Abstract
This study examined the relationship between individual differences in executive functions (EF; assessed by measures of working memory, Stroop, trail making, and verbal fluency) and ability to down-regulate and up-regulate responses to emotionally evocative film clips. To ensure a wide range of EF, 48 participants with diverse neurodegenerative disorders and 21 older neurologically normal aging participants were included. Participants were exposed to three different movie clips that were designed to elicit a mix of disgust and amusement. While watching the films they were either instructed to watch, down-regulate, and up-regulate their visible emotional responses. Heart-rate and facial behaviors were monitored throughout. Emotion regulatory ability was operationalized as changes in heart-rate and facial behavior in the down- and up-regulation conditions, controlling for responses in the watch condition. Results indicated that higher verbal fluency scores were related to greater ability to regulate emotion in both the down-regulation and up-regulation conditions. This finding remained significant even after controlling for age and general cognitive functioning. No relationships were found between emotion regulation and the other EF measures. We believe these results derive from differences among EF measures, with verbal fluency performance best capturing the complex sequence of controlled planning, activation, and monitoring required for successful emotion regulation. These findings contribute to our understanding of emotion-cognition interaction, suggesting a link between emotion-regulatory abilities and individual differences in complex executive functions.
Keywords: emotion regulation, executive functions, emotional behavior, Stroop, trail making, verbal fluency, working memory, FTLD, AD, cognition-emotion interaction, cognitive influences on emotion
The ability to monitor and modify ongoing behavior is critical across a variety of cognitive, emotional and social situations. Executive functions (EF) underlie behavioral goal-directed actions and the modification of hard-wired reactive responses (Lezak, Howieson, Loring, Hannay, & Fischer, 2004; Norman & Shallice, 1986; Smith & Jonides, 1999). EF is an umbrella term (Zelazo, Carter, Reznick, & Frye, 1997) that captures several separable, yet interrelated processes, including keeping information in mind, alternating between streams of different information, inhibiting responses, anticipating outcomes, and planning and monitoring ongoing activity (Miyake et al., 2000).
The role EF plays in behavioral regulation has traditionally been studied in the domains of cognition (Royall et al., 2002), but another class of regulatory processes, emotion regulation, also relies heavily on EF. Emotion regulatory processes are goal directed behaviors functioning to modify dynamic features of emotion such as the magnitude and duration of behavioral, experiential, and physiological responses (Thompson & Gross, 2007). Emotion regulation is essential for successful social interactions, and as a consequence, is used frequently in daily life. In fact, most people report using emotion-regulation every day (Gross, Richards, & John, 2006), and more than half the time, they do so by modifying the expression of emotions in their face, voice, and posture. Viewed in this way, successful emotion regulation can be construed as a form of behavioral regulation and likely relies heavily on EF processes such as remembering behavioral goals, anticipating outcomes, planning, monitoring and executing responses (e.g., Banfield, Wyland, Macrae, Münte & Heatherton, 2004; Denckla, 1996; Zelazo & Cunningham, 2007). For example, in order to reduce and hide visible signs of an emotional response (e.g., hide disappointment over a gift that is not as grand as anticipated), one has to construe the situation, anticipate responses, devise a plan (e.g., keep one’s facial muscles and breathing steady) and execute that plan by continuously monitoring and calibrating emotional responses. Similarly, in order to enhance visible signs of emotions (e.g., teach a child that food from the floor is not safe to eat), one needs to integrate perceptual cues, anticipate one’s natural responses, devise an implementation plan (e.g., stretch facial muscles more, move body and torso to exaggerate the response) and continually monitor the outcome.
EF and emotion regulation
Evidence for the relationship between EF and regulation of emotional responses comes from multiple lines of research. Intact EF abilities are associated with the integrity of the frontal lobes, which are believed to be critical in EF (Gazzaley & D’Esposito, 2007; Royall et al., 2002). Lesion studies that report diminished emotion regulatory functioning among patients with circumscribed frontal lobe damage also support this notion (Baddeley, 1986; Stuss & Levine, 2002). Theoretical accounts suggest that frontal lobe damage and resultant EF deficits compromise abilities to integrate emotional cues into decision making (e.g. Eslinger & Damasio, 1985) and to evaluate the relevance of emotional cues to the task at hand (Rule, Shimamura & Knight, 2002). Furthermore, results of neuroimaging studies demonstrate that there is a functional overlap between frontal brain structures believed to support EF processes and the conscious control and cognitive reappraisal of emotional stimuli (e.g., Beauregard, Levesque, & Bourgouin, 2001; Goldin, McRae, Ramel, & Gross, 2008; Ochsner, Bunge, Gross, & Gabrieli, 2002, Ochsner et al., 2004).
Studies examining individual differences in regulating behavior in emotionally laden situations point to the importance of EF. Across studies, higher EF ability is associated with reduced prejudiced behaviors (von Hippel, Silver, & Lynch, 2000), lower expressions of disgust in response to someone’s distasteful behavior (von Hippel & Gonsalkorale, 2005), reduced expressions of biased opinions (Payne, 2005), and a greater ability to delay gratification (Eigsti et al., 2006). Of particular relevance to the present study, there have been two recent reports of a direct link between EF abilities and specific emotion regulation capacities. In a series of four studies, Schmeichel and colleagues (Schmeichel, Volokhov, & Demaree, 2008) demonstrated that those higher in working-memory were more successful in down-regulation of facial expressive behaviors of disgust and amusement. Furthermore, they reported that participants higher in working-memory were better able to use antecedent focused emotion regulatory strategies and re-frame disgust related film clips, and reported feeling less negative emotions as a result. We (Gyurak et al., 2009) assessed EF and its relation to emotion regulation and found that higher EF (measured as performance on a test of verbal fluency) was related to greater reduction is visible signs of emotional behaviors in response to a loud noise both when participants were explicitly instructed to hide their emotional responses and when they down-regulated their emotional responses spontaneously.
