Abstract
Despite substantial data elucidating the roles of cholesterol in lipid rafts at the plasma membrane, the roles of cholesterol and related lipids in lipid raft microdomains at the level of subcellular membrane, such as the endoplasmic reticulum (ER) membrane, remain less understood. Growing evidence, however, begins to unveil the importance of cholesterol and lipids on the lipid raft at the ER membrane. A few ER proteins including the sigma-1 receptor chaperone were identified at lipid raft-like microdomains of the ER membrane. The sigma-1 receptor, which is highly expressed at a subdomain of ER membrane directly apposing mitochondria and known as the mitochondria-associated ER membrane or MAM, has been shown to associate with steroids as well as cholesterol. The sigma-1 receptor has been implicated in ER lipid metabolisms/transports, lipid raft reconstitution at the plasma membrane, trophic factor signalling, cellular differentiation, and cellular protection against β-amyloid-induced neurotoxicity. Recent studies on sigma-1 receptor chaperones and other ER proteins clearly suggest that cholesterol, in concert with those ER proteins, may regulate several important functions of the ER including folding, degradation, compartmentalization, and segregation of ER proteins, and the biosynthesis of sphingolipids.
Keywords: Cholesterol, Steroid, Sigma-1 receptor chaperone, Endoplasmic reticulum, Mitochondria-associated ER membrane, Lipid raft, Detergent-resistant microdomain, Trophic factor
13.1 Introduction
Cholesterol is an important molecule not only for constituting the biological membrane but also for regulating a spectrum of cellular processes including gene transcription and signal transduction. Cholesterol serving as a universal precursor of steroidgenesis can regulate gene transcriptions indirectly via steroid receptors. Cholesterol can also directly activates cholesterol–sensing transcription factor, the sterol regulatory element binding proteins (SREBPs), thus regulating transcriptions of a spectrum of lipid enzymes (Brown and Goldstein, 1997; Bengoechea-Alonso and Ericsson, 2007; Lavoie and King, 2009). Further, cholesterol, by forming lipid microdomains with sphingolipids (also called as detergent-insoluble microdomains or lipid rafts), can regulate membrane curvature, protein sorting, vesicle transport, endocytosis, and trophic factor-induced signal transduction at the plasma membrane (Simons and Ikonen, 1997; Simons and Toomre, 2000). Lipid rafts composed of cholesterol and sphingolipids compartmentalize signalling molecules (e.g., receptors, kinases) at the phospholipids bilayer, thus promoting selective and efficient signal transduction (Pike, 2003).
The discovery of the lipid raft microdomain shed lights on the importance of cholesterol as a direct modulator of signal transduction. Roles of cholesterol, particularly those in plasma membrane lipid rafts, have been thus extensively examined by employing relevant models such as raft-residing trophic factor receptors and lipid raft-dependent endocytosis (particularly that via caveolae, a subtype of rafts containing caveolins) (Ikonen and Vainio, 2005; Lajoie and Nabi, 2007). On the other hand, roles of cholesterol-rich lipid microdomains at subcellular membranes, except for Golgi apparatus, have been less well-known.
Growing number of studies, however, has begun to elucidate the existence and importance of lipid microdomains at subcellular organelles, which include mito-chondria and the endoplasmic reticulum (ER). Although lipid rafts are considered as ubiquitous constituents of plasma membrane or Golgi, which is in agreement with the enrichment of lipid rafts in these loci, recent studies indicated that lipid rafts are similarly formed at the subcellular membranes with relatively low concentrations of cholesterol and glycosphingolipids (van Meer and van Genderen, 1994; van Meer, 2000). For example, it is known that mitochondrial membranes contain lipid rafts that are remodeled and redistributed during apoptotic and immune reaction, thus initiating cell death signals derived from mitochondria (Malorni et al., 2007).
Most recent studies also demonstrated that the ER membrane, although contains considerably low levels of cholesterol and glycosphingolipids compared to other organelles, can form lipid raft-like microdomains. A few ER proteins were identified as being present in lipid raft-like microdomains, that include ER lipid raft protein (erlin)-1 and -2, PrP, and the sigma-1 receptor chaperone (Hayashi and Su, 2003a,, 2004b; Sarnataro et al., 2004; Hayashi and Su, 2005; Browman et al., 2006; Campana et al., 2006; Hoegg et al., 2009). Erlin, the family of pro-hibitin domain-containing (PHB) proteins including the prohibitins, the stomatins and the flotillins, was found exclusively at detergent-resistant microdomains of the ER membrane (Browman et al., 2006). The biological function of erlin, however, remains elusive. A few recent studies implicated the potential role of ER rafts in protein folding of prion protein (PrP). Campana et al. (2006) found that processing of the PrPsc, the pathogenic glycoprotein causing neurodegenerative Creutzfeldt-Jakob disease, depends on the association of PrP with lipid microdomains at the ER. The pathogenic mutant PrP T182A mainly retains at lipid raft-like domains at the ER. Importantly, the association of the protein with lipid rafts promotes the folding of the protein, thus inhibiting scrapie-like conversion of PrP mutants and protecting cells (Campana et al., 2006).
A line of recent study from our laboratory and others has demonstrated that cholesterol plays important roles at the ER together with the ER protein sigma-1 receptor chaperone. We reported that sigma-1 receptor chaperones targeting ER rafts regulate protein degradation and lipid transports (Hayashi and Su, 2003b, 2007). The sigma receptor is a protein originally proposed as a subtype of opi-oid receptors, but later confirmed not to be an opioid receptor, rather being a novel protein mainly localized at the ER (Su and Hayashi, 2003). Based on the ligand-binding profile, it has been postulated that the sigma receptor consists of at least two subtypes: sigma-1 and sigma-2. The sigma-1 receptor possesses high affinity for the (+)-isomer of prototypic ligands such as (+)-SKF10047, whereas the sigma-2 receptor does it for the (−)-isomers (Su and Hayashi, 2003). Recent studies demonstrated that both type-1 and -2 sigma receptors are present at lipid rafts (Hayashi and Su, 2003b, 2004b; Gebreselassie and Bowen, 2004). Especially, the sigma-1 receptor, which was cloned in 1996 (Hanner et al., 1996), has been implicated in lipid metabolisms, transports, and cellular survival (Hayashi and Su, 2005; Hayashi et al., 2009). Further, recent studies begin to unveil the cholesterol-binding characteristic of the sigma-1 receptors (Palmer et al., 2007). These findings given from the sigma receptor research indeed provide several interesting aspects in terms of roles of cholesterol at the ER. Thus, we seek in this chapter to revisit recent findings on the sigma-1 receptor that may ultimately help understanding roles of cholesterol and lipid microdomains at the ER as well as those in pathophysiology of certain human diseases.
