Abstract
N,N-dimethyltryptamine (DMT) is a hallucinogen found endogenously in human brain that is commonly recognized to target the 5-hydroxytryptamine 2A receptor or the trace amine–associated receptor to exert its psychedelic effect. DMT has been recently shown to bind sigma-1 receptors, which are ligand-regulated molecular chaperones whose function includes inhibiting various voltage-sensitive ion channels. Thus, it is possible that the psychedelic action of DMT might be mediated in part through sigma-1 receptors. Here, we present a hypothetical signaling scheme that might be triggered by the binding of DMT to sigma-1 receptors.
Some amino acid metabolites are biogenic amines that, unlike the major neurotransmitter amines, such as dopamine, nore-pinephrine, and 5-hydroxytryptamine (5-HT), are typically present at low concentrations and accumulate in high amounts only if the amine-digestive enzyme monoamine oxidase is inhibited. These trace amines (TAs) include β-phenylethylamine, tyramine, octopamine, synephrine, and tryptamine, as well as some of their metabolites or derivatives. TAs are purported to be involved in several human diseases (1). Here, we focus on findings related to N,N-dimethyltryptamine (DMT), a tryptamine metabolite with psychedelic effects. DMT is the main ingredient in the hallucinogenic beverage called “ayahuasca,” which has been brewed (by boiling the bark of Banisteriopsis caapi together with the leaves of Psychotria viridis) and used by indigenous people around the South American Amazon basin (2, 3). Using purified DMT, Szara and colleagues first reported the psychoactive effect of the compound in humans (4, 5). Saavedra and Axelrod then demonstrated the formation of DMT in rat and human brain (6), leading both groups to propose that DMT was an endogenous hallucinogen (4–6). Several studies have since confirmed the psychedelic properties of DMT in humans (7–14).
DMT is generally believed to exert its psychedelic effects through the 5-HT receptor, specifically the 5-HT2A subtype, which was identified by using the semisynthetic hallucinogen lysergic acid diethylamide (LSD) (15). However, certain behaviors seen in rats treated with DMT (0.5 to 35 mg/kg administered intraperitoneally), such as jerking, retropulsion, and tremor, do not involve the 5-HT system or other monoaminergic systems (16). Micro-molar concentrations of DMT enhances phosphatidylinositol production in a manner that is not blocked by the 5-HT2A receptor antagonist ketanserin (17), which suggests that part of the action of DMT is not mediated through 5-HT receptors. With the discovery of the G protein–coupled TA-associated receptors (TAARs), which activate adenylyl cyclase and cause cyclic adenosine monophosphate (cAMP) accumulation (18, 19), it was speculated that TAARs mediated part of the pharmacological or psychedelic effect of trace amines, including DMT, as well as LSD. (19). Although DMT at 1 μM is as potent in eliciting cAMP accumulation as the prototypic trace amine tryptamine or LSD (19), it is unclear whether TAARs mediate the psychedelic effect of trace amines, including DMT, because TAAR antagonists have not been tested in humans in this regard. Furthermore, gene association studies attempting to link TAARs and psychiatric disturbances have generated conflicting results as to whether TAARs are involved in schizophrenic symptomatologies, including hallucination. TAAR1 knockout mice display a deficit in “prepulse inhibition” (PPI), or the ability to suppress the magnitude of startle induced by an incoming acoustic signal that had been previously experienced (20). They are therefore a relevant animal model for schizophrenia because the PPI is typically impaired in schizophrenic patients (20). In addition, a genetic study has demonstrated associations between polymorphisms in the TAAR4 subtype with susceptibility to schizophrenia (21); however, conflicting reports later emerged that demonstrated a lack of association between the TAAR4 or TAAR6 gene and schizophrenia (22, 23). Thus, it remains to be fully established whether TAARs mediate the psychotomimetic action of DMT.
