Abstract
Despite significant improvement in the malaria situation of the Greater Mekong Subregion (GMS), malaria control for the region continues to face a multitude of challenges. The extremely patchy malaria distribution, especially along international borders, makes disease surveillance and targeted control difficult. The vector systems are also diverse with dramatic differences in habitat ecology, biting behavior, and vectorial capacity, and there is a lack of effective transmission surveillance and control tools. Finally, in an era of heavy deployment of artemisinin-based combination therapies, the region acts as an epicenter of drug resistance, with the emergence of artemisinin resistant P. falciparum posing a threat to both regional and global malaria elimination campaigns. This problem is further exacerbated by the circulation of counterfeit and substandard artemisinin drugs. Accordingly, this Southeast Asian Malaria Research Center, consisting of a consortium of US and regional research institutions, has proposed four interlinked projects to address these most urgent problems in malaria control. The aims of these projects will help to substantially improve our understanding of malaria epidemiology, vector systems and their roles in malaria transmission, as well as the mechanisms of drug resistance in parasites. Through the training of next-generation scientists in malaria research, this program will help build up and strengthen regional research infrastructure and capacities, which are essential for sustained malaria control in this region.
Keywords: malaria, the Greater Mekong Subregion, epidemiology, vector systems, drug resistance, counterfeit drugs
1. Introduction
Malaria is a significant public health problem and has greatly impaired socioeconomic development in Southeast (SE) Asia, especially in countries of the Greater Mekong Subregion (GMS). GMS is comprised of Cambodia, China’s Yunnan province, Lao PDR, Myanmar, Thailand, and Viet Nam (Socheat et al., 2003). Malaria epidemiology in this region is characterized by immense geographical heterogeneity in disease endemicity, differential prevalence of four malaria species (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae and Plasmodium ovale) which require different drug treatments, and diverse vector systems with different vectorial capacities for the parasites. In recognition of the malaria burden in this region, the WHO Mekong Malaria Program was initiated in 1999. The aim of this program is to significantly reduce malaria-associated morbidity and mortality in the region, as well as to curb the spread of multidrug resistant (MDR) falciparum parasites. In recent years, fueled by governmental investments and international support such as the Global Fund to fight AIDs, Tuberculosis and Malaria (GFATM), the malaria situations in GMS nations have improved greatly.
Current malaria control practices mainly include rapid diagnostic tests (RDTs) at local community levels, artemisinin-based combination therapies (ACTs) to treat falciparum malaria, and long-lasting insecticide-treated bed nets (LLINs) and indoor insecticide residue sprays (IRS) to control vectors. However, the effectiveness of these measures is diminished due to the inherent characteristics of malaria epidemiology in this region. First, in most of the malaria-endemic regions of the GMS, the coexistence of P. falciparum and P. vivax presents difficulties for malaria diagnosis and treatment. Except some regions in Myanmar, most malaria-endemic areas in the GMS are of low endemicity, which is characterized by an annual incidence below 1/1000 (WHO, 2008). In such low-endemicity areas, RDTs for diagnosing vivax malaria are often not sensitive enough (Wongsrichanalai et al., 2003). Also, treatment of vivax malaria still relies on chloroquine (CQ) and primaquine. Whereas CQ treatment is largely effective for vivax malaria in this region, CQ resistance has been detected in Myanmar (Guthmann et al., 2008; Marlar-Than et al., 1995; Myat-Phone-Kyaw et al., 1993). Despite this, the CQ-primaquine combination is still the treatment of choice for vivax malaria. In addition, CQ treatment of vivax malaria often leads to the appearance of falciparum malaria in patients due to the prevalence of mixed-species infections (Krudsood et al., 1999, Mayxay et al., 2001). Primaquine is the only registered drug for eliminating vivax hypnozoites in the liver, but the high prevalence of glucose-6-phosphate dehydrogenase deficiency in the human populations compromises the use of this drug (Wiwanitkit, 2008). Second, ACTs are generally effective in the management of MDR falciparum malaria, but the efficacy of certain