13.2 Structure and Subcellular Localization of the Sigma-1 Receptor
In agreement with a fact that the N-terminus of the sigma-1 receptor has a double-arginine ER retention signal (Hanner et al., 1996), several immunocytochemical or biochemical studies have demonstrated the ER localization of sigma-1 receptors (Alonso et al., 2000; Hayashi and Su, 2003a; Jiang et al., 2006; Dun et al., 2007). Although a number of studies found that sigma-1 receptors modulate functions of proteins at the plasma membrane (e.g., K+ channel) (Aydar et al., 2002; Fontanilla et al., 2009; Johannessen et al., 2009), the possibility of sigma-1 receptors in localizing at the plasma membrane has not been thoroughly clarified at present. Importantly, sigma-1 receptors at the ER show typically a punctate staining pattern in immunocytochemistry (Hayashi and Su, 2003a, 2004b), that is distinctive from the pattern seen with other ER proteins such as cytochrome P450 reductase that typically show the reticular or diffuse cytoplasmic pattern in immunostaining. A recent study identified the ER subdomains enriched with sigma-1 receptors as the mitochondria-associated ER membrane (MAM) (Hayashi and Su, 2007), the ER membrane physically associating with the outer membrane of mitochondria (Rusinol et al., 1994). The MAM plays critical roles in supplying Ca2+ directly from the MAM via inositol 1,4,5-trisphosphate receptors (IP3 receptors) to mito-chondria, thus regulating metabolism and bioenergetics in mitochondria (Rizzuto et al., 1999; Hajnoczky and Hoek, 2007; Duchen et al., 2008). The MAM is also important for lipid synthesis. The MAM express high levels of enzymes involved in syntheses of phospholipids, cholesterol, neutral lipids, and ceramides (Vance, 1990; Rusinol et al., 1993, 1994; Bionda et al., 2004). Phosphatidylserine (PtSer) synthesized at the MAM is transported to mitochondria via intermembrane lipid transport (without a demand of energy and transport vesicles) for the synthesis of phosphatidylethanolamine (PtEt) (Voelker, 2000). On the other hand, the role of the MAM in cholesterol transport is poorly understood. Nevertheless, since the MAM accommodates enzymes involved in cholesterol biosynthesis and since cholesterol transported from ER to mitochondria is the primary step in steroidgenesis, the MAM is proposed to serve as one of loci operating the cholesterol transport from the ER to mitochondria (Hayashi et al., 2009). A study demonstrated that the MAM, which was visualized by expressing sigma-1 receptors fused to the yellow fluorescent protein, accommodates the much higher level of free cholesterol when compared to other ER membranes (Hayashi and Su, 2003b).
The sigma-1 receptor is a 24-kDa protein possessing two transmembrane domains at the N-terminus and the center of the protein. The sigma-1 receptor also possesses one membrane-anchoring domain at the C-terminus. A study analyzing membrane topology of the sigma-1 receptor found the sigma-1 receptor possessing one cytosolic loop flanked by two transmembrane domains with a long ER lumenal domain at the C-terminus (Hayashi and Su, 2007). A recent study identified the sigma-1 receptor as a new class of molecular chaperone forming a complex with another ER chaperone BiP/GRP78 (Hayashi and Su, 2007). The long ER lumenal domain at the C-terminus is demonstrated to possess chaperone activity, which is negatively regulated by the association with BiP (Hayashi and Su, 2007). The molecular function of the sigma-1 receptor will be discussed later with more details.
Several studies have sought to identify the ligand-binding site(s) of sigma-1 receptors including that for steroids. A study showed that the amino acids in the second transmembrane domain are responsible for binding of the ligands with a slight difference in responsible amino acids for binding to agonists and antagonists, respectively (Yamamoto et al., 1999). On the other hands, a few charged amino acids in the ER lumenal domain at the C-terminus are shown to contribute to the formation of the ligand-binding site as well (Ganapathy et al., 1999; Seth et al., 2001). The amino acids in the membrane-anchoring domain seem to be responsible for binding of cocaine or progesterone (Chen et al., 2007; Pal et al., 2007, 2008; Fontanilla et al., 2008). Therefore multiple domains, including the transmem-brane domains or amino acids in vicinity to the ER membrane, may constitute the ligand/sterol-binding pocket of the sigma-1 receptor (Pal et al., 2008). Whether the pocket is composed within a single molecule of the sigma-1 receptor or by homo oligomerization of the protein is not examined yet.
13.3 The Potential Link Between the Sigma-1 Receptor and Sterols
Sigma-1 receptors ubiquitously express in several organs of mammals including brain, liver, pancreas, testis, overlay, placenta, and adrenal gland, as well as in malignant tumors (Vilner et al., 1995; Hanner et al., 1996; Spruce et al., 2004). A number of studies using selective sigma receptor ligands have demonstrated that sigma-1 receptors are involved in regulations of morphogenesis of neuronal cells (e.g., synaptogenesis, neuronal differentiation, myelination), neuroprotection, pain, pathophysiology of certain human diseases including depression, drug abuse, Alzheimer’s disease and cancer (Nakazawa et al., 1998; Goyagi et al., 2001; Maurice, 2004; Spruce et al., 2004; Liu et al., 2005; Marrazzo et al., 2005; Achison et al., 2007; Bermack and Debonnel, 2007; Dun et al., 2007; Martin-Fardon et al., 2007; Mei and Pasternak, 2007; Renaudo et al., 2007; Hayashi and Su, 2008; Smith et al., 2008; Tchedre and Yorio, 2008). Sigma-1 receptors bind a variety of psy-chotropic drugs and progesterone with submicromolar Ki (Su and Hayashi, 2003); particularly the finding on progesterone raised first time a possibility that sigma-1 receptors may interact with sterols (Su et al., 1988).