Taken together, the research reviewed above suggests a link between EF and emotion regulatory ability. However, to date studies have only investigated EF in relation to down-regulation of emotions. While reducing emotional experience is certainly critical, up-regulation of emotions is also essential for effective social functioning. For example, amplifying disgust responses when teaching a child that food is not fit to eat, or showing happiness when one is less than pleased with a gift can enhance social functioning. Furthermore, except for our own work, studies have only assessed the relationship between a single EF component and emotion regulation. While EF measures tend to be inter-related, they are also somewhat separable (Miyake et al., 2000), reflecting their mediation by different neural networks (Alvarez & Emory, 2006).
Multiple EF functions
The need for reliable and valid measures of EF has led to the development of a number of neuropsychological tests that putatively assess related but dissociable facets of EF (Alvarez & Emory, 2006; Miyake et al., 2000; Royall et al., 2002). In the present study, we utilized tests commonly used to measure four such facets: (a) working memory capacity, (b) inhibition, (c) task switching, and (d) fluency.
Working-memory capacity refers to a set of processes that enable maintenance and manipulation of information in short-term memory in the service of a particular goal (Baddeley, 1986; Norman & Shallice, 1986). Working memory is measured by having individuals memorize and manipulate groups of items (e.g., letters or numbers). Working memory might assist emotion regulation by enabling storage and manipulation of perceptual and contextual information. Inhibition at the broadest level, refers to the ability to suppress one behavioral response in favor of another (e.g, Cohen, Dunbar, & McClelland, 1990). There are various conceptualizations of inhibition that further parcellate the concept into related but separable sub-processes (Friedman & Miyake, 2004; Nigg, 2000). Empirical evidence suggests it is useful to distinguish between inhibition over motor responses and inhibition over cognitive processes (Bernal & Altman, 2009). In this paper we focus on inhibition over cognitive processes, in particular on a subprocess termed resistance to distractor interference (Friedman & Miyake, 2004). This process is typically assessed by instructing individuals to respond to one aspect of a stimulus (e.g., ink color) and not to another more compelling aspect (e.g., color word). Inhibition plays a role in emotion regulation by enabling suppression of the more automatic, hardwired aspects of emotional responding. Task switching refers to the ability to redirect attention quickly between tasks. It is typically measured by having individuals engage in activities with alternating instruction sets (e.g., tracing mazes by alternating between letters and numbers). Task switching is important in emotion regulation because of the need to switch attention between various competing stimuli (e.g., the emotion-eliciting cue, other contextual cues, and the appropriate type of emotional behavior). Finally, fluency (Lezak et al., 2004; Ruff, Light, Parker, & Levin, 1997) is the ability to generate responses quickly in keeping with predetermined criteria. Fluency is typically measured with tasks that require participants to generate lists of words or objects that meet some criteria continually (e.g., for 1 minute). For example, in the phonemic fluency test, individuals are asked to generate words starting with the letter A (Lezak et al., 2004). Fluency is important for emotion regulation because it requires a sequence of cue-based activation, monitoring of output for errors, suppressing previously recalled items, and searching strategically for things that are consistent with the current goal (Rosen & Engle, 1997). Fluency tasks, particularly phonemic fluency have been shown to relate to working memory capacity and other EF measures and are thus considered to be “complex” EF measures (Miyake et al., 2000; Rosen & Engle, 1997).
The present study: EF and emotion regulation
The present study was designed to determine whether the four aspects of EF reviewed above (working memory, inhibition, task switching, and fluency) are related to the ability to regulate emotional responding in response to emotion-eliciting films. EF was measured using standard neuropsychological testing procedures. Emotion regulation was measured in the laboratory using procedures that generate strong emotions and assess both emotion down- and up-regulatory abilities (Levenson, 2007). A subset of the current participants (69% or 48 participants) was part of our previously reported study of emotion down-regulation in response to an aversive acoustic startle stimulus (Gyurak et al., 2009); however, the data from the tasks used in the present study have not been presented elsewhere.
Similar to our previous work (Gyurak et al., 2009), to ensure that participants had a wide range of EF, we included patients with a variety of neurodegenerative brain diseases – frontotemporal lobar degeneration (FTLD) or Alzheimer’s disease (AD) – as well as neurologically normal aging participants. Deficits in EF and in emotion regulation have been documented in neurodegenerative disorders that impact the frontal lobes (Baddeley, 1986; Duke & Kaszniak, 2000; Goodkind et al., 2010). FTLD is characterized by neuronal loss in the frontal and anterior temporal lobes (Kertesz, Davidson, & Munoz, 1999; Neary et al., 1998). Because degeneration in FTLD may begin in the right or left frontal or temporal lobes, patients may show diverse emotional deficits and cognitive symptoms (Kertesz et al., 1999). Although degeneration in AD typically starts in medial temporal areas, it often spreads to include the frontal and parietal lobes (Tikofsky, Hellman, & Parks, 1993). Based on these anatomical features, FTLD and AD patients were expected to manifest varying degrees of EF deficits as compared to controls.
In our previous work, we studied emotion down-regulation in response to an aversive acoustic startle stimulus, a very simple emotion elicitor that elicits primitive defensive responding. In the present study we used film clips to elicit emotion. Films require more extensive and complex cognitive processing to extract and appraise the emotional information. We have validated the effectiveness of these films for eliciting emotion in dementia patients (Levenson, 2007) and these kinds of emotion regulation procedures have been used by us (Kunzmann, Kupperbusch, & Levenson, 2005) and by others (e.g., Schmeichel et al., 2008; Demaree et al., 2006). The particular film clips used in the present study (described below under Methods) were selected to elicit disgust primarily; however, participants often also display signs of amusement (laughter and smiles).