combinations such as artesunate-mefloquine has declined considerably, especially along the Thai-Cambodian and Thai-Myanmar borders (Carrara et al., 2009, Na-Bangchang et al., 2010, Rogers et al., 2009, Wongsrichanalai and Meshnick, 2008). The emergence of artemisinin-resistant falciparum parasites in these regions poses a great threat to the regional and global malaria control campaigns (Dondorp et al., 2009, Noedl et al., 2008, 2009). It is not clear whether such parasites have already spread to other areas or whether the enhanced malaria control efforts supported by WHO are able to contain the artemisinin-resistant parasites (Dondorp et al., 2010; WHO, 2011). The problem of drug resistance is further exacerbated by irrational drug use (e.g., artemisinin monotherapy and inability to adhere to treatment regimes) and the circulation of counterfeit/substandard drugs in private sectors (Newton et al., 2001), which not only lead to treatment failures but also promote the development of drug resistance. Third, in terms of vector control, LLINs and IRS may only be able to prevent indoor malaria transmission and are less effective for vector mosquitoes with exophillic biting behavior. There is a deficiency in our knowledge about the mosquito biting behavior and its role in outdoor malaria transmission. Besides, the effectiveness of the traditional vector control measures is also compromised due to the prevalence of insecticide resistance. However, the distribution of insecticide resistance in major malaria vectors is patchy and mechanism is poorly understood. Finally, although malaria control in many GMS countries targets international borders, these efforts are often thwarted by poor accessibility and high population mobility and subsequent parasite reintroduction in the border areas.
Despite improvement in the malaria situation of the GMS since the inception of the WHO’s Mekong Malaria Program in 1999, the socioeconomic burden of malaria in the region remains heavy (Socheat et al., 2003, WHO, 2008). Malaria control in the GMS is met with major knowledge gaps and challenges. The distribution of malaria is extremely uneven and our epidemiological information is far from accurate, making it difficult to establish cost-effective targeted control. Concentrations of malaria incidence along international borders and heavy cross-border migratory activities make malaria monitoring and control very difficult. For example, more than 10 million crossings were recorded in 1996 between China’s Yunnan province and three neighboring countries, Lao PDR, Vietnam, and Myanmar (Hu et al., 1998). Cross-border human population migration from highly-endemic countries poses a great threat to countries such as China, which are entering the phase of malaria elimination. Our knowledge of vector biology and ecology is still superficial and there is a lack of effective transmission surveillance and control tools. Additionally, the GMS is a breeding ground for MDR falciparum parasites and emerging resistance to artemisinins in P. falciparum is a major concern for regional and global malaria control. Furthermore, the mode of action and the mechanisms of artemisinin resistance are poorly understood, and close resistance surveillance is necessary. Finally, circulation of counterfeit and substandard artemisinin drugs requires close monitoring, and there is an urgent need for simple detection tools for field applications.
Based on these challenges in malaria control in the overall region and the unique countrywide malaria situations (WHO, 2008), we have strategically chosen Myanmar, Thailand and China as our study sites. Our rationale for selecting these sites is that they are representative of malaria epidemiology in the whole region, with shared phenomena such as “border malaria”, MDR parasites, intense malaria transmission in remote areas, diverse vector systems, etc. They also allow us to address urgent problems such as artemisinin resistance, which are pertinent to global malaria control.
2. Linking research activity to control practice
Malaria situations vary greatly among the GMS countries, with national strategies ranging from malaria elimination to malaria control (Feachem et al., 2010). These countries also differ considerably in both technical and operational feasibilities, which depend heavily on local financial, demographic, political, and health-system constraints (Tatem et al., 2010). Additionally, the extent of stable vivax malaria transmission in some nations presents a major challenge for malaria elimination. All the GMS nations are supported, to different degrees, by the GFATM, which provides grants to fill critical funding gaps in national plans.
