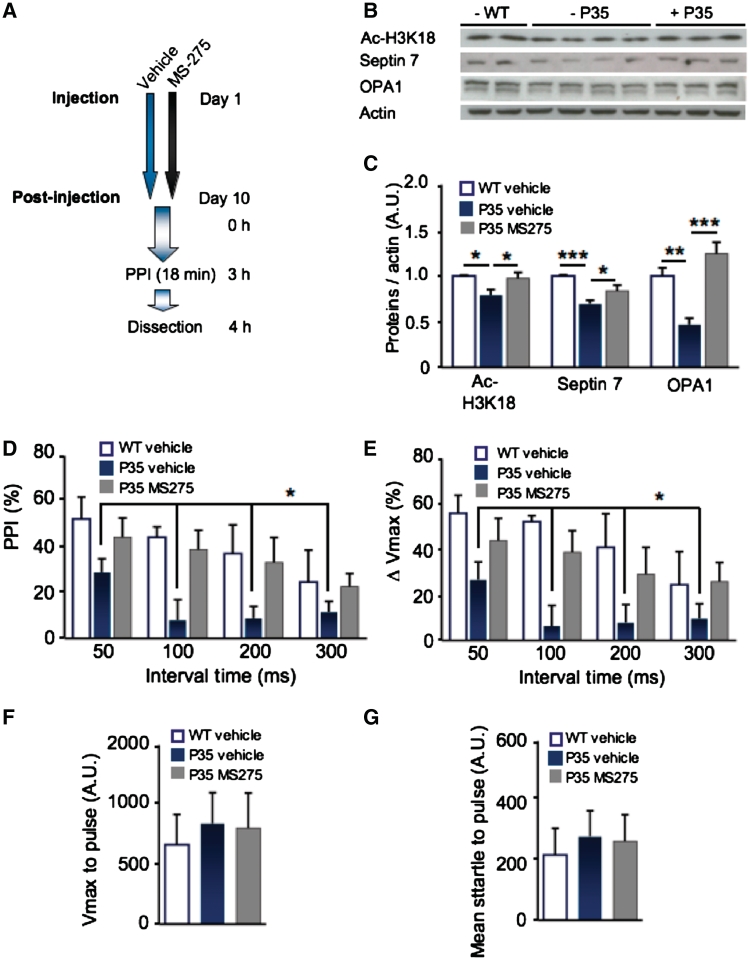
p35+/− mice show protein expression changes similar to post-mortem schizophrenia brain and they have impaired cognitive endophenotypes of schizophrenia. Further, the acetylation of the HDAC1 substrate H3K18 correlated with cognitive and molecular endophenotypes in p35+/− mice (Fig. 4) and administration of an HDAC1-inhibitor increased the expression of p35-regulated proteins in wild-type mice (Fig. 5). Hence, we tested whether systemic administration of the selective HDAC1 inhibitor MS-275 can rescue molecular changes and prepulse inhibition deficits in p35+/− mice. The prepulse inhibition paradigm was chosen for the rescue attempt because of its temporal accuracy. Other tasks such as water maze training and alternation tasks have a larger duration and hence may not be suitable for the current protocol of MS-275 administration. Only females were used for MS-275 treatment (Fig. 6A) since female, but not male, p35+/− mice were impaired in prepulse inhibition (Fig. 3A–F).
Figure 6.
Administration of MS-275 rescues molecular endophenotypes and prepulse inhibition in female p35+/− mice. (A–G) p35+/− mice and wild-type (WT) littermates were injected intraperitoneally for 10 days with either the HDAC1-inhibitor MS-275 or vehicle. (A) Scheme of the experiment. (B and C) Total hippocampal lysates were western blotted against acetyl-H3K18, septin 7 and OPA1. (C) There was a significant reduction in expression of septin 7 and OPA1 and H3K18 acetylation in p35 mutants, which was rescued by administration of MS-275. (D and E) Prepulse inhibition (PPI) was tested with a protocol comprising four different interval times. Weight was analysed as covariate as weight correlated with detected startle amplitudes and therefore affected the measurements. There was a significant treatment by genotype interaction for the prepulse inhibition as measured by change in the mean (ΔPPI) and the maximum (ΔVmax) startle amplitudes. Post hoc analysis revealed that this change arises from reduced prepulse inhibition in female p35+/− mice in the vehicle group as well as from an effect of drug treatment within p35 mutants and within wild-type mice. (F and G) The baseline startle response was not affected by administration of MS-275 [− = vehicle; + = MS-275; n = 4–8 per group; (D and E) asterisks represent sex by genotype interaction and post hoc data; *P < 0.05; **P < 0.01; ***P < 0.001; average ± SEM shown].