In this study we presented three different film clips under three different conditions designed to create different emotion regulatory demands. In the “watch” condition, participants were instructed to watch the clip naturally. This condition did not test regulatory ability but rather was used as a covariate to control for individual differences in baseline emotional responding. In the down-regulation condition, participants were told to watch the clip, but try to hide their feelings so that no one would know what they were feeling. In the up-regulation condition, participants were told to watch the clip, but try to show what they felt during the clip (see procedures below). In a previous study using similar emotion regulation manipulations, heart rate responses were found to track the direction of instructed regulation (i.e., accelerations of the heart-rate during up-regulation and slowing of the heart-rate in down-regulation; e.g., Demaree et al., 2006) likely reflecting the different levels of muscle activity in the two conditions and the associated metabolic demands. Although we have previously found in studies with healthy individuals that instructed down-regulation of emotion is associated with greater sympathetic cardiovascular response (e.g., a composite measure in Gross & Levenson, 1993), heart rate by itself typically tracks the level of motor activity associated with the regulation conditions. Consistent with this, Demaree et al., (2006) reported heart rate decelerations during down-regulation of disgust and amusement.
We expected to find a relationship between EF and ability to both down- and up-regulate emotion. Based on the role that EF plays in emotion regulation, we hypothesized that deficits in EF would be associated with poor ability to down-regulate emotional responding in all groups (FTLD patients, AD patients and controls). In addition, based on our previously reported findings (Gyurak et al., 2009), we hypothesized that performance on the fluency task, a complex measure of EF that indexes the sequence of controlled planning, activation, and monitoring required for successful emotion regulation, would show the strongest relationship with emotion regulatory ability. By including the down-regulation condition, we were able to test the reproducibility of our previously reported findings using different stimuli and emotional response. The up-regulation condition extended this to test the relationship between EF and the ability to amplify visible signs of emotional responses.
Method
Participants
FLTD (N=41) and AD (N=7) patients were recruited through the Memory and Aging Center at the University of California San Francisco (UCSF). Neurologically normal aging participants (N=21) were selected through newspaper ads. Participants were evaluated using clinical interviews, questionnaires, MRI structural brain scans, and neuropsychological measures (including EF tests). FTLD diagnoses were determined using the Neary clinical criteria (Neary et al., 1998). AD diagnoses were determined using the National Institute of Neurological and Communication Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (McKhann, et al., 1984). Normal control participants were free of neurological and psychiatric symptoms. Demographics and general cognitive status of the participants are described in Table 1.
Table 1.
Demographics and general cognitive status of the sample
| Normal control (n = 21) | AD (n = 7) | FTLD (n = 41) | Test statistics | |
|---|---|---|---|---|
| Males | 8 | 5 | 25 | χ2(2, N = 69) = 1.18, ns |
| Age (SD) | 68.63a (8.17) | 63.55b (8.07) | 61.41b (7.85) | F(2,68) = 5.79, p < .05 |
| MMSE (SD) | 29.76a (.44) | 23.28b (3.68) | 26.58c (3.32) | F(2,68) = 16.31, p < .05 |
Note. Groups with different subscripts differed from each other at p < .05.
General cognitive functioning was assessed using the Mini-Mental State Examination (MMSE; Folstein, M. F. Folstein, S. E. & McHugh, 1975) test. In general, MMSE scores below 25 are indicative of cognitive decline. Table 1 shows means and SDs for each group and indicates that dementia participants had lower MMSE scores than normal controls. To control for the effects of general cognitive functioning, all analyses were repeated while controlling for MMSE scores. Furthermore, despite attempts to match the three groups on age, normal older participants were significantly older than FTLD patients; thus, age was used as an additional covariate in all major analyses.
Procedure
Following patient intake, a trained staff member at UCSF administered the EF tests and the MMSE in a testing room using standard neuropsychological testing procedures. This assessment was part of a comprehensive evaluation conduction by a team of neurologists, neuropsychologists, and other clinical staff. Emotion testing took place at the University of California, Berkeley in a separate experimental session scheduled within 3 months of the UCSF assessment. On arrival, participants signed the consent forms, were seated in a chair located 1.25 m from a 48-cm color television monitor, and had sensors attached for physiological monitoring. The full experimental protocol consisted of a series of tasks designed to assess emotional functioning (e.g., emotional reactivity, emotion regulation, and empathy; see Levenson, 2007 for details) in neurological patients. The present article focuses on the three trials during which participants were presented with the emotion-eliciting film clips.
EF tests
Descriptive statistics and correlations among these tests are presented in Table 2.
Table 2.
Descriptive statistics and intercorrelations among EF measures
| Mean (SD) | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| 1. Working memory (Working memory) | 21.81 (3.92) | - | |||
| 2. Stroop (Inhibition; res.) | 0 (30.01) | −.33* | - | ||
| 3. Trail Making (Task switching; res.) | 0 (43.34) | −.15 | .15 | - | |
| 4. Verbal fluency (Fluency) | 31.66 (16.43) | .35* | −.31* | −.08 | - |
Note. N = 69. res. = residualized; Uncorrected mean reading time on the critical Stroop inference card was 79.88 seconds (SD = 33.35). Uncorrected mean time to completion on the numbers-to-letters trail making card was 132.94 seconds (SD = 66.07).
p = .05 or below.
Digit and spatial span
The Digit Span (forward and backward) and its visual analog, the Spatial Span subscales of the Wechsler Adult Intelligence Scale (Version III, Wechsler, 1997) were used to assess working memory. Scores were summed across the correctly completed trials on the digit and spatial span trials to create a single composite score, with higher scores indicating greater working memory capacity. Reported reliabilities of both the digit and spatial span subscales are high, having test-retest correlations of .83 and .71 respectively (Wechsler, 1997).
