Abstract
Opioids have been demonstrated to play an important role in CNS development by affecting proliferation and differentiation in various types of neural cells. This study examined the effect of a stable delta opioid peptide [d-Ala(2), d-Leu(5)]-enkephalin (DADLE) on proliferation and differentiation in an AF5 CNS neural progenitor cell line derived from rat mesencephalic cells. DADLE (1 pM, 0.1 nM, or 10 nM) caused a significant growth inhibition on AF5 cells. The opioid antagonist naltrexone at 0.1 nM also caused growth inhibition in the same cells. When DADLE and naltrexone were both added to the AF5 cells, the resultant growth inhibition was apparently additive. DADLE alone or DADLE in combination with naltrexone did not cause apoptosis as evidenced by negative TUNEL staining. The cell-cycle progression analysis indicated that both DADLE (0.1 nM) and naltrexone (0.1 nM) caused an arrest of AF5 cell cycle progression at the G1 checkpoint. Neuronal marker indicated that DADLE- or naltrexone-treated AF5 cells tend to differentiate more when compared to controls. Results demonstrate the nonopioid action of both DADLE and naltrexone on cell cycle arrest and differentiation in a CNS neural progenitor cell line. Results also suggest some potential utilization of DADLE and/or naltrexone in stem cell research.
Keywords: delta opioid, DADLE, naltrexone, dopamine (DA), AF5, differentiation
INTRODUCTION
It is well known that the in certain systems the opioid antagonist attenuates the agonist-induced effect whereas in others the agonist and the antagonist exhibit the same effect. Whether the unilateral effect caused by opioid agonists and antagonists involve cloned mu, kappa, or delta opioid receptors is not totally clear. An earlier report indicated that both opioid peptides and the opioid antagonist naloxone stimulated cellular growth (Ilyinsky et al., 1987). On the contrary, Persson et al. (2003a,b) reported that opioid agonists induced proliferation whereas opioid antagonists decreased proliferation in rat adult hippocampal progenitors. Kozlova and Kalentchuk (1994) reported that both opioid peptides and naloxone were stimulatory on the survival and cellular adhesion in cultured rat spinal cord cells. The inability of naltrexone to block opioid agonists in certain biological systems implicated nonopioid actions of opioid agonists/antagonists.
We have been investigating physiological functions of a stable delta opioid peptide DADLE in several biological systems. We found that DADLE enhanced the survival of cultured rat fetal ventral mesencephalic dopaminergic neurons (Borlongan et al., 2000), protected against and reversed the methamphetamine-induced loss of dopaminergic terminals in mice (Tsao et al., 2000), and promoted the survival of PC12 cells via the MEK-ERK pathway (Hayashi et al., 2002). Some of these effects of DADLE involve delta opioid receptors (Hayashi et al., 2002) whereas some are less clear in this regard (Borlongan et al., 2000; Tsao et al., 2000).
The AF5 cell line, a rat progenitor neural cell line, was established by immortalization with an N-terminal fragment of SV40 large T (Truckenmiller et al., 2002). The AF5 cell line is considered to be a useful model for cellular proliferation and differentiation of the central nervous system (CNS) since this cell line exerts CNS progenitor characters in phenotypic plasticity and developmental properties and yet still retains the ability to differentiate. Our previous study showed that DADLE promoted the survival of cultured rat fetal ventral mesencephalic dopaminergic neurons when those neurons were grafted into 6-OHDA-treated host animals (Borlongan et al., 2000). Because cellular proliferation/differentiation is important for cellular survival after grafting, we thus were interested in examining the effect of DADLE in a neural progenitor cell line. In the present study, we investigated the cellular proliferation and the cell cycle progression effects of DADLE and naltrexone (NTX) in the neural progenitor AF5 cells.
MATERIALS AND METHODS
Cell culture
CNS progenitor cell line AF5 was cultured in medium containing Dulbecco’s Modified Eagle’s Medium/Ham’s F12 (DMEN/F12, 1:1, Gibco Life Technologies, Gaithesrburg, MD), 10% fetal calf serum (FSC), 2 mM l-glutamine, 100 U/ml penicillin G, and 100 µg/ml streptomycin at 37°C in a 5% CO2 humidified atmosphere.