The success of cloning of the sigma-1 receptor provided another striking evidence supporting the link between the sigma-1 receptor and sterols. The sequence of the sigma-1 receptor, although having no homology to any mammalian proteins, shares a similarity to that of the yeast sterol C8-C7 isomerase (Hanner et al., 1996). In spite of the high homology between these proteins, following studies however negated the sigma-1 receptor serving as a mammalian C8-C7 sterol isomerase; the sigma-1 receptor lacks the enzymatic activity and the cloned mammalian sterol isomerase shares homology with neither the yeast sterol isomerase nor with the sigma-1 receptor (Hanner et al., 1996; Moebius et al., 1997; Bae et al., 2001). Nevertheless, the structural similarity, particularly that between the membrane-spanning domain of the sigma-1 receptor and the sterol-binding pocket of the yeast sterol isomerase (Hanner et al., 1996), argues the possibility that the sigma-1 receptor possesses the sterol-binding domain.
13.4 Sigma-1 Receptors Interact with Cholesterol
Although sigma-1 receptors were shown to bind some steroids in early in vitro binding assays, the possibility of sigma-1 receptors associating with cholesterol has just begun to be examined recently. Immunocytochemical studies have revealed sigma-1 receptors highly compartmentalized at the cholesterol-enriched subdomains of the ER membranes (i.e., MAM) (Hayashi and Su, 2003b; Hayashi and Su, 2004b). The cholesterol content in the ER membrane is generally kept at a considerably low level when compared to that in the plasma or Golgi membrane. However, filipin staining visualizing subcellular distributions of free cholesterol found that cholesterol is accumulated at the MAM and co-localized with sigma-1 receptors (Hayashi and Su, 2003b). The MAM was originally found as a specialized membrane domain for transports of lipids between ER and mitochondria (Vance, 1990). Thus, it is intriguing that the cholesterol is exceptionally enriched at the MAM. Transfection of dominant-negative sigma-1 receptors, which fail to target the MAM, is shown to disrupt the compartmentalization of cholesterol at MAM, leading to the diffuse distribution of cholesterol over the ER membrane (Hayashi and Su, 2003b). Seemingly, sigma-1 receptors regulate, at least in part, the accumulation of free cholesterol at the MAM.
Existence of lipid rafts at the ER has been under a debate for several years. However, growing evidence supports the existence of lipid rafts at the ER membrane (Hayashi and Su, 2003a, b, 2004b, 2005; Sarnataro et al., 2004; Browman et al., 2006; Campana et al., 2006; Hoegg et al., 2009). Sigma-1 receptors were found to reside in lipid rafts in NG108 neuroblastoma × glioma hybrid cells (Hayashi and Su, 2003b). In oligodendrocytes, sigma-1 receptors also accumulate at lipids rafts by forming the complex with cholesterol and galactosylceramides (Hayashi and Su, 2004b). In light of exceptional enrichment of cholesterol and sphingolipids (e.g., ceramides) at MAM among bulk ER membranes (Vance, 1990; Rusinol et al., 1993, 1994; Bionda et al., 2004), it is conceivable for the cell to form lipid rafts at the specialized subdomains. The identity of sphingolipid(s) forming rafts at the MAM is however not clarified, except for that in oligodendrocytes (Hayashi and Su, 2004b). In the sucrose-floatation centrifugation with Triton X-100, the ER-lipid rafts generally show lower buoyancy than that of plasma membrane rafts that mostly contain gangliosides such as GM1 (Hayashi and Su, 2003b). Interestingly, a certain class of caveolin shows similar buoyancy to ER rafts. Calveolin-2, but not caveolin-1 which is enriched at the plasma membrane and Golgi, was indeed shown to co-localize with sigma-1 receptors at the MAM (Hayashi and Su, 2003b).
Lipid rafts at the plasma membrane was classically proposed as platforms of the signal transduction, which compartmentalize receptors and signalling molecules for promoting protein-protein interactions (Simons and Ikonen, 1997; Simons and Toomre, 2000). Several trophic factor receptors, upon stimulation, dimerize and translocate into or out from lipid rafts to pursue activation of downstream signallings (Simons and Ikonen, 1997; Simons and Toomre, 2000). Similarly, sigma-1 receptors at ER rafts, upon stimulation with agonists, translocate from rafts to non-raft ER membranes (Hayashi and Su, 2003b; Palmer et al., 2007). Treatment with selective sigma-1 receptor agonist (+)pentazocine or (+)SKF10047 has been shown to shift sigma-1 receptors from Triton X-100-insoluble to the soluble fractions in a sucrose gradient centrifugation (Hayashi and Su, 2003b; Palmer et al., 2007). As discussed below, sigma-1 receptor agonists replace cholesterol binding to sigma-1 receptors (Palmer et al., 2007) and promote translocation of the receptor at the ER (Hayashi and Su, 2003b; Hayashi and Su, 2003a). Thus, the ligand-free, cholesterol-associating form of sigma-1 receptors seems to associate with ER rafts.
Although the above-mentioned evidence supports the possibility of sigma-1 receptors to bind cholesterol, the direct demonstration of cholesterol binding to sigma-1 receptors has begun to be examined recently. Ruoho and colleagures iden-tified two potential sterol-binding domains (SBDL-1, SDBL-II) of the sigma-1 receptors by using a series of photoaffinity labelings (Chen et al., 2007; Pal et al., 2007, 2008; Fontanilla et al., 2008). They found that derivatives of fenpropimorph (e.g., [125I]IAF), the inhibitor of yeast sterol isomerase that has also a high affinity for sigma-1 receptors, can selectively photolabel amino acids 91–109 and 176–194 with showing a single population of binding sites for [125I]IAF to interact with the sigma-1 receptor (Pal et al., 2007). These data propose a model in which the SBDL-I and SBDL-II are juxtaposed to form a sterol-binding site of the sigma-1 receptor (Pal et al., 2008). Palmer et al. (2007) by using the sequence matching analysis found that the sigma-1 receptor possesses amino acid sequences similar to the cholesterol-binding motif of the benzodiazepine receptor (i.e., L/V-X1–2-Y-X1–5-K/R). They postulated two potential cholesterol-binding domains on the sigma-1 receptors: residues 171–175 and 199–208. They demonstrated that the synthesized peptides containing these putative cholesterol-binding motifs (a.a. 161–180, a.a. 191–210) indeed bind cholesterol on immobilized nitrocellulose membranes (Palmer et al., 2007). The binding of cholesterol in the same system is reduced by co-incubation with sigma-1 receptor agonist (+)-SKF10047 (Palmer et al., 2007). Following energy minimization by Universal Force Field prediction, they proposed that tyrosine173 and 206 at the surface of the lipid bilayer are critical for the cholesterol-binding property of the sigma-1 receptors, since the substitutions of tyrosine to serine at the sites abolished the cholesterol-binding property of the two peptides (Palmer et al., 2007). These findings support the notion that sigma-1 receptors may directly interacts with cholesterol at lipid rafts of the MAM. Further, the ability of the ligands in altering the sigma-1 receptor-cholesterol association may partly explain the underlying mechanism in ligand-induced translocation of sigma-1 receptors from rafts to non-raft ER membranes (Hayashi and Su, 2003b; Hayashi and Su, 2003a).