Stroop
The Stroop task from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) was used to assess inhibition. This tasks consists of two trials: color words (e.g. “red,” “green,” “blue”) are either printed in congruent ink colors (e.g., the word “red” printed in red ink) or incongruent ink colors (e.g. the word “red” printed in green ink) and the participant is asked to name the ink color. To control for individual differences in color naming speed, an overall inhibition score was created by predicting incongruent color word reading time from congruent color word reading time and saving the residuals.1 These residual scores were then used as a measure of inhibition, with shorter times indicating better performance. Reliability of the Stroop is reasonable, with test-retest correlations of .70 – .79 (Delis et al., 2001).
Trail Making
Task switching was assessed with the D–KEFS Trail Making Test (Delis et al., 2001). Participants are instructed to alternate between connecting letters and connecting numbers printed in scrambled order on a card and to work sequentially (connect from “1” to “A” to “2” to “B”, etc.). If the participant makes an error, the examiner points it out and the participant is told to return to the correct location. To assess individual differences in number and letter processing speed, participants complete two trials where the task is to connect letters-to-letters and numbers-to-numbers sequentially. Performance on these processing speed tasks is then used to compute a task switching score by predicting time to completion on the numbers-to-letters trials from the letters-to-letters and numbers-to-numbers trials and saving the resulting residuals. These residual scores are used as a measure of task switching, with shorter times and lower scores indicating better task-switching. Reliability of the Trail Making test is reasonable, with test-retest correlations of .70 – .79 (Delis et al., 2001).
Verbal fluency
The phonemic version of the verbal fluency test (Delis et al., 2001) was used to assess fluency. On separate one-minute trials, participants were asked to generate words that began with the letters “F”, “A”, and “S” (excluding proper nouns and not repeating the same word with different suffixes). Verbal fluency was calculated as the total number of correct words produced across the three trials, with a higher score and greater production of words indicating greater fluency. Reliability of the verbal fluency test is high, with a test-retest correlation of .90 (Delis et al., 2001).
Means on the EF variables are presented in Table 3 for the FTLD, AD, and normal controls. As expected, these results show lower levels of EF for patients compared to controls. This was particularly the case for verbal fluency. We also examined the distribution of each EF test for outliers and found no extreme values (> 2.5 SDs from the mean) on any of the EF measures.
Table 3.
Performance and differences between groups on EF tests
| Normal control (n = 21) Mean (SD) | AD (n = 7) Mean (SD) | FTLD (n = 41) Mean (SD) | Test statistics | |
|---|---|---|---|---|
| 1. Working memory (Working memory) | 22.95 (2.80) | 20.00 (4.97) | 21.54 (4.15) | F(2,67) = 1.77, ns |
| 2. Stroop (Inhibition) | −10.79 (23.02) | 13.52 (51.87) | 3.22(27.55) | F(2,67) = 2.40, p = .10 |
| 3. Trail Making (Task switching) | −3.91(42.51) | 2.36 (47.80) | 1.60 (43.99) | F < 1 |
| 4. Verbal fluency (Fluency) | 45.90a (8.67) | 26.14b (11.71) | 25.32b (15.74) | F(2,67) = 16.52, p < .001. |
Note. Scores on Stroop and Trail Making are residualized. Groups with different subscript differed from each other at p < .05 or below.
Emotional reactivity, down- and up-regulation
Each of the three trials began with a 1-minute baseline period, during which the participant viewed an “X” on the TV screen and was instructed to relax and clear his or her mind. On the first, watch trial, participants were instructed to watch the film. Specifically, participants were told: “First you will see an ’X’ for 60 seconds and then you will see the film clip.” The instructions were given by an experimenter before the trial started, and they were also repeated on the screen after the baseline period, immediately preceding the clip. At the end of the 60-second baseline, the instructions “WATCH” appeared on the TV screen for 5 seconds. The clip was a 69-second long segment of the movie “Trainspotting.” The segment depicts a young male rushing into a filthy, public restroom and forcefully defecating into a dirty toilet. He then reaches into the toilet (and the fecal matter) to search for drugs.
On the second trial (down-regulate), participants were told by the experimenter and on the television screen following the baseline period to not show visible signs of their emotional responses. Specifically, the experimenter explained: “Now we will show you a short film clip. First you will see an X for 60 seconds and then you will see the film clip. This time, if you have any feelings as you watch the film clip, I want you to hide your feelings. An example of this is to pretend that someone is watching you and you do not want them to be able to tell that you are feeling anything about the film.” At the end of the 60-second baseline, the instructions “HIDE YOUR FEELINGS” appeared on the TV screen for 5 seconds. The down-regulate clip was a 34-second segment from the movie “Vampire’s Kiss.” The actor is shown as he chases down a cockroach on the kitchen stove, grabs the bug with his hand, examines it closely and then places the bug in his mouth to chew it for several seconds while the bug is clearly visible in his mouth.
On the third trial (up-regulate), participants were instructed by the experimenter before the trial and on the television screen following the baseline period to show visible signs of their emotional responses. Specifically, the experimenter explained: “Now you will watch another film clip. This time if you have any feelings as you watch the film clip, show your feelings as much as you can.” At the end of the 60-second baseline, the instructions “AMPLIFY YOUR FEELINGS” appeared on the TV screen for 5 seconds. The clip was an 85-second segment from the movie “Pink Flamingos.” The segment showed an actor dressed in drag as he follows a poodle on the street. When the dog defecates, the actor scoops up the feces, smears it over his face and tongue, licks and chews it, and then swallows the fecal matter. The three movie clips can be viewed at http://www.ocf.berkeley.edu/~agyurak/disgustclips/.
The fixed order of trials merits some explanation. To get a relatively unbiased measure of “natural” reactivity, the watch condition has to be administered first. Otherwise the regulation instructions would likely carry over into subsequent trials. For the two regulation conditions, we reasoned that there would be some habituation to the emotional content over the course of the three films. If the third condition was down-regulation, this habituation would make the task easier. In contrast, having the third condition be the up-regulation condition made the task a bit more challenging and worked against any habituation effects.