To facilitated malaria control and elimination in the GMS, an International Center of Excellence for Malaria Research (ICEMR) was recently created with financial support from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. The three target countries of our ICEMR are entering the phases of malaria control (Myanmar), pre-elimination (Thailand) and elimination (China), respectively. This program is expected to contribute greatly to the fulfillment of the goals for each country. The overall goal of China is zero malaria-associated mortality by 2013 and, except in the border counties of Yunnan and Tibet, malaria elimination by the end of 2015 (Ministry of Health of China, 2009). The main interventions are composed of early and accurate diagnosis; prompt, effective, and safe treatment in both public and private sectors; appropriate vector control measures (IRS and LLINs); and better outreach efforts to the populations at risk (such as residents of remote communities and migrant workers). Malaria in Thailand is endemic only in the remote, hilly, and forested regions near international borders (mostly with Myanmar and Cambodia). Therefore, the GFATM program aims at improving access to prevention measures, early diagnosis and treatment of malaria, increasing effective utilization of LLINs, and better outreach efforts to promote prompt treatment-seeking behaviors among fever cases. Since Thailand is at the epicenter of MDR falciparum parasites, close monitoring of drug resistance is essential to ensure effective treatment of malaria. With dramatic decline in malaria incidence in China and Thailand, both nations need to strengthen their malaria surveillance systems and to ensure adequate outbreak response capability through improved case detection and reporting as well as entomological and antimalarial resistance monitoring. The malaria burden in Myanmar is the heaviest among all GMS nations. The GFATM program provides malaria control support in highly endemic areas such as development project sites, resettlement areas, and epidemic prone areas. Major interventions include scaled-up use of LLINs and IRS for vector control, improved access to quality diagnosis and treatment for malaria, and efficient supply chain management. The technical feasibility of malaria elimination in Myanmar is very low and requires enhanced health education, supportive supervision, monitoring, and evaluation.
In each country, the GFATM funded grants are expected to meet specific goals that are measured by country-specific metrics. For example, in China, the performance of the program is measured by indicators such as the number of malaria patients treated according to national policy, the number of active foci that received IRS, the number of feverish border crossers tested for malaria, etc. In Thailand, the indicators, reflecting an emphasis on “border malaria”, include the number of village malaria posts providing malaria diagnosis and treatment in high transmission areas, the number of confirmed cases receiving proper diagnosis and antimalarial treatment, the number of functional sentinel sites for monitoring drug resistance, etc. Therefore, metrics such as those for accurate determination of malaria epidemiology and monitoring of cross border migration malaria are shared among countries. In recognition of these requirements, our ICEMR program comprehensively targets these areas to assist in the aspects of epidemiology, vector control, and drug resistance management. It embraces many technological breakthroughs in the field and brings together expertise in epidemiology, disease ecology and modeling, vector biology and control, diagnostics, as well as parasite genomics and population biology. This program fosters and strengthens collaborations with institutions from endemic countries, some of which (e.g., vector-borne disease training center of Thailand, Yunnan Institute of Parasitic Diseases) are major players in carrying out the projects designated by the GFATM. Therefore, the research activities of the ICEMR program directly link with the malaria control practices, allowing more efficient dissemination of our findings. In addition, the participation of regional experts in malaria control in the scientific advisory group of this center enables tailoring of the research priorities to the needs of regional malaria control activities.