Analysis of the prepulse inhibition data showed that there was a significant treatment effect for both the mean startle amplitude and the maximum startle amplitude at varying interval times (all P < 0.05 or less; Fig. 6D and E). Post hoc analysis demonstrated that this effect was based on a reduction of prepulse inhibition in vehicle-treated p35+/− mice (all P < 0.05 or less), confirming our previous data that untreated, female p35+/− mice have reduced prepulse inhibition (Fig. 3A–F). MS-275 treated p35+/− mice were indistinguishable in their performance from vehicle-treated wild-type mice (Fig. 6F and G). Furthermore, MS-275 also rescued the protein expression changes in female p35+/− mice (Fig. 6B and C). Note, that here septin 7 expression is reduced in female mutants, possibly because the mice were previously trained in prepulse inhibition that may have increased septin 7 expression in wild-type mice analogous to training in other hippocampal tasks (Engmann et al., 2011), but not in the mutants. Taken together, we show that molecular endophenotypes and prepulse inhibition deficits in female p35+/− mice can be restored by treatment with the drug MS-275, which is currently being used as cancer treatment.
Discussion
We found that in schizophrenia, a disorder associated with impaired cognition, p35 expression is reduced by ∼50% in relevant brain regions. Furthermore, the expression of two p35-regulated proteins implicated in spine formation, septin 7 and OPA1, are decreased in schizophrenia in a specific pattern. These molecular changes are modelled in heterozygous p35 knockout mice and they were found to cause cognitive endophenotypes, including deficits in prepulse inhibition. The molecular and behavioural endophenotypes in p35 heterozygotes correlate with acetylation of the HDAC1 target site H3K18. Administration of the HDAC1 inhibitor MS-275, which is clinically used as treatment for cancer, restores molecular changes and prepulse inhibition deficits in the mouse model. Taken together, our findings propose that in schizophrenia, reduced p35 expression contributes to cognitive impairments, possibly by epigenetic dysregulation of septin 7 and OPA1 expression.
Our findings suggest impaired p35 signalling in schizophrenia. Specifically, we demonstrate by western blot that the expression of p35 is decreased in hippocampus and prefrontal cortex of schizophrenia post-mortem samples. Furthermore, our immunohistochemical analysis suggests that in schizophrenia there is not only a reduction of p35 expression, but there might also be a translocation of p35. Despite an overall decrease of the expression, the remaining p35 might translocate into the nucleus, in analogy to a translocation of proteins into the nucleus found in other neurological diseases (e.g. Nishimura et al., 2010). Follow-up studies will need to test whether there is altered p35 translocation in p35 heterozygotes. We found that the p35-regulated proteins septin 7 and OPA1 are reduced in prefrontal cortex and hippocampus in schizophrenia. A recent post-mortem study also found reduced septin 7 expression at the messenger RNA level in prefrontal cortex in schizophrenia (Ide and Lewis, 2010). Our studies with the p35 mouse model suggest that the reduced p35 expression causes epigenetic dysregulations that impact on septin 7 and OPA1 expression in schizophrenia. Specifically, we have shown that acetylation at the HDAC1 site H3K18 is reduced in p35 heterozygotes. The HDAC1 inhibitor MS-275 restores not only acetylation of H3K18 in p35 heterozygotes, but also the expression of septin 7 and OPA1. Unfortunately, we could not investigate histone acetylation with our schizophrenia samples due to instability of histone acetylation during post-mortem delay. Nonetheless, we have some evidence that there is epigenetic dysregulation in the genes encoding septin 7 and OPA1. A study examining DNA methylation differences in monozygotic twins discordant for schizophrenia showed a significant difference in methylation of CpG islands in the septin 7 gene and in sufficient close proximity to the OPA1 gene promoter between affected and unaffected twins (Supplementary Table 3). These changes in DNA methylation suggest that there are epigenetic dysregulations in schizophrenia that may cause the reduced expression of OPA1 and septin 7. Notably, histone acetylation and DNA methylation have been shown to work in concert to regulate genes associated with synaptic plasticity (Miller et al., 2008). A link between acetylation of HDAC1 target sites and DNA methylation in p35 mutants will be further explored in a future study.