Cell proliferation assay
AF5 cells were plated on the black-bottomed 96-well plate (Greiner bio-one) at a density of 5 × 103 cells/well. Cells were maintained in 0.5% FBS medium and allowed to grow for 24 h before treatments. On the treatment day, DADLE (final concentrations at 1 pM, 0.1 nM, and 10 nM), naltrexone (0.1 nM), and DADLE/naltrexone, respectively were added to the cells. Cells were then allowed to grow for another 24, 72, or 120 h before the cellular proliferation assay was performed with the CyQUANT® Cell Proliferation Assay Kit (Molecular Probes. Eugene, OR). DADLE or naltrexone was added to the culture whenever the culture medium was replenished.
Detection of apoptosis
AF5 cells were plated on Lab-Tek II Chamber Slides (Nalge Nunc International) at a density of 5 × 104 cells/well. Cells were maintained and treated as described above. Twenty-four hours after treatments with DADLE or naltrexone, AF5 cells were fixed and stained by using the ApoAlert™ DNA Fragmentation Assay Kit (BD Biosciences. Palo Alto, CA). AF5 cells treated with 1 µg/ml of DNase I (Roche) served as positive controls for the detection of apoptosis. In the final washing step, cells were incubated with the nuclear dye Hoechst 33342 (1:1000 from stock at 10 mg/ml; Molecular Probe) for 10 min. Immunofluorescence was detected and processed using a Zeiss Axiovert 100 fluorescence microscope.
FACS analysis
The effects of DADLE and naltrexone on cell cycle progression were determined by using the FACS analysis of propidium iodide (PI)-stained AF5 cells. AF5 cells were plated in T75 tissue culture flasks at a density of 106 per flask. Cells in cultures were then synchronized regarding their growth phase by starving them in a serum-free medium for 24 h. At the end of the 24 h, DADLE, naltrexone, or DADLE plus naltrexone was added to the cells. Cells were then maintained in the 0.5% FBS medium for an additional 24 h. Cells were harvested by trypsinization, washed twice with ice-cold PBS, and spun down for 5 min at 300g. The pellets were resuspended in Vindeloves PI staining solution (100 ml Vindeloves PI containing 121 mg Trizma Base, 58 mg NaCl, 5 mg PI, 100 µl NP-40, and 70 Kunitz U RNAse) and stored at 4°C for at least 2 h. AF5 cells were then analyzed by flow cytometry by using a FACSCaibur flow cytometer (Becton Dickinson, San Jose, CA). The proportion of cells in the G0/G1, S, and G2/M fractions of the cell cycle was determined by using the ModFit LT software (Verity Software, Topsham, ME).