3. Goal and overall plan of our ICEMR
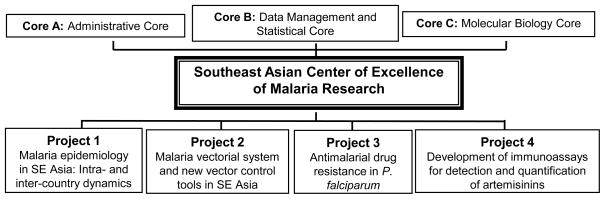
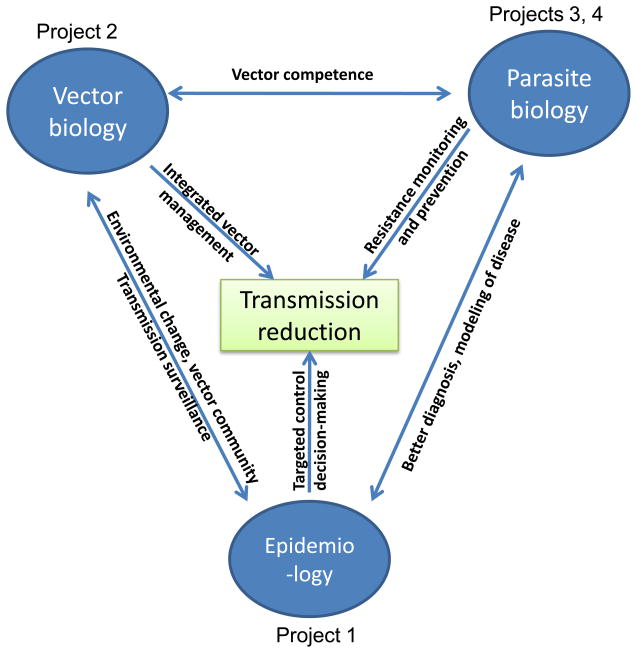
In moving towards malaria control and subsequent elimination, the GMS nations face major challenges and important scientific knowledge gaps which reduce the technical feasibility of malaria elimination. Accordingly, the central goal of our ICEMR is to improve our understanding of malaria epidemiology, vector systems and their roles in malaria transmission, and the mechanisms of drug resistance in parasites. With this knowledge, and through a combination of accurate disease surveillance, integrated vector control, and more effective chemotherapy, the nations of the GMS can achieve more effective, integrated malaria control. In recognition of the major problems in malaria control in the region, we have designed a multidisciplinary research program with four research projects to address significant knowledge gaps in each component of the vector-parasite-epidemiology triad (Fig. 1). The four projects are intrinsically linked, addressing the most urgent malaria problems shared by most SE Asian malaria-endemic nations: dynamic and asymmetric distribution of malaria that requires improved surveillance, substantially understudied vectorial systems, MDR parasites and emerging resistance to artemisinins, and circulation of counterfeit and substandard drugs (Fig. 2).
Fig. 1.
Projects and supporting cores of the Southeast Asian ICEMR.
Fig. 2.
The interrelated projects of the ICEMR address the three essential components of malaria transmission with the goal of ultimate transmission reduction.
In the GMS, malaria epidemiology is characterized by generally low incidence (<1/1000 population at risk), but large geographical asymmetry with numerous high transmission foci. As many countries are planning to enter the elimination phase, an essential problem lies in ascertaining accurate malaria incidence distributions and mobilizing the limited resources for targeted control. In addition, “border malaria” is a difficult issue in many countries of this region, where malaria transmission is concentrated along international borders that are often hard to access and cross-border migratory malaria is difficult to monitor and control. Based on these problems, Project 1 of our ICEMR studies the inter- and intra-country dynamics of malaria, and aims to develop and field-validate integrated malaria management strategies to further reduce malaria burden in these countries. This project will perform close surveillance of malaria in three international border areas and study sites with dramatically different patterns of malaria epidemiology. The proposed sentinel sites in China, Thailand and Myanmar vary in malaria vector and parasite species composition and transmission intensity, and offer a unique opportunity to compare malaria control strategies in multiple sites that exhibit varying epidemiology. Through the use of mathematical modeling and economic analysis, we aim to develop a malaria control decision system which will be evaluated through close collaborations with local public health authorities. Since the different patterns of malaria epidemiology in these countries are representative of many malaria-endemic areas in SE Asia, knowledge of malaria epidemiology gained from these selected countries will be applicable to other malaria-endemic regions in the GMS.