Measures
Facial expressive behaviors
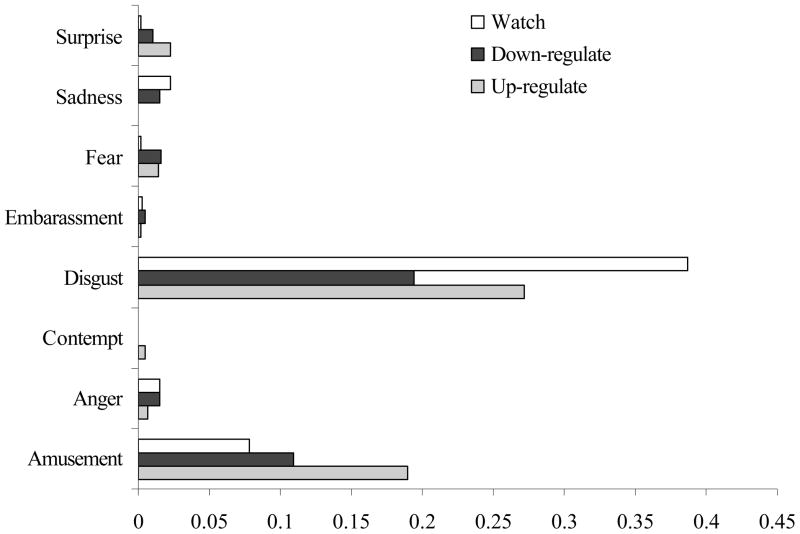
To assess facial expressive behaviors, a front-view of the participant’s face was videotaped using a partially concealed, remotely-controlled camera. For each trial, a group of emotion research experts identified the 30 consecutive seconds of each film clip that were deemed to be the most emotionally evocative. A team of research assistants coded facial expressive behavior from the videotapes using the Emotional Expressive Behavior Coding System (Gross & Levenson, 1993). Two coders who were blind to diagnostic status of the participants viewed each tape without sound. Coders rated the intensity of basic emotional expressions of anger, contempt, disgust, fear, amusement, embarrassment, sadness and surprise on a second-by-second basis using a 4-point intensity scale (0=absent, 3= strong) during the most evocative 30-second segment of the movie clips. Inter-coder reliability for the full set of codes was high (α = .79). Analysis of the mean intensity for each emotional facial expressive behavior for each trial confirmed that as intended, the films primarily elicited emotions of disgust and amusement. Figure 1 depicts the mean intensity across the sample for each coded emotion.
Figure 1.
Mean level of each coded emotional expression in the three trials.
Heart rate
Demaree and colleagues (Demaree et al., 2006) found that, among a number of physiological measures, heart rate was most reliably associated with up- and down-regulation of emotional responses in response to film clips that elicited disgust and amusement responses. Thus, we focused on heart-rate responding in the present study. Continuous recordings of heart rate were obtained using a system of Grass Model 7 polygraph and a computer equipped for processing multiple channels of analog information. The electrocardiogram was measured using two Beckman miniature electrodes with Redux paste placed in a bipolar configuration on opposite sides of the participant’s torso. Heart rate was quantified as the inter-beat interval in milliseconds between successive R waves using software written by one of the authors (R.W.L.). As customary in our laboratory, eight additional measurements of autonomic nervous system activity were collected (skin conductance, finger temperature, finger pulse transmission time, ear pulse transmission time, respiration period, respiration depth, blood pressure, and general somatic activity). We explored our main questions of interest with these variables in preliminary analyses but found no reliable effects, thus the reported results focus on heart-rate.
Data reduction and analysis
Emotional responses
Past research shows that performance on laboratory-based emotion regulation tests can be reliably measured with facial expressive behaviors and cardiovascular physiological responses (e.g., Gross & Levenson, 1993). Thus, in this study, we derived three variables to index emotional responding: (a) disgust and amusement facial expressions; (b) heart rate, and (c) composite emotional responding. Facial expressions and heart rate were averaged separately for the most emotionally-evocative 30 seconds of the film clips. Cronbach’s alpha coefficients of facial expressive behaviors and heart-rate were, α’s .31, .28, and .56 during watch, down-regulation and up-regulation trials respectively. Based on theoretical (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005) and empirical (Demaree et al., 2006) data, we believe that composites that capture reasonably coherent channels of emotional responding can be formed even when alpha coefficients are not high. For our main analyses we formed a single composite measure of emotional responding by normalizing and averaging facial expressive behaviors and heart-rate. To make sure that this composite represented the results adequately we conducted follow-up analyses for facial expressive behaviors and heart-rate separately.
Statistical analyses were performed using the SAS statistical package (Version 9.1; SAS Institute Inc, Cary, NC). Similar to our previous work (Gyurak et al., 2009), when examining the relationship between EF and emotional responding, data from all participants were pooled (this maximized variability in EF and also in emotion regulatory performance).
Relationships between performance on the watch trial and EF were examined using correlations. For the regulation trials, we correlated performance on each EF test measure with the overall emotional responding composite, using the overall emotional responding composite on the watch trial as a covariate. Controlling for responding on the watch trial enabled us to examine responding in the two regulation trials free of any individual differences in the magnitude of emotional responding (Ekman, Friesen, & Simons, 1985; Gyurak et al., 2009).
Where significant relationships were found between EF and the emotional responding composite, we followed up with a GLM analysis (a test that is more robust for handling unequal sample sizes than traditional ANOVA) that tested the interaction of diagnostic group and EF scores to determine whether diagnostic group moderated the relationship between EF and emotional responding. The primary analyses were then re-examined while controlling for age and general cognitive functioning (MMSE). Furthermore, when significant relationships between an EF measure and the composite emotional responding were found, we examined the unique predictive ability of the EF measure in separate analyses after we partialled out performance on the other EF measures. We conducted exploratory analyses between EF and facial expressive behaviors and heart-rate changes separately. To control for multiple statistical tests, we applied a Bonferroni adjustment and adopted a more conservative significance level of .025 for a priori two-tailed tests.