A report now demonstrates that DMT targets a receptor called the sigma-1 receptor (Sig-1R) (24). DMT binds to the Sig-1R with a moderate affinity at about 14 μM (24). Although this affinity is not impressive when compared to other Sig-1R ligands, such as (+)pentazocine (which has an affinity in nanomolar range), high concentrations of DMT (100 μM, about 7 times as high as its affinity for Sig-1R) could nonetheless inhibit voltage-gated sodium channels (24), a hallmark action of Sig-1R ligands and Sig-1Rs (25). Sig-1R knockout mice, which reacted normally to the locomotor stimulating effect of methamphetamine, did not become hyper-active in response to DMT (24), a phenomenon also observed with the prototypic Sig-1R agonist N-allylnormetazocine, an opiate analog better known as SKF-10047 (26). Furthermore, the locomotor-stimulating action of DMT resembles that of SKF-10047 (24, 26). These results definitively link the action of DMT to the Sig-1R.
The Sig-1R was originally thought to be the opiate receptor subtype that mediated the psychotomimetic or drug-induced psychotic-like effect of SKF-10047 in animals (27). However, the same laboratory later found that the psychotomimetic effect of SKF-10047 was not reversed by naloxone, a universal antagonist for all opiate receptor subtypes (28). Thus, the Sig-1R was recognized to be a nonopiate receptor (29–31) that might mediate the psychotomimetic effect not only of SKF-10047 but also of the dissociative anesthetic phencyclidine (PCP) (28, 32). However, PCP is thought to induce its mind-altering effect through the N-methyl-D-aspartate (NMDA) receptor, and systematic behavioral studies are needed to differentiate between the SKF-10047– and PCP-induced effects mediated by the Sig-1R versus the NMDA receptor. In addition to their postulated psychotomimetic action, Sig-1Rs have been implicated in diseases such as addiction, depression, amnesia, pain, stroke, and cancer (33).
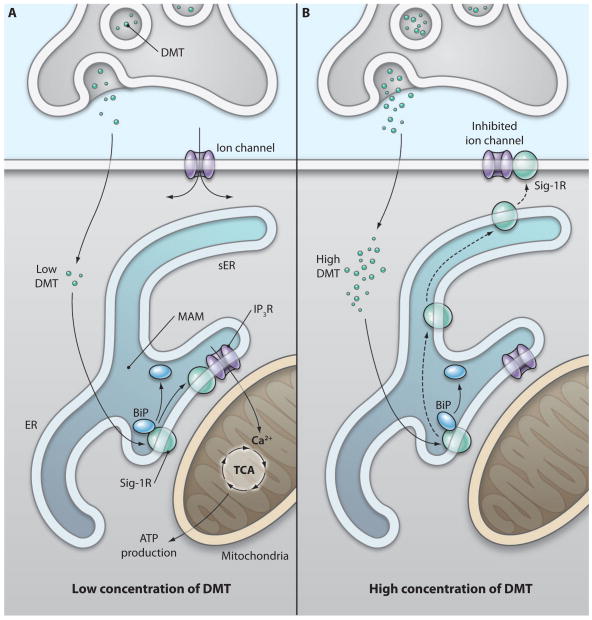
Sig-1Rs localize at the interface between the endoplasmic reticulum (ER) and mitochondrion, which is known as the mitochondria-associated ER membrane (MAM). Sig-1R agonists at affinity concentrations (i.e., close to their Ki values) cause Sig-1Rs to disassociate from another ER chaperone, binding immunoglobulin protein (BiP), allowing them to act as molecular chaperones to inositol 1,4,5-trisphosphate (IP3) receptors. By stabilizing IP3 receptors, Sig-1Rs at the MAM enhance Ca2+ signaling from the ER into mitochondria (34, 35), thereby activating the tricarboxylic acid (TCA) cycle and increasing the production of adenosine triphosphate (ATP) (35) (Fig. 1). Although Sig-1Rs reside primarily at the ER, they can translocate from the MAM to the plasma membrane (also termed the plasmalemma) or the subplasma membrane area when stimulated by higher concentrations (e.g., at approximately 10-fold Ki) of Sig-1R ligands or when Sig-1Rs are overexpressed in cells (36–38) (Fig. 1). This may explain why higher concentrations of Sig-1R ligands result in the inhibition of various ion channels at the plasma membrane and, in particular, why the channel-inhibiting concentration of DMT is almost 10 times as high as its affinity concentration (24). By triggering the translocation of Sig-1Rs from the MAM to the plasma membrane or subplasma membrane, high concentrations of Sig-1R ligands may allow Sig-1Rs to directly interact with and inhibit channel proteins (24, 38). High concentrations of Sig-1R ligands tonically inhibit the small conductance K+ (SK) channel, which in turn leads to the potentiation of NMDA receptors (39). The NaV1.5 channel (24, 25), the KV1.4 channel (38), the voltage-gated N-, L-, and P/Q-type Ca2+ channels (40), the acid-sensing ion channel (41), and the volume-regulated Cl− channel (42) are also inhibited by high concentrations of Sig-1R ligands.