Vector control is one of the most important strategies in malaria control and elimination, and it must be built on a thorough understanding of vector biology, including ecology, behavior, and genetics. In the GMS, malaria vectors are highly diverse in species composition, population dynamics, ecological niche requirements, host feeding preference, and vectorial competence. As malaria vector control programs in this region largely rely on IRS and LLINs, the emergence and spread of insecticide resistance in mosquito vectors have reduced the effectiveness of insecticide-based malaria prevention programs. Further, some major vector species with outdoor biting behavior are also not amenable to IRS or LLIN control measures. Therefore, Project 2 aims to understand the malaria vectorial system and its regulation by environmental factors in multiple sites with different eco-epidemiological situations, and to develop innovative malaria transmission surveillance and control tools. This project will first address how the rapid environmental change and deforestation occurring in the GMS has affected the species composition and dynamics of malaria vectors, and the impact of these changes on malaria transmission. Major malaria vectors in this region are mostly species complexes that differ in bionomics, behavior, and perhaps vectorial competence. By focusing on species complexes that are common vectors in the entire region and sampling in multiple sites spanning the three endemic countries, we hope to generate a broad picture of parasite population genetics and parasite response to the intensified vector control programs. We will explore mosquito host-seeking behavior and use a combination of neurophysiological and behavioral approaches to identify candidate compounds that may be used as environmentally-safe and cost-effective repellents for disrupting outdoor transmission or for application in traps for surveillance and control purposes. By studying the mechanisms of pyrethroid resistance, we will develop a more convenient and accurate method for resistance surveillance so that we can predict the efficacy of the insecticide-based vector control programs. Meanwhile, we will develop new alternative vector control tools such as long-lasting larvicides for larval control that are beyond LLIN and IRS.
The emergence and spread of drug resistance in malaria parasites is a major impediment to effective malaria control and sustained management. This problem is particularly severe in the GMS, where MDR P. falciparum parasites are highly prevalent. As most falciparum-endemic countries have changed to ACTs for treating P. falciparum (Bosman and Mendis, 2007), resistance monitoring and study of resistance mechanism are a high priority. Recently, clinical artemisinin resistance has been observed in the GMS, a historical epicenter of MDR parasites (Dondorp, et al., 2009; Noedl, et al., 2008, 2009). Additionally, reduced efficacy of ACTs has been observed at our study sites (Na-Bangchang, et al., 2010; Wongsrichanalai and Meshnick, 2008; Yang et al., 2003), suggesting that P. falciparum resistance to artemisinins is emerging. Thus, Project 3 will address the urgent issue of antimalarial drug resistance, taking advantage of the unique features of our selected study sites, including MDR genetic background, long histories of artemisinin monotherapy, and detected reduction in susceptibility of parasites to artemisinins. We will systematically collect and culture-adapt P. falciparum field clinical samples from the three countries, where reduced susceptibility to artemisinins has already been observed. We will accurately determine the parasites’ drug response profile to a panel of known antimalarials. Using a custom-built single nucleotide polymorphism array, we will perform high-throughput genotyping of these parasite isolates (Mu et al., 2009). Using the genotyping and drug sensitivity data, we will perform a genome-wide association study to identify genetic loci that are associated with drug resistance. This approach will allow us to identify potential loci in the genome with signatures of drug selection, without a priori knowledge of the drug resistance mechanism, contributing to more efficient surveillance of drug resistance in malaria-endemic regions.
Malaria control relies heavily on the use of effective antimalarial medicines. ACTs are massively deployed in most falciparum-endemic countries to deal with MDR parasites. However, circulation of counterfeit and substandard artemisinins is a major threat to the success of malaria control. Fake artemisinins, containing few or no active ingredients, are life-threatening (Newton et al., 2006), and substandard drugs encourage resistance development. This problem is particularly serious in many GMS countries (Newton et al., 2003), where > 50% of artesunate drugs sold in certain regions are either fake or substandard (Newton et al., 2001). This problem may “spread” to Africa since counterfeit or substandard artemisinins have already been detected in some African countries (Atemnkeng et al., 2007). Artemisinin drug quality control is urgently needed. Therefore, Project 4 will aim to develop convenient and reliable immunoassays for rapid and semi-quantitative field monitoring of artemisinin drug quality.