13.5 Molecular Function of the Sigma-1 Receptor
Since the 1970 s, a numerous number of studies have demonstrated a variety of pharmacological and physiological effects of sigma-1 receptors and their ligands in both in vitro and in vivo systems; that include neuroprotection, anti/pro-apoptotic action, cellular differentiation, potentiation of Ca2+ signalling via IP3 receptors, regulation of ion channels such as K+ channel and NMDA receptor, potentiation of trophic factor signalling (NGF, EGF, BDNF and MAPKs), cellular proliferation, protein secretion, carcinogenesis, long-term potentiation of hippocampal neurons, learning and memory, mood and cognition, and drug-dependence and craving (Maurice et al., 2002; Takebayashi et al., 2002, 2004a, b; Matsumoto et al., 2003; Su and Hayashi, 2003; Chen et al., 2006; Yagasaki et al., 2006; Martina et al., 2007; Hayashi and Su, 2008; Sabeti and Gruol, 2008; Fontanilla et al., 2009; Hayashi et al., 2009). However, the basic molecular function of the sigma-1 receptor has been elusive until the recent discovery of the innate activity. The sigma-1 receptor is a ligand-operated molecular chaperone at MAM (Hayashi and Su, 2007). Under the physiological concentration of Ca2+ in the ER lumen (0.5 mM), the lumenal domain of the sigma-1 receptor forms a complex with BiP, an ER homologue of heat-shock protein 70 (Hsp 70), in a Ca2+/Mn2+-dependent manner (Hayashi and Su, 2007). Sigma-1 receptors forming the complex with BiP are basically at the dormant state, thus minimizing the chaperone activity (Hayashi and Su, 2007). The depletion of ER Ca2+ by activation of IP3 receptors or inhibition of the ER Ca2+ pump by thapsigargin triggers the dissociation of sigma-1 receptors from BiP, which in turn fully activates sigma-1 receptor chaperones (Hayashi and Su, 2007; Hayashi et al., 2009).
Because of their specific localization at the MAM, sigma-1 receptor chaperones are assumed to stabilize mostly MAM-residing proteins. So far, the type-3 IP3 receptor is only protein identified as a substrate of the sigma-1 receptor chaperone. Knockdown of sigma-1 receptors causes rapid ubiquitination and degradation of type-3 IP3 receptors residing at MAM, thus causing decrease of direct Ca2+ influx from MAM to mitochondria (Hayashi and Su, 2007). Ca2+ uptaken into mitochon-dria in turn activates dehydrogenases in the TCA cycle, leading to potentiation of the ATP production in mitochondria (Rizzuto et al., 1999; Hajnoczky and Hoek, 2007; Duchen et al., 2008), the stabilization of IP3 receptors by sigma-1 receptor chaperones is thus postulated to contribute to bioenergetics and cellular survival. In this context, the role of cholesterol potentially specifying the MAM localization of sigma-1 receptors seems vast.
13.6 Ligand-Binding Profile of the Sigma-1 Receptor
The prominent uniqueness of the sigma-1 receptor is that the innate chaperone activity can be activated/inactivated pharmacologically by synthetic compounds or by sterols. Sigma-1 receptor agonists promote the dissociation of sigma-1 receptors from BiP, which in turn activates chaperone activity of sigma-1 receptors in an ER Ca2+-independent manner (Hayashi and Su, 2007). The action of the agonists is blocked by antagonists. Structurally diverse compounds including steroids and some clinically used psychotropic drugs and immunesuppressants exert high affinities for sigma-1 receptors (Table 13.1). A recent study found that endogenous hallucinogen dimethyltryptamine binds to sigma-1 receptors (Fontanilla et al., 2009). Selective sigma-1 receptor agonists have been demonstrated to possess therapeutic actions in animal models of depression, schizophrenia, and strokes (Hayashi and Su, 2008). Some steroids that have affinities to sigma-1 receptors indeed exert antiamnesic or antidepressant-like actions via sigma-1 receptors (Hayashi and Su, 2008). Whether cholesterol at the MAM may affect the chaperone activity of the sigma-1 receptor as a potential endogenous ligand is an interesting unsolved question. The recent find-ing showing ER rafts regulating folding of PrP (Campana et al., 2006), however, raises a tempting speculation that ER cholesterol may gain the folding capability of the ER by either mimicking a molecular chaperone by itself or by regulating activity of ER chaperone proteins.
Table 13.1.
Ligands of the sigma-1 receptor chaperone
| Synthetic compounds |
| (+)Pentazocine |
| (+)Dextromethorphan |
| (+)SKF10047 |
| Haloperidol |
| Fluvoxamine |
| Imipramine |
| Donepezil |
| SA31747 |
| Natural/endogenous compounds |
| Progesterone |
| Dehydroepiandrosterone sulfate |
| Pregnenolone sulfate |
| Hyperforin/hypericin |
| Dimethyltryltamine |
13.7 Roles of Sigma-1 Receptors in Subcellular Distribution of Lipids and Reconstitution of Lipid Rafts
Studies exploring roles of sigma-1 receptors in lipid biology has just emerged. Recent data suggest that sigma-1 receptors play important roles in the subcellular distribution of cholesterol as well as its partitioning in lipid rafts at the plasma membrane (Hayashi and Su, 2005). For example, overexpression of sigma-1 receptors, although they are mainly at the ER (e.g., MAM), can increase cholesterol at the plasma membrane rafts (Takebayashi et al., 2004b). Further, expression of dominantly negative sigma-1 receptors decreases plasma membrane cholesterol with concomitantly increasing cholesterol at the ER (Hayashi and Su, 2003b). Thus, sigma-1 receptors at the ER seem to be involved in export of cholesterol from ER to the plasma membrane.