Immunoblotting
Forty microgram of total protein lysates were subjected to SDS PAGE for analysis. Blots were labeled with primary antibodies rabbit β-III-tubulin (Promega) and rabbit GAPDH (Sigma), respectively. Fluorescent western blots were labeled with the secondary antibodies goat anti mouse IRDye 700 (red) and goat antirabbit IRDye 800 (green) and analyzed using the LI-COR Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Statistical analysis
All values were expressed as group means ± the standard error of the means (SEM). Group means were compared by using Student’s t-test or by analysis of variance (ANOVA). For all tests, the significance level was set at P < 0.05.
RESULTS
Effects of DADLE on AF5 cellular proliferation
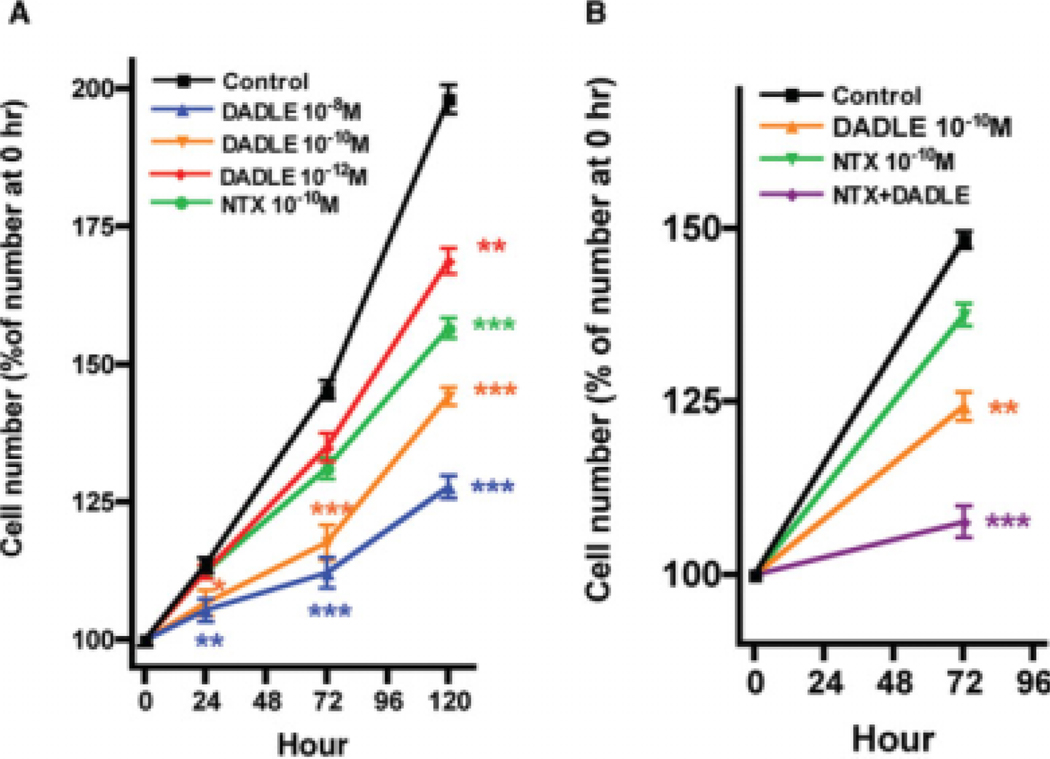
To investigate the effect of DADLE on cellular proliferation in AF5 cells, cells were grown in 0.5% FBS medium before the addition of various concentrations of DADLE (1 pM, 0.1 nM, or 10 nM). Cells were then allowed to grow for an additional 24, 72, or 120 h. DADLE at all concentrations examined significantly inhibited the cellular proliferation especially at the 120 h time point (Fig. 1A). The opioid antagonist naltrexone alone was tested. Naltrexone at 0.1 nM also induced a significant antiproliferative effect on AF5 cells (Fig. 1A).
Fig. 1.
Effects of DADLE and naltrexone on the proliferation of AF5 cells. (A) DADLE treatment. The AF5 cells were maintained in 0.5% FBS medium for 24 h before the addition of DADLE (10−12, 10−10, or 10−8M) or naltrexone (10−10M). Cells were then cultured for another 24, 72, and 120 h. At the 24, 72, and 120 h time points, cells were counted by using a CyQUANT® Cell Proliferation Assay Kit. (B) Effect of DADLE (10−10M) alone, naltrexone (10−10M) alone, or DADLE plus naltrexone on the AF5 proliferation. Data (means ± SEM) represent results from five independent experiments. **P < 0.01 and ***P < 0.001 compared with the control group. P = 0.0517 between DADLE and DADLE + naltrexone.
We next tested the effect of DADLE in combination with naltrexone on the proliferation of AF5 cells. Naltrexone (0.1 nM) was added to cultured cells 1 h before the addition of DADLE (0.1 nM). Results showed that the resultant antiproliferative effect of DADLE plus naltrexone is apparently additive when compared with that seen for each drug administered alone (Fig. 1B).
Growth inhibition not due to apoptosis
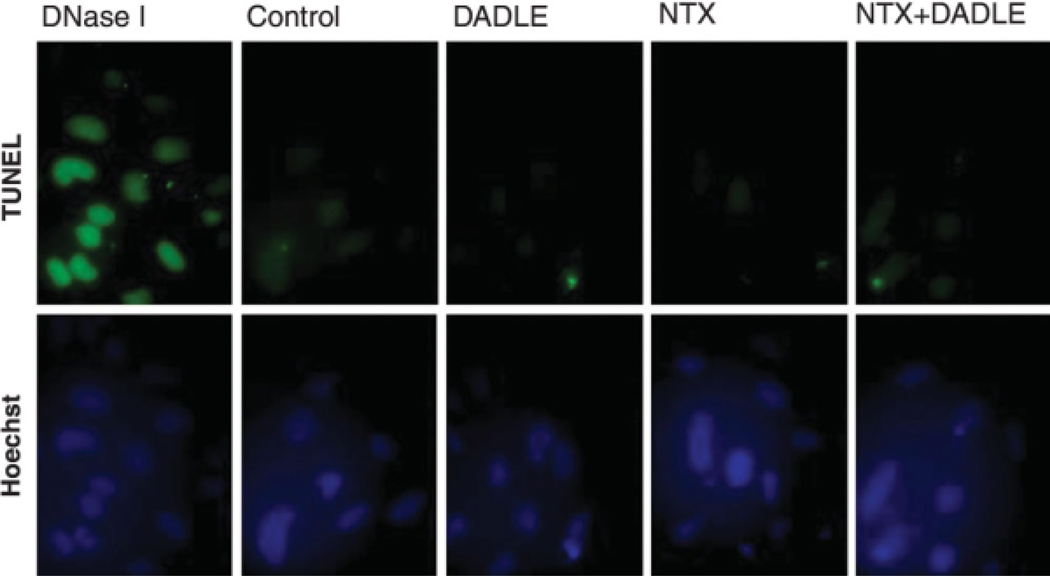