Results
Film effects
A series of one-sample t-tests among normal control participants indicated that the films reliably elicited emotional responding. Facial expressive behaviors differed from zero during the watch trial, t(20) = 4.69, p = .0002, down-regulate trial, t(20) = 3.08, p = .01, and up-regulate trial, t(20) = 7.57, p <.0001. Furthermore, to determine the effects of regulation instructions, we compared facial expressive behaviors during the down-regulate and up-regulate trials, finding that they were significantly different in the expected direction (down-regulate M = .18, SD = .24; up-regulate M = .31, SD = .19, t(20) = 2.05, p = .04).
EF and watch trial
We hypothesized that EF would not be related to responses on the watch trial. Consistent with this, correlations showed that none of the EF measures were related to the overall emotional responding composite on the watch trial; correlations were: working memory r(67) = −.03, ns, R2 = .0009; Stroop r(67) = −.02, ns, R2 = .0004; trail making r(67) = .16, ns, R2 = .03; and verbal fluency r(67) = .007, ns, R2 = .00005. Furthermore, the results did not change after we partialled out age and MMSE scores in these analyses.
Additionally, exploratory analyses were then conducted examining facial expressive behavior and heart rate during the watch trial separately. There were no significant correlations between performance on the four EF measures and facial expressive behaviors: working memory r(67) = .05, ns, R2 = .0025; Stroop r(67) = −.03, ns, R2 = .0009; trail making r(67) = .13, ns, R2 = .02; and verbal fluency r(67) = .15, ns, R2 = .02. Similarly, there were no significant correlations between performance on the four EF measures and heart rate change during the watch trial: working memory r(67) = .07, ns, R2 = .0049; Stroop r(67) = −.05, ns, R2 = .0025; trail making r(67) = −.13, ns, R2 = .01; and verbal fluency r(67) = .10, ns, R2 = .01.
EF and emotion regulation trials
We hypothesized that higher levels in EF would be associated with better emotion regulation performance on the two regulation trials. Emotional response during the watch trial was controlled for in these analyses. Because we examined both down- and up-regulation, we expected that higher EF levels (better performance) would be related to lower emotional responding on the down-regulation trials and higher emotional responding on the up-regulation trial. As described under “Measures,” better performance on EF is defined as higher scores on working memory and verbal fluency and lower scores on trail making and the Stroop tasks. We examined these questions using a series of correlations and partial correlations as described above under “Data reduction and analysis.”
Down-regulation of responding
Composite emotional responding
The only EF measure that predicted emotional responding was verbal fluency. Consistent with our hypothesis, higher verbal fluency scores were related to less emotional responding, r(66) = −.26, p = .01, R2 = .07. We next examined the interaction term between verbal fluency and diagnostic group membership and found no evidence of an interaction (F < 1). Furthermore, the relationship between verbal fluency and emotional responding remained significant when age and MMSE scores were controlled for, r(64) = −.34, p = .005, R2 = .11. Finally, we also examined the unique predictive ability of verbal fluency above and beyond the other three measures of EF in a partial correlation in which we controlled for working memory, Stroop, trails, age and MMSE scores. These results showed that the relationship between verbal fluency and emotional responding in the down-regulate condition remained significant, r(61) = −.31, p = .01, R2 = .10. Results of these analyses for all four EF measures are presented in Table 4.
Table 4.
Partial correlations between EF test measures and emotional responding in the down-regulation and up-regulation trials
| Down-regulate | Up-regulate | |||||
|---|---|---|---|---|---|---|
| Facial expressive behavior | Heart-rate | Emotional responding composite | Facial expressive behavior | Heart-rate | Emotional responding composite | |
| Working memory (Working memory) | −.03, p = .83 | −.16, p = .21 | −.14, p = .27 | .04, p = .73 | −.07, p = .59 | r = −.01, p = .91 |
| Stroop (Inhibition) | .23, p = .06 | .14, p = .27 | .23, p = .06 | −.03, p = .78 | −.17, p = .18 | −.12, p = .35 |
| Trail Making (Task switching) | −.13, p = .28 | .07, p = .59 | −.03, p = .83 | .05, p = .72 | .00, p = .99 | .04, p = .78 |
| Verbal fluency (Fluency) | −.11, p = .35 | −.36, p = .003 | −.26, p = .02 | .38, p = .001 | .28, p = .02 | .39, p = .001 |
Note. Emotional responding in the watch trial, age and MMSE scores were held constant in all analyses2, r(67), Values that appear in bold are significant at Bonferroni adjusted p = .025 or below.
Facial expressive behaviors
None of the EF measures were related to facial expressive behaviors in the down-regulate trial. The relationship between verbal fluency performance and facial expressive behaviors was in the expected direction, but did not reach statistical significance. Results of these analyses for all four EF measures are presented in Table 4.
Heart-rate
Consistent with our hypothesis, results indicated that verbal fluency performance was related to slowing of the heart-rate during the down-regulate trial, r(66) = −.36, p = .01, R2 = .13. None of the other EF measures showed a significant relationship to heart-rate changes. Results of these analyses for all four EF measures are presented in Table 4.
Up-regulation of responding
Composite emotional responding
For the up-regulate trial, the only EF measure that predicted emotional responding was verbal fluency. Consistent with our predictions, higher verbal fluency scores were related to more emotional responding, r(66) = .39, p = .001, R2 = .15. Paralleling results from the down-regulation trials, no significant interaction was found between verbal fluency and diagnostic group membership (F < 1) and verbal fluency scores predicted emotional responding in the up-regulate trial even after controlling for age and MMSE scores, r(64) = .31, p = .01, R2 = .01. Finally, verbal fluency scores significantly predicted emotional responding even after the other three EF measures were controlled for in a partial correlation, r(61) = .34, p = .006, R2 = .11. Results of these analyses for all four EF measures are presented in Table 4.