Fig. 1.
Hypothetical scheme illustrating the signaling of N,N-dimethyltryptamine through sigma-1 receptors. (A) Sigma-1 receptors (Sig-1Rs) at the mitochondrion-associated endoplasmic reticulum (ER) membrane (MAM) function as ligand-activated molecular chaperones, particularly when ligands are present at concentrations close to their affinities (34). Sig-1R ligands, including DMT, at concentrations close to their Ki values, cause the dissociation of Sig-1Rs from another ER chaperone, binding immunoglobulin protein (BiP) (34), allowing Sig-1Rs to chaperone inositol 1,4,5-trisphosphate receptors (IP3Rs) at the MAM (34). This enhances Ca2+ signaling from the ER into mitochondria (34, 35), activates the tricarboxylic acid (TCA) cycle, and increases adenosine triphosphate (ATP) production (35). (B) Higher concentrations of DMT cause the translocation of Sig-1Rs from the MAM to the plasma membrane, leading to the inhibition of ion channels. Thus, Sig-1R ligands might shift the site of action of Sig-1R chaperones from the center of the cell to its periphery. In the present scheme, Sig-1Rs and related molecules or organelles are illustrated in the postsynaptic region for the sake of simplicity, although they may also be present presynaptically or in glia.
So, do sigma-1 receptors mediate the psychedelic effect of DMT? First, we need to specify that Sig-1Rs have not been firmly established as being involved in causing psychotomimesis. Secondly, moderate concentrations of selective Sig-1R ligands, including (+)pentazocine and PRE-084, are not reported to cause psychotomimetic-like effects in animals (43). However, the possibility that Sig-1Rs are involved in psychotomimesis cannot be totally excluded at present. We therefore speculate that Sig-1Rs may partially mediate the psychotomimetic effects of DMT, such as visual hallucinations in humans (7–14). PCP and SKF-10047 cause animals to behave as if they are hallucinating (they move their heads and eyes as if they are tracking objects in the air) (28). Could the psychotomimetic effect caused by PCP and SKF-10047 in animals (28) be explained by PCP or SKF-10047 blocking NMDA receptors and not by their binding to Sig-1Rs? It might not be, because it might be difficult to distinguish the psychedelic effect mediated by the NMDA receptor blockade from that mediated by Sig-1Rs in animal studies. A clearer differentiation of the effects mediated by the two different receptors might come only from human studies. Results from previously mentioned clinical studies, although not designed to answer this question, might provide some interesting clues.
DMT, which we know now is also a Sig-1R ligand, has been used as a 5-HT2A agonist by Gouzoulis-Mayfrank et al. to compare the psychedelic effect of DMT with that of ketamine, which is also an NMDA receptor blocker like PCP (11–14). DMT effects relate more to the paranoid-type psychoses with particular positive formal thought disorders—including loosening of associations, derailment, and distractibility—than to the neurocognitive impairment seen with ketamine (11, 14). Thus, DMT effects in humans might be mediated through Sig-1Rs or 5-HT2A receptors and not through blockade of NMDA receptors. In this regard, it would be interesting to examine whether Sig-1R antagonists block the psychedelic effect of DMT in humans.
Based on the current understanding of the cell biological actions of Sig-1Rs (34, 35, 38), we propose a hypothetical scheme for the molecular mechanism by which DMT signals through sigma-1 receptors (Fig. 1). Like other Sig-1R agonists (34), DMT at affinity concentrations (14 μM) (24) might cause the dissociation of Sig-1Rs from the Sig-1R-BiP complex (34, 35) (Fig. 1A), and at higher concentrations (100 μM) (24) might cause Sig-1Rs to translocate from the MAM to the plasma membrane (36, 37) (Fig. 1B). By doing so, DMT might first unleash the chaperone activity of the free form of Sig-1Rs at the MAM (34) and then cause the receptors to translocate (36, 37) to the plasma membrane to inhibit voltage-gated ion channels (24, 38–42). We do not know at present whether the chaperone activity of Sig-1Rs contributes to ion channel inhibition or whether Sig-1Rs associate with ion channels at the subplasma ER membrane or at the plasma membrane. Nor do we know whether the chaperone-unleashing action seen at affinity concentrations of DMT or the ion channel–inhibiting action caused by high concentration of DMT relate to the psychedelic effect induced by DMT. More studies are needed to provide answers to these questions.