To test whether DADLE- or naltrexone-induced growth inhibition was due to apoptotic cell death, the DNA fragmentation assay was performed in DADLE/naltrexone-treated cells. AF5 cells were grown in the 0.5% FBS medium containing DADLE (0.1 nM), naltrexone (0.1 nM), or a combination of DADLE and naltrexone for 24 h. AF5 cells grown in the 0.5% FBS medium and in the 0.5% FBS medium containing DNaseI (1 µg/ml) respectively served as negative and positive controls for apoptosis. The AF5 cells were then subjected to DNA fragmentation assays using the ApoAlert™ kit. The TUNEL staining thus obtained showed that DADLE- or naltrexone treated-AF5 cells were negative in DNA fragmentation when compared with controls. These results indicate that the DADLE- or naltrexone-induced cell growth inhibition was unrelated to apoptosis (Fig. 2).
Fig. 2.
Detection of Apoptosis in DADLE- or naltrexone-treated cells. AF5 Cells were stained for fragmented DNAs (green; upper panels) by using the ApoAlert kit. Dnase I-treated AF5 cells served as TUNEL-positive controls. The first column panels show positive apoptotic controls in which cells were treated with Dnase I. Cells in the second column received only medium and thus served both as the negative controls for apoptosis and as the controls for DADLE- or naltrexone-treated cells. Note that no apoptosis was found in controls, DADLE (0.1 nM)-, naltrexone (0.1 nM)-, or DADLE (0.1 nM)/naltrexone (0.1 nM)-treated AF5 cells. To confirm cell numbers, cells were also stained with Hoechst 33,342 that labels nuclei (blue; lower panels).
Effects of DADLE and naltrexone on cell-cycle progression
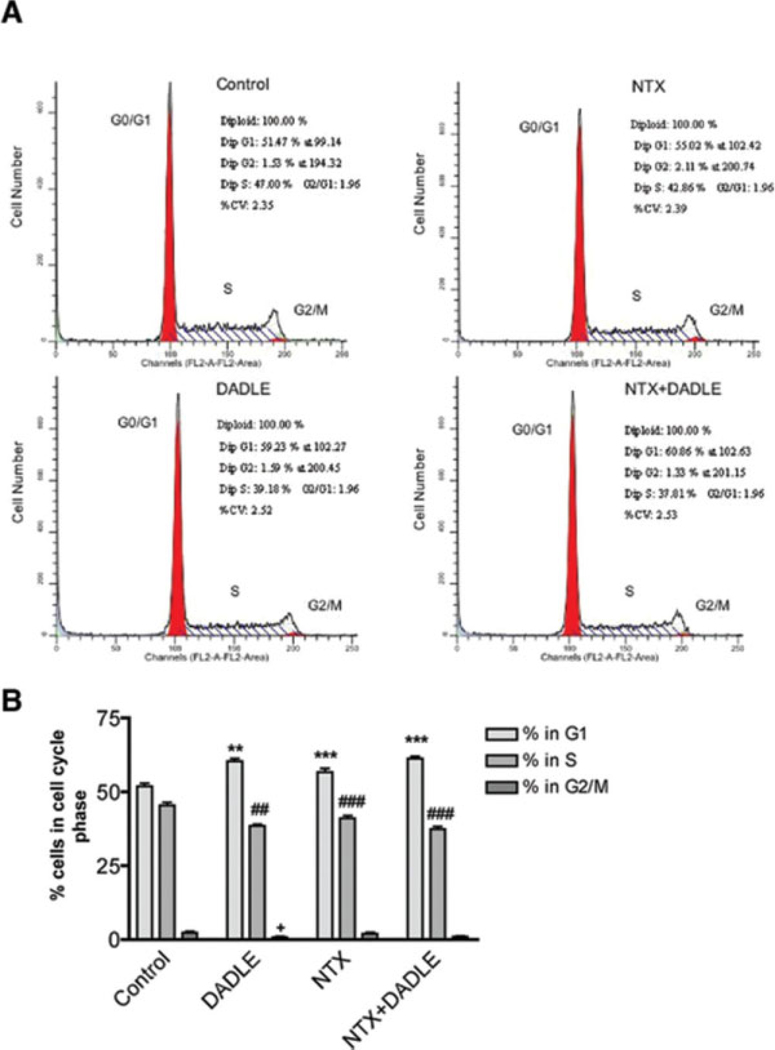
Apart from apoptosis, cellular growth inhibition might be related to the cell cycle arrest caused by test agents. We reasoned therefore that the growth inhibitory effect of DADLE or naltrexone on AF5 cells might be related, at least in part, to the cell cycle-arresting effect of the compound. To provide evidence for this hypothesis, we examined the effects of DADLE, naltrexone, or DADLE plus naltrexone on cell-cycle progression by using the fluorescence-activated cell-sorter (FACS). As shown in a representative FACS experiment (Fig. 3A), DADLE, naltrexone, or DADLE plus naltrexone treatment each induced a significantly stronger G1 cellular arrest and a compensatory reduction of cells in the S and G2/M phase when compared with controls. Again, no pharmacological blockade was observed between DADLE and naltrexone. Results from six