We wish to note that the four projects in our ICEMR program are interlinked and that they target the most urgent problems in malaria control in the GMS (Fig. 2). Project 1 will study the inter- and intra-country dynamic epidemiology of malaria, which will serve as a foundation on which other projects are built. The focus of Project 2 will be on vector biology and the development of new vector surveillance and control tools, Project 3 will study antimalarial drug resistance mechanisms and develop resistance surveillance tools, and Project 4 will focus on field-applicable drug quality surveillance tools that will also be integrated into malaria surveillance, a key aim of Project 1. The effects of the innovative tools developed from Projects 2-4 will be ultimately evaluated in Project 1, and the summation of the results will provide comprehensive and accurate information about disease incidence, vectorial systems, epidemiology of drug resistance, efficacy of the current drug regimens, and eventually, effects of the integrated control strategies on disease reduction and elimination. It is also noteworthy that most of our established study sites are located in areas where the most impoverished ethnic minorities reside. Hence, our studies will address problems not only pertinent to the entire region, but also will target areas where malaria research and control are most desperately needed. With extensive research and control efforts undertaken in the endemic countries, we expect that there will be significant changes in malaria epidemiology, drug efficacy, vector community structure, disease diagnosis, novel parasite antigens, new repellents, and control strategy, etc. These new breakthroughs, as well as changing malaria epidemiology, will be addressed in a timely manner by the ICEMR special projects. The involvement of investigators from policy-making organizations in our research allows fast dissemination of the information gleaned from this study on malaria epidemiology, vector biology, drug sensitivity, etc. The collaborative team also serves as an excellent platform for the testing and evaluation of potential products developed from this program.
4. Prospects of the ICEMR
Strong malaria research capacity is essential for the realization and sustainment of the malaria elimination goal in the GMS. Currently, research capacity in the malaria institutions of the GMS is generally weak. To strengthen institutional capacity in the endemic countries, we have built a collaborative network with active involvement of scientists from institutions in the endemic countries. More importantly, this ICEMR has an integrated research and training program to promote career development of junior scientists from the endemic countries. Through the training program, we will recruit and train graduate students, post-doctoral scholars and junior faculty from endemic countries in malaria research. This will be achieved through our established mechanisms of formal degree-oriented training and short-term training workshops, which will be organized and administered through this center. Our consortium recruits scientists and researchers in diverse scientific disciplines with expertise in spatial-temporal modeling of diseases, clinical studies of malaria, drug resistance monitoring and mechanism studies, molecular epidemiology, vector biology and population genetics, vectorial competence, and drug quantification and metabolism. Trainees will therefore receive systematic, multidisciplinary training in malaria research. The goal of this training program is to train a cadre of young scientists and researchers from the endemic countries in the GMS in malaria research; an essential component for sustained malaria control in the target countries.