Stable overexpression of sigma-1 receptors in PC12 cells promotes the alteration of glycosphingolipids in plasma membrane lipid rafts (Takebayashi et al., 2004b). Overexpression of sigma-1 receptors increases ganglioside GD1a in plasma membrane rafts, the endproduct of the ganglioside synthesis, but decreases less-glycosylated precursors of GD1a such as GM1 and GM2 (Takebayashi et al., 2004b). Because the biosynthesis of glycopshingolipids downstream of ceramides is entirely processed at Golgi (van Meer, 2000), this finding suggests the sigma-1 receptor accelerating glycosylation of gangliosides at the Golgi apparatus. The reconstitution of plasma membrane rafts caused by sigma-1 receptors therefore promotes a significant alteration in signal transduction triggered by receptors of trophic factors as mentioned below. How sigma-1 receptors associating with cholesterol at the MAM regulate glycosylation of sphingolipids at Golgi is, however, an open question at present.
Several trophic factor receptors, upon stimulation, dimerize and translocate into lipid rafts for activation of downstream signallings. However, some trophic factor receptors such as the EGF receptor conversely translocate from rafts to non-raft membranes upon the activation; thus lipid rafts serve as both positive and negative regulators of trophic factor-related signals in the receptor-specific manner. Importantly, it is known that the association of receptors with lipid rafts depends on sugar moieties of residing gangliposides; for example, EGF receptors have the highest affinity for GM1 or GM2 ganglioside, but the lowest affinity for highly gly-cosylated GD1a (Miljan et al., 2002). Thus, reconstitution of gangliosides in lipid rafts caused by sigma-1 receptors promotes the relocation of EGF receptors from rafts to non-rafts membranes, leading to potentiation of the EGF receptor activity (Takebayashi et al., 2004b). In such, sigma-1 receptors by modulating lipid components of the plasma membrane may regulate diverse signal transductions mediated by trophic factor receptors as is indeed seen in other systems such as NGF or BFND (Takebayashi et al., 2002; Yagasaki et al., 2006).
13.8 The Sigma-1 Receptor in Human Diseases
13.8.1 Neuropsychiatric Disorders
Since the first prototypic sigma receptor ligand SKF-10047 induces psy-chotomimeric actions and some antipsychotics such as haloperidol have low-nM affinities for sigma receptors (Su and Hayashi, 2003), the sigma-1 receptor lig-ands had been expected to serve as a new class of psychotherapeutic drugs. However, newly synthesized selective sigma-1 receptor ligands do not necessarily have psychotimimetic effects (Hayashi and Su, 2004a). Further clinical studies failed to show significant effect of sigma-1 receptor ligands on positive symptoms of schizophrenia (Volz and Stoll, 2004). On the other hand, recent preclinical studies have accumulated substantial data showing that sigma-1 receptor ligands possess robust neuroprotective action against, for example, β-amyloid- or ischemia (Hayashi and Su, 2008). Sigma-1 receptor agonists also show anti-amnesic and anti-depressant-like actions in animal models (Maurice et al., 2002).
On the analogy between psychostimulant-induced psychosis and schizophrenia, sigma receptor ligands have been extensively examined in animal models of drug-dependence. The use of psychostimulants such as methamphetamine and cocaine acutely causes psychosis in humans similar to positive symptoms of schizophrenia. Further, the long-term use of psychostimulants promotes neuroad-aptative/neuroplastic changes, often those are irreversible and promote hard-to-cure symptoms such as withdrawal symptoms, craving, anxiety, aberrant stress-responses and depression (Hyman et al., 2006). Recent studies have found that sigma-1 receptors are upregulated in particular brains regions relating to dopaminergic reward and motor systems following chronic treatments with psychosimulants (Stefanski et al., 2004; Liu and Matsumoto, 2008).
It is not totally clarified how sigma-1 receptor chaperones at the MAM promote neuronal plasticity that likely involves morphogenesis of cells in the central nervous system. In in vitro studies, however, upregulation of sigma-1 receptors is shown to promote neuronal differentiation and potentiation of BDNF signallings (Hayashi and Su, 2004b; Yagasaki et al., 2006); both have been proven to be implicated in the pathophysiology of depression and drug dependence (Hyman et al., 2006). Indeed, certain antidepressants promote neuritegenesis by activating sigma-1 receptors in PC12 cells (Takebayashi et al., 2002). The action in activating neuronal differentiation may involve at least in part the action of sigma-1 receptors to promote reconstitution of plasma membrane lipid rafts that alters signal transduction of the trophic factor receptors (Hayashi and Su, 2005). Recent studies demonstrate that cholesterol is de novo synthesized in the brain, which is independent of cholesterol supplied by the blood circulation. Further, cholesterol provided by astrocytes is shown to serve as a potent inducer of neuronal morphogenesis including synap-togenesis (Barres and Smith, 2001; Suzuki et al., 2007). In contrast, the aberrant metabolism of cholesterols is known to cause neuronal dysfunction and degeneration (Pregelj, 2008). Thus, sigma-1 receptors regulating cholesterol transport and raft formation may play important roles in neuroprotection and neuroplasticity in the brain.
13.8.2 Cancer
Early binding studies found that sigma-1 receptors highly express in many cancer cells (Vilner et al., 1995). Recent studies demonstrated that some sigma-1 receptor ligands inhibit unstrained proliferation of carcinoma both in vitro and in vivo (Spruce et al., 2004). A recent study provides an intriguing mechanism of sigma-1 receptors in regulating proliferation in cancers. The study found that sigma-1 receptor ligands cause the dissociation of cholesterol from sigma-1 receptors, which subsequently leads to reconstitution of plasma membrane rafts (Palmer et al., 2007). The reconstitution of lipid rafts promotes dramatic inhibition of cell adhesion mediated by β-integrin, the strength is indicative of invasiveness of cancers (Palmer et al., 2007). Knockdown of sigma-1 receptors by siRNA shows the similar phenotype. The effect of sigma-1 receptor ligands was abolished if the composition of lipid rafts is altered by using drugs that affect lipid raft formation such as methyl- β-cyclodextrin (2% for 30 min) (Palmer et al., 2007). These findings further suggest that cholesterol trafficking and raft formation regulated by sigma-1 receptors may be involved in promoting pathogenic processes involved in human diseases.