independent experiments indicate that 52% of the control cells remained in the G1 phase, 46% in the S phase, and 2.48% in the G2/M phase (Fig. 3B). In contrast, DADLE-, naltrexone-, and DADLE plus naltrexone-treated cells were arrested significantly higher (a 16.43%, 9.23%, and 18.12% increment, respectively) at the G1 phase when compared with controls (Fig. 3B). The corresponding decreases of cells in the S phase were also apparent (Fig. 3B). These findings indicate that DADLE and naltrexone cause cell cycle arrest and suggest that growth inhibition of AF5 cells induced by DADLE or naltrexone is related in part to the cell cycle-arresting effect the drug.
Fig. 3.
Cell cycle progression regulated by DADLE and naltrexone. (A) Flow cytometry analysis of DNA contents in AF5 cells. AF5 cells were plated in T75 tissue culture flasks at a density of 106 cells per flask. Cells were then synchronized by replacing the culture medium with a serum-free medium for 24 h. At the end of the 24 h, DADLE, naltrexone, or DADLE plus naltrexone, respectively, were added to the cells and cells were maintained in 0.5% FBS medium for an additional 24 h before assayed for the cell cycle progression analysis using the fluorescence-activated cell-sorter (FACS). The control cells received only the 0.5% FBS medium. DADLE, cells received 10−10 M of DADLE; NTX, cells received 10−10 M of naltrexone; NTX + DADLE, cells received a combination of 10−10 M of DADLE and 10−10 M of nlatrexone. (B) Summary of the results of six independent FACS determinations. The percentage of cells in different phases of the cell cycle is included in each treatment condition. **P < 0.01 and ***P < 0.001 compared to the control group in G1 phase; ##P < 0.01 and ###P < 0.001 compared with the control group in S phase; and +P < 0.05 compared with the control group in G2/M phase.
DADLE or naltrexone enhances neuronal differentiation in AF5 cells
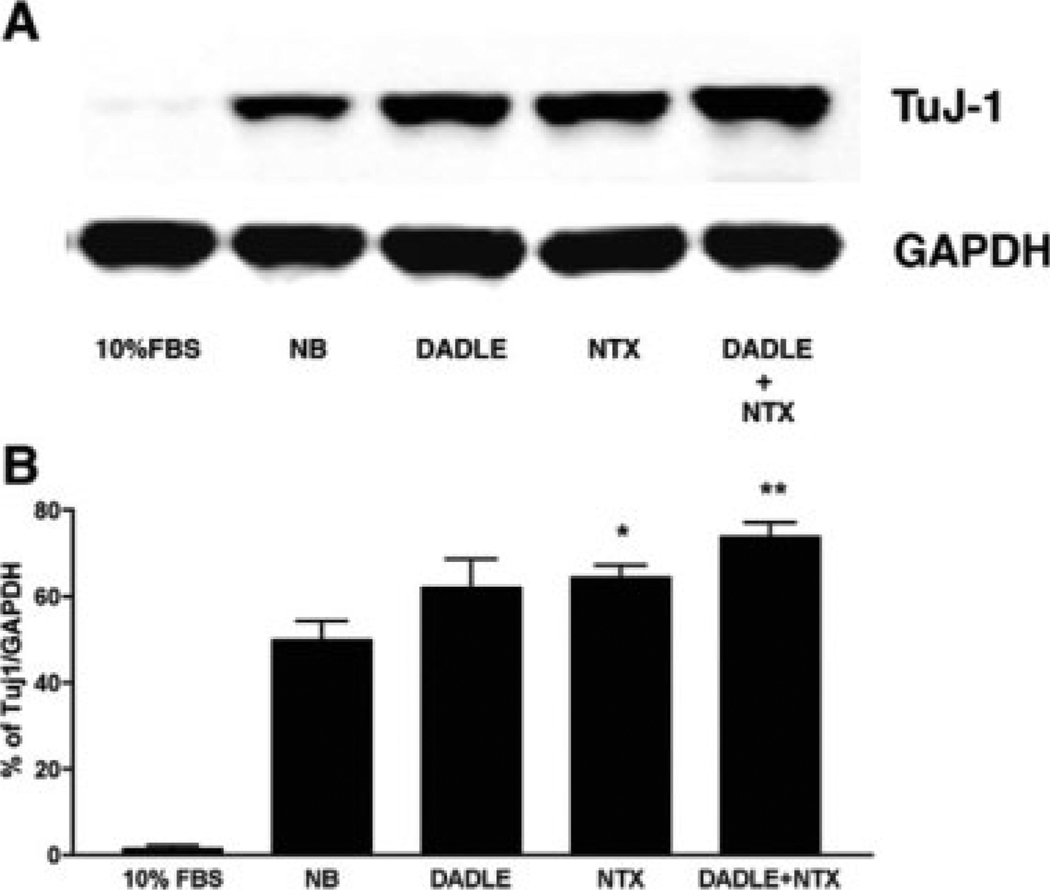
Neuronal differentiation is closed related to cell cycle arrest specifically when cells are arrested at the G1 phase. Differentiated AF5 cells were previously shown to increase in the expression of neuronal and GABAergic markers (Sanchez et al., 2006). We speculated that DADLE and naltrexone, by virtue of causing cell cycle arrest at the G1 phase in AF5 cells, might increase the differentiation of AF5 cells. Thus, we examined the expression of a neuronal marker β-III-tubulin in cells treated with DADLE, naltrexone, or DADLE plus