Almost 30 years after the initial description of a psychotomimesis-related sigma receptor (27–32), investigators have identified DMT as an endogenous hallucinogen that targets a new site of action (24). The characterization of Sig-1Rs as ligand-regulated chaperone receptors (34) and the discovery of the endogenous hallucinogen DMT as a Sig-1R ligand (24) represent potential breakthroughs in drug abuse research. Yet many questions remain, the most important being: What is the physiological importance or relevance of the DMT signaling through Sig-1Rs? Furthermore, Sig-1Rs are present not only in the central nervous system, but also in peripheral organs such as the liver, heart, lung, adrenal gland, spleen, and pancreas (34). For example, the enzyme that synthesizes DMT from its precursor tryptamine (44) and Sig-1Rs (34) are particularly abundant in lung tissue. Thus, it will be important to delineate the roles of Sig-1Rs and their associated ligands, including DMT, within the context of the physiology or pathophysiology of human diseases related to those organs. It is hoped that future research will increase our understanding of these roles.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, Department of Health and Human Services of the United States.
References and Notes
- 1.Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 3.Gambelunghe C, Aroni K, Rossi R, Moretti L, Bacci M. Identification of N,N-dimethyltryptaime and beta-carbolines in psychotropic ayahuasca beverage. Biomed Chromatogr. 2008;22:1056–1059. doi: 10.1002/bmc.1023. [DOI] [PubMed] [Google Scholar]
- 4.Szara I, Sai-Halasz A, Boszormenyi Z. Dimethyltryptamine as a new psychotic agent. Acta Physiol Hung. 1957;11:78–79. [PubMed] [Google Scholar]
- 5.Sai-Halasz A, Brunecker G, Szara S. Dimethyl-tryptamine: A new psychoactive drug. Psychiatr Neurol (Basel) 1958;135:285–301. [PubMed] [Google Scholar]
- 6.Saavedra JM, Axelrod J. Psychotomimetic N-methylated tryptamines: formation in brain in vivo and in vitro. Science. 1972;175:1365–1366. doi: 10.1126/science.175.4028.1365. [DOI] [PubMed] [Google Scholar]
- 7.Strassman RJ, Qualls CR. Dose-dependent study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch Gen Psychiatry. 1994;51:85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- 8.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 9.Riba J, Rodrihuez-Fornells A, Urbano G, Morte A, Antonijoan R, Montero M, Callaway JC, Barbanoj MJ. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology (Berlin) 2001;154:85–95. doi: 10.1007/s002130000606. [DOI] [PubMed] [Google Scholar]
- 10.Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite secretion, and pharmacokinetics. J Pharmacol Exp Ther. 2003;306:73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- 11.Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar KA. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): A double blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 12.Heekeren K, Neukirch A, Daumann J, Stoll M, Obradovic M, Kovar KA, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N-dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol. 2007;21:312–320. doi: 10.1177/0269881107077734. [DOI] [PubMed] [Google Scholar]
- 13.Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, Waberski TD, Gouzoulis-Mayfrank E. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berlin) 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daumann J, Heekeren K, Neukirch A, Thiel CM, Moller-Hartmann W, Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berlin) 2008;200:573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- 15.Bennett JP, Jr, Snyder SH. Serotonin and lysergic acid diethylamide binding in rat brain membranes: Relationship to post-synaptic serotonin receptors. Mol Pharmacol. 1976;12:373–389. [PubMed] [Google Scholar]
- 16.Jenner P, Marsden CD, Thanki CM. Behavioural changes induced by N,N-dimethyltryptamine in rodents. Br J Pharmacol. 1978;63:380P. [PMC free article] [PubMed] [Google Scholar]
- 17.Deliganis AV, Pierce PA, Peroutka SJ. Differential interactions of dimethyltryptamine (DMT) with 5-HT1A and 5-HT2 receptors. Biochem Pharmacol. 1991;41:1739–1744. doi: 10.1016/0006-2952(91)90178-8. [DOI] [PubMed] [Google Scholar]