13.9 Conclusions
In this book chapter, we introduced a novel sterol-binding ER protein, the sigma-1 receptor chaperone. Sigma-1 receptor chaperones localize at the MAM, an ER subcompartment apposing to mitochondria (Hayashi and Su, 2007), where sigma-1 receptors associate with lipid rafts (Hayashi and Su, 2005). The association of sigma-1 receptors with cholesterol is altered by the ligand treatment, leading the relocation of sigma-1 receptors at the ER membrane (Hayashi and Su, 2005; Palmer et al., 2007). The sigma-1 receptor regulates the ER distribution of cholesterol as well as the formation of lipid rafts at the plasma membrane (Hayashi and Su, 2005). Further, sigma-1 receptors accelerate glycosylation of gangliosides at Golgi (Hayashi and Su, 2005). It is unclear at present how exactly the innate activity of the sigma-1 receptors (i.e., molecular chaperone activity) contributes to the regulations of cholesterol transport, ganglioside syntheses, and reconstitution of lipid rafts at the plasma membrane. Nevertheless, the regulation of lipid redistribution and metabolisms certainly affect a variety of signal transductions mediated by receptors of several trophic factors (e.g., EGF, NGF, BDNF), thus regulating cellular survival, differentiation, and cell adhesion.
The role of ER cholesterol in regulation of SREBP has been exhaustively examined, and elucidated evidence constitutes a clear picture wherein cholesterol regulates one of fundamental functions of the ER: the lipid biosyntheses. However, information for depicting roles of cholesterol in other basic ER functions (e.g., protein synthesis, protein folding, protein/lipid secretion, post-translational protein modifications, Ca2+ signalling), if any, is considerably scarce. Thus, one ultimate goal of this chapter is to cast fresh light on potential functions of cholesterol at the ER by reviewing the sigma-1 receptor chaperone, and to provide a scope for future investigations. The data from sigma-1 receptor chaperones and others clearly suggest the potentials of ER cholesterol in regulating 1) protein folding and degradation at the ER, 2) compartmentalization/segregation of ER resident proteins, 3) the function of mitochondria via the ER-mitochondria contact, and 4) sphingolipid biosyntheses.
Fig. 13.1.
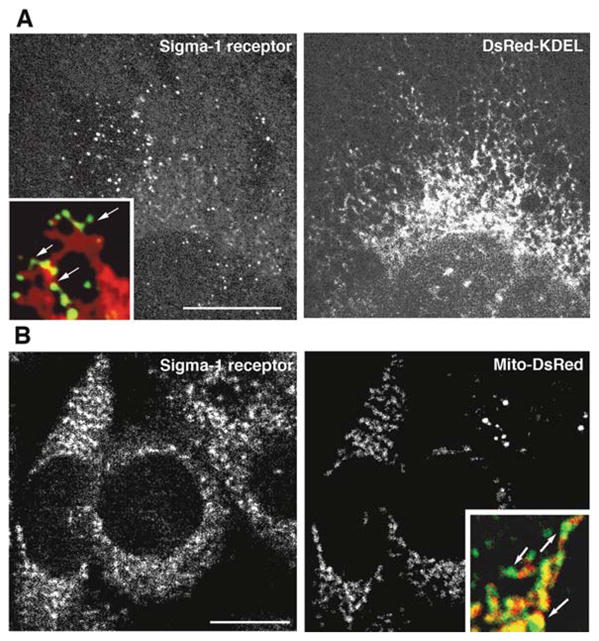
Mitochondria-associated ER membrane (MAM). A Spatial localization of MAM and bulk ER membranes. MAM is visualized by immunofluorescence using anti-sigma-1 receptor antibodies. ER membranes are visualized by expressing DsRed-tagged KDEL in the CHO cell. Bar=10 μm. Note that sigma-1 receptors accumulated at punctate substructures of ER membranes (arrows in the inset at higher magnification). B Spatial localization of MAM and mitochondria. MAM is visualized by immunofluorescence using anti-sigma-1 receptor antibodies. Mitochondria were visualized by expressing mitochondria-targeting red fluorescent proteins (Mito-DsRed) in the CHO cell. Bar=10 μm. MAM expressing sigma-1 receptors apposes to mitochondria (arrows in the inset at higher magnification)
Fig. 13.2.
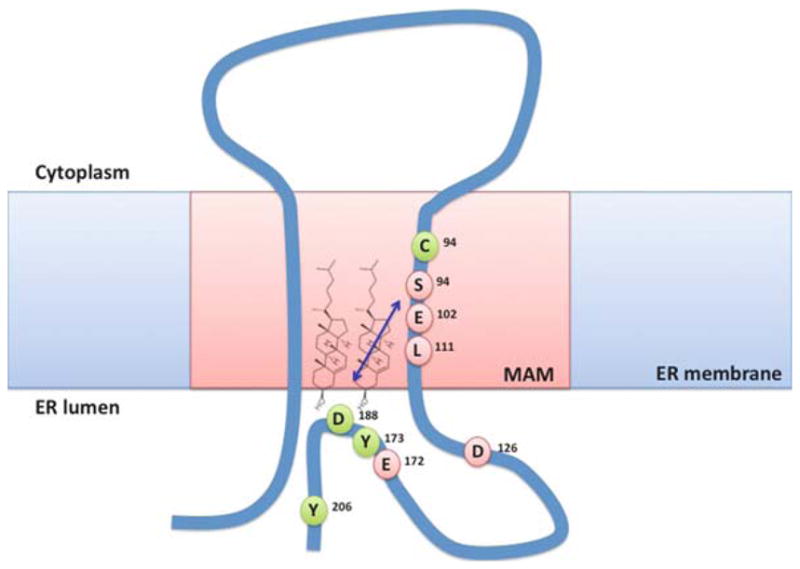
ER lipid rafts composed of cholesterol and sigma-1 receptors. The mitochondria-associated ER membrane (MAM) contains the relatively high level of cholesterol, when compared to other bulk ER membranes, thus forming lipid rafts as reported by recent studies. The sphin-golipid counterpart(s) constituting lipid rafts with cholesterol at the ER is not well defined. In oligodendrocytes, galactosylceramide appears to represent a major sphingolipid component of ER rafts. The sigma-1 receptor, which possesses two transmembrane domains, resides preferentially at lipid rafts of the MAM. Amino acids marked with pink are shown to be involved in ligand-binding of the sigma-1 receptor. Those in green are proposed to constitute sterol or cholesterol-binding domains. Sigma-1 receptors at ER rafts have been shown to regulate lipid transport/metabolism, Ca2+ signalling, reconstitution of plasma membrane lipid rafts, ganglioside synthesis, cellular survival and differentiation. The arrow indicates the domains in the juxtaposed position as demonstrated by sulfhydryl-reactive, radioiodinated photo-crosslinking
Acknowledgments
This work is supported by Intramural Research Program, NIDA, NIH, DHHS.