naltrexone. AF5 cells were allowed to grow either under subconfluent condition in the DMEM/F-12 medium or under the differentiation condition in the Neurobasal/B-27 medium as previously reported (Sanchez et al., 2006). DADLE, naltrexone, or DADLE plus naltrexone were added to the differentiation medium during the observation period for 4 days. Western blottings revealed that the DADLE, naltrexone and DADLE plus naltrexone treatments tend to increase β-III-tubulins in AF5 cells that were cultured in the Neurobasal/B-27 differentiation medium (Fig. 4).
Fig. 4.
DADLE or naltrexone tends to enhance differentiation as indicated by the expression of neural marker β-III-tubulin. AF5 cells were cultured to subconfluency in the DMED/F12 medium (FBS) or in the Neurobasal/B27 differentiation medium (NB) post-confluency for 4 days. DADLE, naltrexone, or DADLE plus naltrexone were present in the differentiation medium during the observation period of 4 days. (A) Western blottings indicate the tendency of an increased expression of neuron specific β-III-tubulin (TuJ-1) in DADLE (0.1 nM)- or naltrexone (0.1 nM)-treated cells. (B) Statistic analysis of β-III-tubulin expression. β-III-tubulin protein expression was normalized to GAPDH. n = 3. *P < 0.05 and **P < 0.01 compared to differentiated samples (NB). P = 0.09 between NB and DADLE.
DISCUSSION
In the present study we demonstrated that both DADLE and naltrexone exerted an antiproliferative effect on dopaminergic progenitor AF5 cells and that the effects were apparently additive when both drugs were used. The antiproliferative effect seen with DADLE and naltrexone is certainly nonopioid in nature. Opioids have previously been shown to exert proliferative or antiproliferative effect via an opioid or nonopioid action (Ilyinsky et al., 1987; Kornyei et al., 2003; Martin-Kleiner, 2002; Persson et al., 2003a,b; Zagon and McLaughlin, 2003; McLaughlin et al., 2003). Our results showing the unidirectional action of DADLE and naltrexone on AF5 cells strengthen the notion of the nonopioid nature of certain actions of opioid peptides in cellular systems. Further, inasmuch as DADLE’s affinity for cloned opioid receptors is at best about 10 nM, DADLE’s antiproliferative property in AF5 cells as observed in this study cannot be explained by its action on cloned opioid receptors. DADLE at 0.1 nM would occupy only less than 0.1% of the delta opioid receptors of which DADLE has the highest affinity among the subtypes of opioid receptors.