Both septin 7 and OPA1 are implicated in spine formation (Chen et al., 2007; Tada et al., 2007; Xie et al., 2007). Therefore, the reduced expression of septin 7 and OPA1 might contribute to the aberrant spine density that has been detected in prefrontal cortex and hippocampus in patients with schizophrenia (Glantz and Lewis, 2000; Kolomeets et al., 2005). As OPA1 is also involved in mitochondrial fusion (Cipolat et al., 2004), the reduced OPA1 expression also suggests that mitochondrial abnormalities are associated with schizophrenia. Interestingly, such abnormalities have recently been found in the striatum (Somerville et al., 2010).
p35+/− mice show not only reduced expression in p35, septin 7 and OPA1 similar to post-mortem schizophrenia brain, but they also display cognitive deficits, including prepulse inhibition and reversal learning impairments, reminiscent of other mouse models for cognitive endophenotypes occurring in schizophrenia. Thus, the p35+/− mice model various aspects of schizophrenia. A reduction of p35 in mice is sufficient to alter H3K18 acetylation, most likely via reduced HDAC1 inhibition (Kim et al., 2008; Guan et al., 2009). In the p35 mouse model, inhibition of HDAC1 by MS-275 administration restored not only prepulse inhibition deficits, but also molecular endophenotypes of schizophrenia. Our studies are in agreement with the suggestion that epigenetic dysregulation contributes to schizophrenia. In spite of difficulties in detecting post-translational modifications and enzymatic activities post-mortem, HDACs are coming increasingly into focus as treatments for psychiatric illnesses (Fischer and Sananbesi, 2009). We show for the first time that changes in histone acetylation impact on prepulse inhibition, a behavioural endophenotype of schizophrenia. Therefore, our findings encourage further studies of HDAC1 inhibitors in the context of schizophrenia and other disorders with related cognitive deficits.
Sex differences for cognitive impairments associated with schizophrenia have been described (e.g. Weiser et al., 2000; Rubin et al., 2008). Furthermore, sex-specific impairments in prepulse inhibition have been found in patients with bipolar disorder, a disease that is closely related to schizophrenia (Gogos et al., 2009). Accordingly, mouse models for candidate genes of pathways of schizophrenia, such as inducible Disrupted in schizophrenia 1 missense mice (Pletnikov et al., 2008) and Nurr 1 mice (Rojas et al., 2007) show sex-specific behavioural or molecular alterations as well. As in these mouse models, we found sex-specific molecular and behavioural changes in the p35 mouse model. Although the basis of these sex differences is not clear to date, this effect is not uncommon during work with mouse models but may be overshadowed by the fact that most mouse studies only employ male mice (Arguello and Gogos, 2006; Mizuno and Giese, 2010). Unfortunately, our post-mortem samples did not allow a detailed comparison of molecular changes between sexes in the schizophrenia cohort.
Previous studies with high doses of the HDAC1 inhibitor MS-275 (50 mg/kg, 10 days, compared with our dose of 12.5 mg/kg for 10 days) suggested that reduced HDAC1 activity and as a result, increased H3K18 acetylation is neurotoxic (Kim et al., 2008). However, the same study showed that HDAC1 activity was reduced in inducible p25 transgenic mice at a condition that improves memory formation (Fischer et al., 2007; Kim et al., 2008). Therefore, HDAC1 targets may be dysregulated in a dose-dependent manner, resulting in either improved memory formation or increased neurotoxicity. This is supported by the fact that no neurotoxicity was observed by Kim and colleagues (2008) for the protocol used in the study presented here. More importantly, we show that H3K18 acetylation was increased under physiological conditions during spatial memory formation. Therefore, it is unlikely that H3K18 acetylation is associated solely with neurotoxic events. Moreover, we show that the memory-enhancing, unspecific HDAC inhibitor SAHA (Fischer et al., 2007) can enhance moderate p25 formation. Taken together, it needs to be emphasized that for future studies of both H3K18 acetylation and p25-formation, physiological doses have to be studied, as overdoses of both p25 and HDAC1-inhibitors result in aversive effects (Fischer et al., 2005; Kim et al., 2008), while mild inhibition of HDAC1 and formation of low p25 doses are beneficial for memory (Kim et al., 2008; Engmann et al., 2011).
In summary, we propose a signalling pathway of p35 and downstream proteins, which may be affected in schizophrenia. We suggest, based on a mouse model with reduced p35 expression, that cognitive and molecular endophenotypes of schizophrenia can be rescued by altering histone acetylation with a clinically approved, selective HDAC1 inhibitor.