Facial expressive behaviors
Only verbal fluency was related to facial expressive behaviors. Consistent with our hypothesis, higher performance on verbal fluency was related to more facial expressive behaviors during the up-regulate trial, r(66) = .39, p = .001, R2 = .15. Results of these analyses for all four EF measures are presented in Table 4.
Heart-rate
Consistent with our hypothesis, results indicated that verbal fluency performance was related to speeding of the heart-rate during the up-regulate trial, r(66) = .29, p = .02, R2 = .08. None of the other EF measures showed a relationship to heart-rate changes. Results of these analyses for all four EF measures are presented in Table 4.
Discussion
Executive functioning underlies the regulation of thought and action. In this research we tested the hypothesis that EF performance would be related to the ability to regulate emotion. Results support this hypothesis and indicate that, among a number of established measures of EF (working memory, Stroop, trails, verbal fluency), verbal fluency performance is most strongly and reliably related to emotion regulatory ability as assessed by our laboratory-based procedures. These results replicate and extend our previous findings (Gyurak et al., 2009) that verbal fluency performance was related to down-regulation of a relatively primitive emotional reflex, the defensive response to an aversive, loud noise. The present study extended this relationship, showing that verbal fluency performance also predicted emotion regulatory ability using different and more complex stimuli (film clips), different emotional responses (disgust and amusement), and an additional direction of regulation (up-regulation). Consistent with our previous findings, higher verbal fluency performance was related to more successful regulation of emotional responses. We also predicted and found that EF measures were not related to emotional reactivity, as assessed by having participants simply watch an emotionally-evocative film clip without any explicit regulation instructions. This result supports prior findings that emotional reactivity and emotion regulation are somewhat separable processes (e.g., Goldin et al., 2008).
The finding that among measures of EF, only verbal fluency performance was related to down- and up-regulation of emotions emphasizes that, even though EF measures are interrelated, they are not identical (Miyake et al., 2000). Previous research (Schmeichel et al., 2008) among healthy young adults found that greater working memory was related to better emotion-regulation. Our previous and current results clearly do not support this finding; however, there are several factors that might account for this discrepancy. First, our populations were different. It is quite possible that the young adults in Schmeichel et al.’s study (2008) used different strategies for regulating their emotions (e.g., young adults might rely less on planning and more on rehearsal) and that these strategies might map on to different aspects of EF performance. Second, our working memory tasks were different. Schmeichel and colleagues (Schmeichel et al., 2008) assessed working-memory using two relatively complex measures of working-memory (i.e., OSPAN and N-back; Unsworth & Engle, 2007), whereas our work focused on two relatively simple measures of working-memory (digit and spatial span). As we noted in our previous work, complex measures of working-memory might show a relationship to emotion regulation because these measures likely tap planning and monitoring abilities critical for emotion regulation. Thus, performance on complex EF measures might provide a better index of the abilities that underlie behavioral regulation. The fact that verbal fluency remained significantly predictive of emotion regulatory abilities even after controlling for performance on the other three, less complex (Miyake et al., 2000) EF measures (working-memory, Stroop and trails) lends further support to this idea.
We found that verbal fluency was related to the composite emotional responding score in both regulation trials. We also separately examined the relationship between EF and facial-expressive behaviors and heart-rate and found that the relationship was stronger for heart-rate than for expressive behaviors. Heart-rate is sensitive to metabolic demands (Obrist, 1981), and has been found to relate to emotion-regulatory success in other studies (Demaree et al., 2006). Our findings support these observations and suggest that these kinds of physiological indicators of emotion-regulation are important to consider along with the more readily visible facial expressive and other behavioral indicators.
Fluency measures have long been considered central to cognitive functioning and were included among measures of intelligence (e.g., Thurstone, 1938). Verbal fluency and certain verbal subtests of traditional IQ measures share a small amount of variance (correlations of .20 to .25; Ardila, Galeano, & Rosselli, 1998), but there is not a substantial relationship between IQ and executive functions. Advances in the measurement of executive functions and the development of executive function batteries further shifted this conceptual overlap between word fluency and IQ. In present day testing, fluency measures are considered part of executive function “proper” (Delis et al., 2001; Lezak et al., 2004). Even though IQ and executive functions are largely unrelated, an important question remains whether measures of general intelligence or of specialized forms of intelligence (e.g., emotional intelligence; Mayer, Roberts, & Barsade, 2008) are related to laboratory measures of emotion regulatory performance.
Limitations
Several limitations may have affected our findings. First, we relied on a sample of older participants, and as a consequence, we do not know if findings would generalize to younger populations. Second, we have only investigated down- and up-regulation, and we do not know whether these results would generalize to other forms of emotion-regulation (e.g., reappraisal). Finally, we assessed emotion regulation in response to movie clips that elicit disgust and amusement and do not know whether the observed relationship between EF and emotion regulation would generalize to other emotions.
Conclusions
We believe that our results help illuminate new aspects of the relationship between cognition and emotion. EF have traditionally been defined and studied within the domains of cognition (e.g., Royall et al., 2002) and argued to aid “cold” cognitive behaviors. Our results paint a more nuanced picture of the interrelations between cognition and emotion. They suggest that the boundaries between them are quite porous, with EF subserving regulation of cognitive and well as emotional processes.
Footnotes
There are numerous ways to calculate interference (Jensen & Rohwer, 1966). We chose residualized scores over other methods because this resulted in a distribution that best approximated normality.