We also demonstrated that the growth inhibition of AF5 cells caused by DADLE and naltrexone was not due to the apoptotic cell death but was deeply related to the drugs’ ability to induce cell cycle arrest. Molecular mechanism(s) underlying the cell cycle-arresting effect of DADLE or naltrexone is unknown at present. The antiproliferative and cell cycle-arresting effects of DADLE and naltrexone, particularly regarding their ability to block the cell cycle at the G1 check point, may represent a potentially important utilization of these agents in certain neurological diseases. We also found that treatment with either DADLE/naltrexone alone or in combination did not enhance apoptosis, indicating that low dose treatment with DADLE or naltrexone would be a useful step in engineering neural progenitor cells for neural transplantation.
Regulation of cell cycle, especially the length of G1 phase, regulate neuronal differentiation from progenitor cells (Calegari and Huttner, 2003; Hodge et al., 2004; Lukaszewicz et al., 2002). Studies on the cell cycle regulators have provided evidence that the lengthening of the G1/S phase enhanced neurogenesis and neurite extension, increased β-III-tubulin-positive cells, and was in conjunction with the expression of the cyclin-dependent kinase inhibitor p27kip1 from neuronal progenitor cells (Kim et al., 2006; Misumi et al., 2008). Neural replacement therapy is one of the most promising treatments for neurodegenerative diseases such as Parkinson’s disease. However, neural transplantation encounters problems such as low survival rate of grafted cells, failure to differentiate into desired phenotype in the graft, tumor formation, and immunological rejection. Among these, cellular proliferation and cellular differentiation are major concerns for the success of neural transplantation therapy. Our present findings with the effect of DADLE and naltrexone may be of use in this regard because these two drugs can arrest the cell growth at the G1 checkpoint which is a critical step leading to cellular differentiation. Many of our previous studies showing the tissue- and cellular survival-promoting effects exerted by DADLE, specifically in the grafting of mesencephalic and substantia nigra dopaminergic neurons (e.g., Borlongan et al., 2000), may have a bearing with the present findings, i.e., the antiproliferative and cell cycle-arresting properties of DADLE.
The nonopioid action of properties of DADLE and naltrexone observed in the present study with the AF5 progenitor cell line may have a potential use in the stem cell research as well. The use of embryonic stem (ES) cells as a source of dopamine neurons for transplantation has been another therapeutic potential for Parkinson’s patients. However, scientists are still finding a way to figure out how to control ES cells that produce useful cells without the danger of overgrowing. Several studies reported successful efforts in converting ES cells to dopaminergic neurons. For example, Kawasaki et al. (2000) reported the development of dopaminergic neurons from mouse ES cells. To prevent tumor formation from residual ES cells after transplantation, cells were treated with mitotic inhibitor mitomycin C in that report. In this study, we demonstrated that DADLE and naltrexone-treated AF5 cells exhibited a reduction in growth rate and a cell cycle arrest at the G1 checkpoint. Thus, the DADLE, naltrexone, or the combination of both drugs may be of value as useful agent in stopping the stem cells from proliferation, thus facilitating the differentiation of stem cells.
The rat AF5 CNS progenitor cell line used in the present study was created by immortalizing the cells with an N-terminal fragment of SV40 large T antigen (T155g) (Truckenmiller et al., 2002). The AF5 cells express nestin, a neural stem cell marker. They also show properties similar to neural progenitor or stem-like cells and retain developmental and plasticity characters of neuronal cells. Depending on the cultural conditions, the AF5 cells could be maintained in undifferentiated state as neurospheres, i.e., spherical aggregates, either in suspensions in serum free medium or as adherent cultures in serum-containing medium. The neurospheres can differentiate in serum-containing medium when they are allowed to grow to confluency. Thus, unlike other tumor-derived cell lines containing a lot of abnormalities, AF5 cell line exhibit stable genetic and growth properties while retaining plasticity to differentiate like primary neural progenitor cells. Thus, our results on the effects of DADLE and naltrexone on AF5 cells may represent valuable information that may be useful in neural stem cell research.