Funding
This work was supported by funding and a PhD studentship of the Medical Research Council, UK (to K.P.G. and O.E.), a grant from the National Institutes of Health (R01-MH-087463-01A1; to K.P.G. and J.M.), a short-term fellowship of the Boehringer Ingelheim Fonds, Germany (to O.E.), and funding from the Deutsche Forschungsgemeinschaft (to M.R.K.). We have no competing financial interest in relation to the work described.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank Drs David Bannerman, Nickolas Rawlins, Tomasz Schneider, Amy Taylor (all Oxford University, UK), David Collier, Walter Lucchesi, Keiko Mizuno (all King’s College London, UK), Jean-Antoine Girault and Matthias Groszer (INSERM Fer a Moulin, France) for helpful advice and Dr Li-Huei Tsai (MIT) for providing the p35 mutant mice.
Glossary
Abbreviations
- Cdk-5
cyclin-dependent kinase 5
- HDAC1
histone deacetylase 1
- SAHA
suberoylanilide hydroxamic acid
References
- Angelo M, Plattner F, Giese KP. Cyclin-dependent kinase 5 in synaptic plasticity, learning and memory. J Neurochem. 2006;99:353–70. doi: 10.1111/j.1471-4159.2006.04040.x. [DOI] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423–31. doi: 10.1046/j.1460-9568.2003.02746.x. [DOI] [PubMed] [Google Scholar]
- Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–96. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Barclay LL, Gibson GE, Blass JP. The string test: an early behavioral change in thiamine deficiency. Pharmacol Biochem Behav. 1981;14:153–7. doi: 10.1016/0091-3057(81)90236-7. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–71. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–62. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–83. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P. Cyclin-dependent kinase 5 regulates dopaminergic and glutamatergic transmission in the striatum. Proc Natl Acad Sci US A. 2004;101:2191–6. doi: 10.1073/pnas.0308652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101:15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat Genetics. 2004;36:131–7. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Engmann O, Hortobágyi T, Thompson AJ, Guadagno J, Troakes C, Soriano S, et al. Cdk5 activator p25 is generated during memory formation and is reduced at an early stage in Alzheimer’s disease. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.04.011. (in press) [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J Neurosci. 2002;22:3700–7. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–38. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–82. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F. The epigenetic bottleneck of neurodegenerative and psychiatric diseases. Biol Chem. 2009;390:1145–53. doi: 10.1515/BC.2009.131. [DOI] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–19. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269–78. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, et al. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–6. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Koovakkattu D, Hayashi K, Anderson AE, Powell CM, Sinton CM, et al. Regulation of hippocampal and behavioral excitability by cyclin-dependent kinase 5. PLoS One. 2009;4:e5808. doi: 10.1371/journal.pone.0005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobágyi T, Troakes C, Nishimura AL, Vance C, van Swieten JC, Seelaar H, et al. Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD-TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol. 2011;121:519–27. doi: 10.1007/s00401-011-0813-3. [DOI] [PubMed] [Google Scholar]
- Humbert S, Dhavan R, Tsai LH. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–83. doi: 10.1242/jcs.113.6.975. [DOI] [PubMed] [Google Scholar]
- Ide M, Lewis DA. Altered cortical CDC42 signalling pathways in schizophrenia: implications for dendritic spine deficits. Biol Psychiatry. 2010;68:25–32. doi: 10.1016/j.biopsych.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–58. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–17. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Lai KO, Ip NY. Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim Biophys Acta. 2009;1792:741–5. doi: 10.1016/j.bbadis.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Liddle PF. Cognitive impairment in schizophrenia: its impact on social functioning. Acta Psychiatr Scand Suppl. 2000;400:11–6. doi: 10.1111/j.0065-1591.2000.007s021[dash]3.x. [DOI] [PubMed] [Google Scholar]