We envision that at the end of this program, we will have contributed to a better understanding of the urgent issues surrounding malaria control in this region, including malaria epidemiology at international borders, vector biology and disease transmission, insecticide resistance in key malaria vectors, the epidemiology of antimalarial drug resistance and the emerging problem of artemisinin resistance. The translational aspects of the research should help provide better solutions to improving the monitoring of migratory malaria, surveillance of insecticide resistance in vectors, surveillance of antimalarial drug resistance, and field applicable diagnostic tools of counterfeit/substandard artemisinin drugs. This ICEMR program will provide a platform for accelerating transfer of advanced technologies to scientists in the endemic countries. Finally, with the built-in training component, our program will train a significant number of young scientists in malaria research, thus helping build up and strengthen regional research infrastructure and capacities.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases, NIH (U19 AI089672). X. Su is supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atemnkeng MA, De Cock K, Plaizier-Vercammen J. Quality control of active ingredients in artemisinin-derivative antimalarials within Kenya and DR Congo. Trop Med Int Health. 2007;12:68–74. doi: 10.1111/j.1365-3156.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Bosman A, Mendis KN. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg. 2007;77:193–197. [PubMed] [Google Scholar]
- Carrara VI, Zwang J, Ashley EA, Price RN, Stepniewska K, Barends M, Brockman A, Anderson T, McGready R, Phaiphun L, Proux S, van Vugt M, Hutagalung R, Lwin KM, Phyo AP, Preechapornkul P, Imwong M, Pukrittayakamee S, Singhasivanon P, White NJ, Nosten F. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. Artemisinin resistance: current status and scenarios for containment. Nat Rev. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- Feachem RG, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmann JP, Pittet A, Lesage A, Imwong M, Lindegardh N, Min Lwin M, Zaw T, Annerberg A, de Radiguès X, Nosten F. Plasmodium vivax resistance to chloroquine in Dawei, southern Myanmar. Trop Med Int Health. 2008;13:91–98. doi: 10.1111/j.1365-3156.2007.01978.x. [DOI] [PubMed] [Google Scholar]
- Hu H, Singhasivanon P, Salazar NP, Thimasarn K, Li X, Wu Y, Yang H, Zhu D, Supavej S, Looarecsuwan S. Factors influencing malaria endemicity in Yunnan Province, PR China (analysis of spatial pattern by GIS). Geographical Information System Southeast Asian. J Trop Med Public Health. 1998;29:191–200. [PubMed] [Google Scholar]
- Krudsood S, Wilairatana P, Mason DP, Treeprasertsuk S, Singhasivanon P, Looareesuwan S. Hidden Plasmodium falciparum infections. Southeast Asian J Trop Med Public Health. 1999;30:623–624. [PubMed] [Google Scholar]
- Marlar-Than Myat-Phone-Kyaw, Aye-Yu-Soe Khaing-Khaing-Gyi, Ma-Sabai Myint-Oo. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg. 1995;89:307–308. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- Mayxay M, Pukritrayakamee S, Chotivanich K, Imwong M, Looareesuwan S, White NJ. Identification of cryptic coinfection with Plasmodium falciparum in patients presenting with vivax malaria. Am J Trop Med Hyg. 2001;65:588–592. doi: 10.4269/ajtmh.2001.65.588. [DOI] [PubMed] [Google Scholar]
- Myat-Phone-Kyaw Myint-Oo, Myint-Lwin Thaw-Zin, Kyin-Hla-Aye Nwe-Nwe-Yin. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma) Trans R Soc Trop Med Hyg. 1993;87:687. doi: 10.1016/0035-9203(93)90294-z. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of China. The People’s Republic of China - From Malaria Control to Elimination: A Revised National Malaria Strategy. 2009 ftp://ftp.wpro.who.int/scratch/R10%20Submitted%20Proposals/CHN/Malaria/R10%20Annexes%201-10/Annex%205%20China%20National%20Malaria%20Strategy%20(2010-2015).doc.