- 18.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle TA, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the cate-cholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 20.Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P, Branchek T, Gerald CP. The trace amine 1 receptor knockout mice: An animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 21.Duan J, Martinez M, Sanders AR, Hou C, Saitou N, Kitano T, Mowry BJ, Crowe RR, Silverman JM, Levinson DF, Gejman PV. Polymorphisms in the trace amine receptor 4 (TAAR4) gene on chromosome 6q23.2 are associated with susceptibility to schizophrenia. Am J Hum Genet. 2004;75:624–638. doi: 10.1086/424887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amann D, Avidan N, Kanyas K, Kohn Y, Hamdan A, Ben-Asher E, Macciardi F, Beckmann JS, Lancet D, Lerer B. The trace amine receptor 4 gene is not associated with schizophrenia in a sample linked to chromosome 6q23. Mol Psychiatry. 2006;11:119–121. doi: 10.1038/sj.mp.4001752. [DOI] [PubMed] [Google Scholar]
- 23.Vladimirov VI, Maher BS, Wormley B, O’Neill FA, Walsh D, Kendler KS, Riley BP. The trace amine associated receptor (TAAR6) gene is not associated with schizophrenia in the Irish case-control study of schizophrenia (ICCSS) sample. Schizophr Res. 2009;107:249–254. doi: 10.1016/j.schres.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng ZX, Lan DM, Wu PY, Zhu YH, Dong Y, Ma L, Zheng P. Neurosteroid dehydroepiandrosterone sulfate inhibits persistent sodium current in rat medial prefrontal cortex via activation of sigma-1 receptors. Exp Neurol. 2008;210:128–136. doi: 10.1016/j.expneurol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Langa F, Condony X, Tovar V, Lavado A, Gimenez E, Cozar P, Cantero M, Dordal A, Hernandez E, Perez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type 1 (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine-and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 28.Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF-10047 in the dog. Eur J Pharmacol. 1983;92:269–274. doi: 10.1016/0014-2999(83)90297-2. [DOI] [PubMed] [Google Scholar]
- 29.Su TP. Evidence for sigma opioid receptor: Binding of [3H]SKF-10047 to etorphine-inaccessible site in guinea pig brain. J Pharmacol Exp Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- 30.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 31.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma-1 and sigma-2 receptors: Characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 32.Snyder SH, Largent BL. Receptor mechanisms in antipsychotic drug action: Focus on sigma receptors. J Neuropsychiatry Clin Neurosci. 1989;1:7–15. doi: 10.1176/jnp.1.1.7. [DOI] [PubMed] [Google Scholar]
- 33.Collier TL, Waterhouse RN, Kassiou M. Imaging sigma receptors: Applications in drug development. Curr Pharm Des. 2007;13:51–72. doi: 10.2174/138161207779313740. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. Trends Cell Biol. 2009;19:81. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors in NG-108-15 cells. J Pharmacol Exp Ther. 2003;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 37.Mavlyutov TA, Ruoho A. Ligand-dependent localization and intracellular stability of sigma-1 receptors in CHO-K1 cells. J Mol Signal. 2007;2:8. doi: 10.1186/1750-2187-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 39.Martina M, Turcotte ME, Halman S, Begeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- 41.Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. Sigma-1 receptor modulation of acid-sensing ion channel a (ASICa) and ASICa-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- 42.Renaudo A, L’Hoste S, Guizouarn H, Borgese F, Soriani O. Cancer cell cycle modulated by a functional coupling between sigma-1 receptors and Cl− channels. J Biol Chem. 2007;282:2259–2267. doi: 10.1074/jbc.M607915200. [DOI] [PubMed] [Google Scholar]
- 43.Urani A, Romieu P, Roman FJ, Maurice T. Enhanced antidepressant effect of sigma-1 receptor agonists in β25-35-amyloid peptide-treated mice. Behav Brain Res. 2002;134:239–247. doi: 10.1016/s0166-4328(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 44.Axelrod J. Enzymatic formation of psychotomimetic metabolites from normally occurring compounds. Science. 1961;134:343. doi: 10.1126/science.134.3475.343. [DOI] [PubMed] [Google Scholar]