In conclusion, the present study reports the nonopioid action of both DADLE and the opioid antagonist naltrexone on cell cycle arrest and differentiation. These results seen with DADLE and naltrexone may shed light on certain therapeutic approaches for treating neurodegenerative diseases. More studies will be carried out to understand the underlying molecular mechanism of DADLE and naltrexone in causing cell cycle arrest and differentiation.
ACKNOWLEDGMENTS
The authors thank Tomohisa Mori for helpful discussion and Stacie Errico for performing the Western blotting in Figure 4.
Contract grant sponsors: Intramural Research Program of the National Institute on Drug abuse, NIH, and DHHS
REFERENCES
- Borlongan CV, Su TP, Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 2000;11:923–926. doi: 10.1097/00001756-200004070-00005. [DOI] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsao LI, Su TP. Antiapoptotic and cytotoxic properties of delta opioid peptide [d-Ala(2),d-Leu(5)]enkephalin in PC12 cells. Synapse. 2002;43:86–94. doi: 10.1002/syn.10019. [DOI] [PubMed] [Google Scholar]
- Hodge RD, D’Ercole AJ, O’Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyinsky OB, Kozlova MV, Kondrikova ES, Kalentchuk VU, Titov MI, Bespalova ZD. Effects of opioid peptides and naloxone on nervous tissue in culture. Neuroscience. 1987;22:719–735. doi: 10.1016/0306-4522(87)90368-x. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Hida H, Jung CG, Miura Y, Nishino H. Treatment with deferoxamine increases neurons from neural stem/progenitor cells. Brain Res. 2006;1092:1–15. doi: 10.1016/j.brainres.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Kornyei JL, Vertes Z, Kovacs KA, Gocze PM, Vertes M. Developmental changes in the inhibition of cultured rat uterine cell proliferation by opioid peptides. Cell Prolif. 2003;36:151–163. doi: 10.1046/j.1365-2184.2003.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlova M, Kalentchuk V. Stimulatory effect of opioid peptides and naloxone on rat spinal cord cells in primary dissociated culture. Int J Dev Neurosci. 1994;12:507–515. doi: 10.1016/0736-5748(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Kennedy H, Dehay C. Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J Neurosci. 2002;22:6610–6622. doi: 10.1523/JNEUROSCI.22-15-06610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Kleiner I. The effect of opioid agonists of delta-class DSLET, mu-class DAMGO, kappa-class U-69593 and an opioid antagonist, naloxone, on MTT activity of NALM-1 leukemic cells. Biomed Pharmacother. 2002;56:458–462. doi: 10.1016/s0753-3322(02)00288-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Levin RJ, Zagon IS. Opioid growth factor (OGF) inhibits the progression of human squamous cell carcinoma of the head and neck transplanted into nude mice. Cancer Lett. 2003;199:209–217. doi: 10.1016/s0304-3835(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Misumi S, Kim TS, Jung CG, Masuda T, Urakawa S, Isobe Y, Furuyama F, Nishino H, Hida H. Enhanced neurogenesis from neural progenitor cells with G1/S-phase cell cycle arrest is mediated by transforming growth factor beta1. Eur J Neurosci. 2008;28:1049–1059. doi: 10.1111/j.1460-9568.2008.06420.x. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci. 2003b;23:360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003a;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Sanchez JF, Crooks DR, Lee CT, Schoen CJ, Amable R, Zeng X, Florival-Victor T, Morales N, Truckenmiller ME, Smith DR, Freed WJ. GABAergic lineage differentiation of AF5 neural progenitor cells in vitro. Cell Tissue Res. 2006;324:1–8. doi: 10.1007/s00441-005-0094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truckenmiller ME, Vawter MP, Zhang P, Conejero-Goldberg C, Dillon-Carter O, Morales N, Cheadle C, Becker KG, Freed WJ. AF5, a CNS cell line immortalized with an N-terminal fragment of SV40 large T: Growth, differentiation, genetic stability, and gene expression. Exp Neurol. 2002;175:318–337. doi: 10.1006/exnr.2002.7898. [DOI] [PubMed] [Google Scholar]
- Tsao LI, Hayashi T, Su TP. Blockade of dopamine transporter and tyrosine hydroxylase activity loss by [d-Ala(2), d-Leu(5)]enkephalin in methamphetamine-treated CD-1 mice. Eur J Pharmacol. 2000;404:89–93. doi: 10.1016/s0014-2999(00)00616-6. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Opioids and the apoptotic pathway in human cancer cells. Neuropeptides. 2003;37:79–88. doi: 10.1016/s0143-4179(03)00007-6. [DOI] [PubMed] [Google Scholar]