- Maekawa S, Leigh PN, King A, Jones E, Steele JC, Bodi I, et al. TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology. 2009;29:672–83. doi: 10.1111/j.1440-1789.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Richer E, Benkovic SA, Hayashi K, Kansy JW, Hale CF, et al. Striatal dysregulation of Cdk5 alters locomotor responses to cocaine, motor learning, and dendritic morphology. Proc Natl Acad Sci USA. 2008;105:18561–6. doi: 10.1073/pnas.0806078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89:599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Giese KP. Towards a molecular understanding of sex differences in memory formation. Trends Neurosci. 2010;33:285–91. doi: 10.1016/j.tins.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Nishimura AL, Zupunski V, Troakes C, Kathe C, Fratta P, Howell M, et al. Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain. 2010;133:1763–71. doi: 10.1093/brain/awq111. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ogura H, Tomizawa K, Hayashi K, Suzuki H, Saito T, et al. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35 mice. J Neurochem. 2005;94:917–25. doi: 10.1111/j.1471-4159.2005.03233.x. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Peterson DW, Ando DM, Taketa DA, Zhou H, Dahlquist FW, Lew J. No difference in kinetics of tau or histone phosphorylation by CDK5/p25 versus CDK5/p35 in vitro. Proc Natl Acad Sci USA. 2010;107:2884–9. doi: 10.1073/pnas.0912718107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F, Angelo M, Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–65. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–86. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, et al. Premorbid intra-individual variability in intellectual performance and risk for schizophrenia: a population-based study. Schizophr Res. 2006;85:49–57. doi: 10.1016/j.schres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Ris L, Angelo M, Plattner F, Capron B, Errington ML, Bliss TV, et al. Sexual dimorphisms in the effect of low-level p25 expression on synaptic plasticity and memory. Eur J Neurosci. 2005;21:3023–33. doi: 10.1111/j.1460-9568.2005.04137.x. [DOI] [PubMed] [Google Scholar]
- Rojas P, Joodmardi E, Hong Y, Perlmann T, Ögren SO. Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry. 2007;12:756–66. doi: 10.1038/sj.mp.4001993. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Haas GL, Keshavan MS, Sweney JA, Maki PM. Sex difference in cognitive response to antipsychotic treatment in first episode schizophrenia. Neuropsychopharmacology. 2008;33:290–7. doi: 10.1038/sj.npp.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BH, Shirley E, Rushworth MF, Bannerman DM. Distinct contributions of frontal areas to emotion and social behavior in the rat. Eur J Neurosci. 2007;26:2315–26. doi: 10.1111/j.1460-9568.2007.05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–9. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonini MV, Camargo LM, Dong E, Maloku E, Veldic M, Costa E, et al. The benzamide MS-275 is a potent, long-lasting brain region-selective inhibitor of histone deacetylases. Proc Natl Acad Sci USA. 2006;103:1587–92. doi: 10.1073/pnas.0510341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, et al. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla KH, Mikhaylova M, Sahin J, Bernstein HG, Bogerts B, Schmitt A, et al. A comparison of the synaptic proteome in human chronic schizophrenia and rat ketamine psychosis suggest that prohibitin is involved in the synaptic pathology of schizophrenia. Mol Psychiatry. 2008;13:878–96. doi: 10.1038/mp.2008.60. [DOI] [PubMed] [Google Scholar]
- Somerville SM, Conley RR, Roberts RC. Mitochondria in the striatum of subjects with schizophrenia. World J Biol Psychiatry. 2010;12:48–56. doi: 10.3109/15622975.2010.505662. [DOI] [PubMed] [Google Scholar]
- Spellmann I, Rujescu D, Musil R, Mayr A, Giegling I, Genius J, et al. Homer-1 polymorphisms are associated with psychopathology and response to treatment in schizophrenic patients. J Psychiatr Res. 2010;45:234–41. doi: 10.1016/j.jpsychires.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–8. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi S, Fujita Y, Hayashi S, Kakita A, Takahashi H, Murayama S, et al. Calpain-mediated degradation of p35 to p25 in postmortem human and rat brains. FEBS Lett. 2001;489:46–50. doi: 10.1016/s0014-5793(00)02431-5. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Delalle L, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–23. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobaashi A, Otsuki K, Yamagata H, Hobara T, et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–72. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Wei FY, Tomizawa K, Ohshima T, Asada A, Saito T, Nguyen C, et al. Control of cyclin-dependent kinase 5 (Cdk5) activity by glutamatergic regulation of p35 stability. J Neurochem. 2005;93:502–12. doi: 10.1111/j.1471-4159.2005.03058.x. [DOI] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Rabinowitz J, Kaplan Z, Merk M, Nahon D, et al. Gender differences in premorbid cognitive performance in a national cohort of schizophrenic patients. Schizophr Res. 2000;45:185–90. doi: 10.1016/s0920-9964(99)00190-5. [DOI] [PubMed] [Google Scholar]
- Wen Y, Planel E, Herman M, Figueroa HY, Wang L, Liu L, et al. Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signalling leads to differential effects on tau phosphorylation and amyloid precursor protein processing. J Neurosci. 2008;28:2624–32. doi: 10.1523/JNEUROSCI.5245-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–51. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.