- Mu J, Myers RA, Jiang H, Liu S, Ricklefs S, Waisberg M, Chotivanich K, Udomsangpetch R, Sattabongkot J, Cui L, Ho M, Ou FZ, Li H, Song J, Li G, Wang X, Seila S, Sokunthea S, Socheat D, Porcella S, Fairhurst R, Wellems TE, Awadalla P, Su X. Genome-wide positive selection, recombination hotspots, and loci associated with Plasmodium falciparum resistance to antimalarial drugs. Nat Genet. 2009;42:268–271. doi: 10.1038/ng.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na-Bangchang K, Ruengweerayut R, Mahamad P, Ruengweerayut K, Chaijaroenkul W. Declining in efficacy of a three-day combination regimen of mefloquine-artesunate in a multi-drug resistance area along the Thai-Myanmar border. Malar J. 2010;9:273. doi: 10.1186/1475-2875-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P, Proux S, Green M, Smithuis F, Rozendaal J, Prakongpan S, Chotivanich K, Mayxay M, Looareesuwan S, Farrar J, Nosten F, White NJ. Fake artesunate in southeast Asia. Lancet. 2001;357:1948–1950. doi: 10.1016/S0140-6736(00)05085-6. [DOI] [PubMed] [Google Scholar]
- Newton PN, Dondorp A, Green M, Mayxay M, White NJ. Counterfeit artesunate antimalarials in southeast Asia. Lancet. 2003;362:169. doi: 10.1016/S0140-6736(03)13872-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PN, McGready R, Fernandez F, Green MD, Sunjio M, Bruneton C, Phanouvong S, Millet P, Whitty CJ, Talisuna AO, Proux S, Christophel EM, Malenga G, Singhasivanon P, Bojang K, Kaur H, Palmer K, Day NP, Greenwood BM, Nosten F, White NJ. Manslaughter by fake artesunate in Asia--will Africa be next? PLoS Med. 2006;3:e197. doi: 10.1371/journal.pmed.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- Noedl H, Socheat D, Satimai W. Artemisinin-resistant malaria in Asia. N Engl J Med. 2009;361:540–541. doi: 10.1056/NEJMc0900231. [DOI] [PubMed] [Google Scholar]
- Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socheat D, Denis MB, Fandeur T, Zhang Z, Yang H, Xu J, Zhou X, Phompida S, Phetsouvanh R, Lwin S, Lin K, Win T, Than SW, Htut Y, Prajakwong S, Rojanawatsirivet C, Tipmontree R, Vijaykadga S, Konchom S, Cong le D, Thien NT, Thuan le K, Ringwald P, Schapira A, Christophel E, Palmer K, Arbani PR, Prasittisuk C, Rastogi R, Monti F, Urbani C, Tsuyuoka R, Hoyer S, Otega L, Thimasarn K, Songcharoen S, Meert JP, Gay F, Crissman L, Cho Min N, Chansuda W, Darasri D, Indaratna K, Singhasivanon P, Chuprapawan S, Looareesuwan S, Supavej S, Kidson C, Baimai V, Yimsamran S, Buchachart K. Mekong malaria. II. Update of malaria, multi-drug resistance and economic development in the Mekong region of Southeast Asia The Southeast Asian. J Trop Med Public Health. 2003;34 (Suppl 4):1–102. [PubMed] [Google Scholar]
- Tatem AJ, Smith DL, Gething PW, Kabaria CW, Snow RW, Hay SI. Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010;376:1579–1591. doi: 10.1016/S0140-6736(10)61301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Malaria in the Greater Mekong Subregion: regional and country profiles. 2008:59. http://www.whothailand.org/EN/Section3/section113.htm.
- WHO. Global Plan for Artemisinin Resistance Containment (GPARC) 2011. p. 87. [Google Scholar]
- Wiwanitkit V. Genetic disorders and malaria in Indo-China region. J Vector borne Dis. 2008;45:98–104. [PubMed] [Google Scholar]
- Wongsrichanalai C, Arevalo I, Laoboonchai A, Yingyuen K, Miller RS, Magill AJ, Forney JR, Gasser RA., Jr Rapid diagnostic devices for malaria: field evaluation of a new prototype immunochromatographic assay for the detection of Plasmodium falciparum and non-falciparum Plasmodium. Am J Trop Med Hyg. 2003;69:26–30. [PubMed] [Google Scholar]
- Wongsrichanalai C, Meshnick SR. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg Infect Dis. 2008;14:716–719. doi: 10.3201/eid1405.071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu D, Yang Y, Fan B, Yang P, Li X, Li C, Dong Y, Yang C. Changes in susceptibility of Plasmodium falciparum to artesunate in vitro in Yunnan Province, China. Trans R Soc Trop Med Hyg. 2003;97:226–228. doi: 10.1016/s0035-9203(03)90127-1. [DOI] [PubMed] [Google Scholar]