Abbreviations
- ER
endoplasmic reticulum
- MAM
mitochondria-associated ER membrane
- SREBP
sterol regulatory element binding protein
- PHB
prohibitin domain-containing
- PrP
prion protein
- erlin
ER lipid raft protein
- IP3 receptors
inositol 1,4,5-trisphosphate receptors
- PtSer
phosphatidylserine
- PtEt
phosphatidylethanolamine
- SBDL
sterol-binding domain-like
- IAF
iodo-azido fenpropimorph
- NGF
nerve growth factor
- EGF
epidermal growth factor
- BDNF
brain-derived neurotrophic factor
- MAPK
mitogen-activated protein kinase
- NMDA
N-methyl-D-aspartate
- Hsp
heat shock protein
- BiP
immunoglobulin binding protein
References
- Achison M, Boylan MT, Hupp TR, Spruce BA. HIF-1alpha contributes to tumour-selective killing by the sigma receptor antagonist rimcazole. Oncogene. 2007;26:1137–1146. doi: 10.1038/sj.onc.1209890. [DOI] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Bae S, Seong J, Paik Y. Cholesterol biosynthesis from lanosterol: molecular cloning, chromosomal localization, functional expression and liver-specific gene regulation of rat sterol delta8-isomerase, a cholesterogenic enzyme with multiple functions. Biochem J. 2001;353:689–699. doi: 10.1042/0264-6021:3530689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Smith SJ. Neurobiology. Cholesterol–making or breaking the synapse. Science. 2001;294:1296–1297. doi: 10.1126/science.1066724. [DOI] [PubMed] [Google Scholar]
- Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19:215–222. doi: 10.1016/j.ceb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Bermack JE, Debonnel G. Effects of OPC-14523, a combined sigma and 5-HT1a ligand, on pre- and post-synaptic 5-HT1a receptors. J Psychopharmacol. 2007;21:85–92. doi: 10.1177/0269881106063996. [DOI] [PubMed] [Google Scholar]
- Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382:527–533. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman DT, Resek ME, Zajchowski LD, Robbins SM. Erlin-1 and erlin-2 are novel members of the prohibitin family of proteins that define lipid-raft-like domains of the ER. J Cell Sci. 2006;119:3149–3160. doi: 10.1242/jcs.03060. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Campana V, Sarnataro D, Fasano C, Casanova P, Paladino S, Zurzolo C. Detergent-resistant membrane domains but not the proteasome are involved in the misfolding of a PrP mutant retained in the endoplasmic reticulum. J Cell Sci. 2006;119:433–442. doi: 10.1242/jcs.02768. [DOI] [PubMed] [Google Scholar]
- Chen L, Dai XN, Sokabe M. Chronic administration of dehydroepiandrosterone sulfate (DHEAS) primes for facilitated induction of long-term potentiation via sigma 1 (sigma1) receptor: optical imaging study in rat hippocampal slices. Neuropharmacology. 2006;50:380–392. doi: 10.1016/j.neuropharm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hajipour AR, Sievert MK, Arbabian M, Ruoho AE. Characterization of the cocaine binding site on the sigma-1 receptor. Biochemistry. 2007;46:3532–3542. doi: 10.1021/bi061727o. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Verkhratsky A, Muallem S. Mitochondria and calcium in health and disease. Cell Calcium. 2008;44:1–5. doi: 10.1016/j.ceca.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-pentazocine, a sigma receptor-1 specific ligand. Invest Ophthalmol Vis Sci. 2007;48:4785–4794. doi: 10.1167/iovs.07-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D, Hajipour AR, Pal A, Chu UB, Arbabian M, Ruoho AE. Probing the steroid binding domain-like I (SBDLI) of the sigma-1 receptor binding site using N-substituted photoaffinity labels. Biochemistry. 2008;47:7205–7217. doi: 10.1021/bi800564j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J Pharmacol Exp Ther. 1999;289:251–260. [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Goyagi T, Goto S, Bhardwaj A, Dawson VL, Hurn PD, Kirsch JR. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Hoek JB. Cell signalling. Mitochondrial longevity pathways. Science. 2007;315:607–609. doi: 10.1126/science.1138825. [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003a;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003b;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsy-chiatric disorders. CNS Drugs. 2004a;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2004b;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. The potential role of sigma-1 receptors in lipid transport and lipid raft reconstitution in the brain: implication for drug abuse. Life Sci. 2005;77:612–1624. doi: 10.1016/j.lfs.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca(2+) Signalling and Cell Survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. An update on the development of drugs for neuropsychiatric disorders: focusing on the sigma 1 receptor ligand. Expert Opin Ther Targets. 2008;12:5–58. doi: 10.1517/14728222.12.1.45. [DOI] [PubMed] [Google Scholar]
- Hoegg MB, Browman DT, Resek ME, Robbins SM. Distinct regions within the erlins are required for oligomerization and association with high molecular weight complexes. J Biol Chem. 2009;284:7766–7776. doi: 10.1074/jbc.M809127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikonen E, Vainio S. Lipid microdomains and insulin resistance: is there a connection? Sci STKE. 2005;2005:pe3. doi: 10.1126/stke.2682005pe3. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest Ophthalmol Vis Sci. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen MA, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. Voltage-Gated Sodium Channel Modulation by Sigma Receptors in Cardiac Myocytes and Heterologous Systems. Am J Physiol Cell Physiol. 2009;296:C1049–C1057. doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 2009 doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:87–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- Malorni W, Giammarioli AM, Garofalo T, Sorice M. Dynamics of lipid raft components during lymphocyte apoptosis: the paradigmatic role of GD3. Apoptosis. 2007;12:941–949. doi: 10.1007/s10495-007-0757-1. [DOI] [PubMed] [Google Scholar]
- Marrazzo A, Caraci F, Salinaro ET, Su TP, Copani A, Ronsisvalle G. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16:1223–1226. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Maurice T. Neurosteroids and sigma1 receptors, biochemical and behavioral relevance. Pharmacopsychiatry. 2004;37(Suppl 3):S171–S182. doi: 10.1055/s-2004-832675. [DOI] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak GW. Modulation of brainstem opiate analgesia in the rat by sigma 1 receptors: a microinjection study. J Pharmacol Exp Ther. 2007;322:1278–1285. doi: 10.1124/jpet.107.121137. [DOI] [PubMed] [Google Scholar]
- Miljan EA, Meuillet EJ, Mania-Farnell B, George D, Yamamoto H, Simon HG, Bremer EG. Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J Biol Chem. 2002;277:10108–10113. doi: 10.1074/jbc.M111669200. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Reiter RJ, Hanner M, Glossmann H. High affinity of sigma 1-binding sites for sterol isomerization inhibitors: evidence for a pharmacological relationship with the yeast sterol C8-C7 isomerase. Br J Pharmacol. 1997;121:1–6. doi: 10.1038/sj.bjp.0701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa M, Matsuno K, Mita S. Activation of sigma1 receptor subtype leads to neuroprotection in the rat primary neuronal cultures. Neurochem Int. 1998;32:337–343. doi: 10.1016/s0197-0186(97)00105-8. [DOI] [PubMed] [Google Scholar]
- Pal A, Chu UB, Ramachandran S, Grawoig D, Guo LW, Hajipour AR, Ruoho AE. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the sigma-1 receptor. J Biol Chem. 2008;283:19646–19656. doi: 10.1074/jbc.M802192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Hajipour AR, Fontanilla D, Ramachandran S, Chu UB, Mavlyutov T, Ruoho AE. Identification of regions of the sigma-1 receptor ligand binding site using a novel photoprobe. Mol Pharmacol. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Pregelj P. Involvement of cholesterol in the pathogenesis of Alzheimer’s disease: role of statins. Psychiatr Danub. 2008;20:162–167. [PubMed] [Google Scholar]
- Renaudo A, L’Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl− channels. J Biol Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- Rusinol AE, Chan EY, Vance JE. Movement of apolipoprotein B into the lumen of microsomes from hepatocytes is disrupted in membranes enriched in phosphatidyl-monomethylethanolamine. J Biol Chem. 1993;268:25168–25175. [PubMed] [Google Scholar]
- Rusinol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269:27494–27502. [PubMed] [Google Scholar]
- Sabeti J, Gruol DL. Emergence of NMDAR-independent long-term potentiation at hip-pocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus. 2008;18:148–168. doi: 10.1002/hipo.20379. [DOI] [PubMed] [Google Scholar]
- Sarnataro D, Campana V, Paladino S, Stornaiuolo M, Nitsch L, Zurzolo C. PrP(C) association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Mol Biol Cell. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Ganapathy ME, Conway SJ, Bridges CD, Smith SB, Casellas P, Ganapathy V. Expression pattern of the type 1 sigma receptor in the brain and identity of critical anionic amino acid residues in the ligand-binding domain of the receptor. Biochim Biophys Acta. 2001;1540:59–67. doi: 10.1016/s0167-4889(01)00117-3. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:1–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smith SB, Duplantier J, Dun Y, Mysona B, Roon P, Martin PM, Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest Ophthalmol Vis Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, O’Neill M, Howie J, Samson J, Watt S, Murray K, McLean D, Leslie NR, Safrany ST, Ferguson MJ, Peters JA, Prescott AR, Box G, Hayes A, Nutley B, Raynaud F, Downes CP, Lambert JJ, Thompson AM, Eccles S. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:4875–4886. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology (Berl) 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci. 2007;27:6417–6427. doi: 10.1523/JNEUROSCI.0690-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Nerve growth factor-induced neurite sprouting in PC12 cells involves sigma-1 receptors: implications for antidepressants. J Pharmacol Exp Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry. 2004a;37(Suppl 3):S208–213. doi: 10.1055/s-2004-832679. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. Sigma-1 receptors potentiate epidermal growth factor signalling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse. 2004b;53:90–103. doi: 10.1002/syn.20041. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Invest Ophthalmol Vis Sci. 2008;49:2577–2588. doi: 10.1167/iovs.07-1101. [DOI] [PubMed] [Google Scholar]
- van Meer G. Cellular organelles: how lipids get there, and back. Trends Cell Biol. 2000;10:550–552. doi: 10.1016/s0962-8924(00)01851-1. [DOI] [PubMed] [Google Scholar]
- van Meer G, van Genderen IL. Intracellular lipid distribution, transport, and sorting. A cell biologist’s need for physicochemical information. Subcell Biochem. 1994;23:1–24. [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:408–413. [PubMed] [Google Scholar]
- Voelker DR. Interorganelle transport of aminoglycerophospholipids. Biochim Biophys Acta. 2000;1486:97–107. doi: 10.1016/s1388-1981(00)00051-2. [DOI] [PubMed] [Google Scholar]
- Volz HP, Stoll KD. Clinical trials with sigma ligands. Pharmacopsychiatry. 2004;37(Suppl 3):S214–220. doi: 10.1055/s-2004-832680. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signalling for glutamate release. J Biol Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Miura R, Yamamoto T, Shinohara K, Watanabe M, Okuyama S, Nakazato A, Nukada T. Amino acid residues in the transmembrane domain of the type 1 sigma receptor critical for ligand binding. FEBS Lett. 1999;445:19–22. doi: 10.1016/s0014-5793(99)00084-8. [DOI] [PubMed] [Google Scholar]