We re-analyzed our results after controlling for diagnostic group membership (dummy coded) and executive function measures. These results showed that the relationship between fluency composite and up-regulation composite was reduced from r(65) = .39 to r(65) = .27, but remained significant (p = .02). Using similar controls, the relationship between fluency composite and down-regulation composite was reduced from r(65) = −.26 to r(65) = −.22, dropping to marginal significance (p = .06). With these controls, null findings between emotion regulation and other executive function measures remained unchanged.
Contributor Information
Anett Gyurak, Departments of Psychiatry and Psychology, Stanford University.
Madeleine S. Goodkind, Department of Psychology, University of California, Berkeley
Joel H. Kramer, Memory and Aging Center, University of California, San Francisco
Bruce L. Miller, Memory and Aging Center, University of California, San Francisco
Robert W. Levenson, Department of Psychology, University of California, Berkeley
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology Review. 2006;16(1):17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Ardila A, Galeano LM, Rosselli M. Toward a model of neuropsychological activity. Neuropsychology Review. 1998;8(4):171–190. doi: 10.1023/a:1021618218943. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. New York, NY, US: Clarendon Press/Oxford University Press; 1986. [Google Scholar]
- Banfield JF, Wyland CL, Macrae CN, Münte TF, Heatherton TF. The cognitive neuroscience of self-regulation. Guilford Press; New York, NY: US: 2004. [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:6993–7000. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Altman N. Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: an fMRI study. The International Journal of Neuroscience. 2009;119(10):1848–1880. doi: 10.1080/00207450802333029. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan executive function scale. San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- Demaree HA, Schmeichel BJ, Robinson JL, Pu J, Everhart DE, Berntson GG. Up- and down-regulating facial disgust: Affective, vagal, sympathetic, and respiratory consequences. Biological Psychology. 2006;71(1):90–99. doi: 10.1016/j.biopsycho.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Denckla MB. A theory and model of executive function: A neuropsychological perspective. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Paul H. Brookes Publishing; Baltimore, MD: US: 1996. pp. 263–278. [Google Scholar]
- Duke LM, Kaszniak AW. Executive control functions in degenerative dementias: A comparative review. Neuropsychology Review. 2000;10(2):75–99. doi: 10.1023/a:1009096603879. [DOI] [PubMed] [Google Scholar]
- Eckart J, Sturm VE, Miller BL, Levenson RW. Evidence for diminished emotional reactivity to disgust in frontotemporal dementia. Poster presented at the Society for Psychophysiological Research; Savannah, GA, U.S.A. 2007. [Google Scholar]
- Eigsti I, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17(6):478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Simons RC. Is the startle reaction an emotion? Journal of Personality and Social Psychology. 1985;49(5):1416–1426. doi: 10.1037//0022-3514.49.5.1416. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: Patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. Journal of Experimental Psychology General. 2004;133(1):101–135. doi: 10.1037/0096–3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Unifying prefrontal cortex function: Executive control, neural networks, and top-down modulation. In: Miller Bruce L, Cummings Jeffrey L., editors. The human frontal lobes: Functions and disorders. 2007. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Gyurak A, McCarthy M, Miller BL, Levenson RW. Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer’s disease. Psychology and Aging. 2010;25(1):30–37. doi: 10.1037/a0018519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Richards JM, John OP. Emotion Regulation in Everyday Life. In: Snyder Douglas K, Simpson Jeffry, Hughes Jan N., editors. Emotion regulation in couples and families: Pathways to dysfunction and health. Vol. 2006. 2006. [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Goodkind MS, Madan A, Kramer JH, Miller BL, Levenson RW. Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective & Behavioral Neuroscience. 2009;9(2):144–152. doi: 10.3758/CABN.9.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR, Rohwer WD., Jr The Stroop color-word test: A review. Acta Psychologica. 1966;25(1):36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Munoz DG. Clinical and pathological overlap between frontotemporal dementia, primary progressive aphasia and corticobasal degeneration: The pick complex. Dementia and geriatric cognitive disorders. 1999;10(Suppl 1):46–49. doi: 10.1159/000051212. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: a comparison of two age groups. Psychology and Aging. 2005;20(1):144–158. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Emotion elicitation with neurological patients. In: Coan JA, Allen JJB, editors. The handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 158–168. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. 4. New York, NY, US: Oxford University Press; 2004. [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528–3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Roberts RD, Barsade SG. Human abilities: Emotional intelligence. Annual Review of Psychology. 2008;59:507–536. doi: 10.1146/annurev.psych.59.103006.093646. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman DA, Folstein MF, Katzman R, Price DL, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDSADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On Inhibition/Disinhibition in Developmental Psychopathology: Views From Cognitive and Personality Psychology and a Working Inhibition Taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation: Advances in research and theory. Vol. 4. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Obrist PA. Cardiovascular Psycholphysiology: A Perspective. Plenum Press; NY: 1981. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;(23):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Payne BK. Conceptualizing control in social cognition: how executive functioning modulates the expression of automatic stereotyping. Journal of Personality and Social Psychology. 2005;89(4):488–503. doi: 10.1037/0022-3514.89.4.488. [DOI] [PubMed] [Google Scholar]
- Rosen VM, Engle RW. The role of working memory capacity in retrieval. Journal of Experimental Psychology General. 1997;126(3):211–227. doi: 10.1037//0096-3445.126.3.211. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, et al. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. The psychological construct of word fluency. Brain and Language. 1997;57(3):394–405. doi: 10.1006/brln.1997.1755. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective & Behavioral Neuroscience. 2002;2(3):264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95(6):1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research Special Issue: Executive Control. 2000;63(3):289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Thompson R, Gross JJ. Emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Thurstone LL. Primary mental abilities. University of Chicago Press; Chicago: 1938. [Google Scholar]
- Tikofsky RS, Hellman RS, Parks RW. Single photon emission computed tomography and applications to dementia. New York, NY, US: Oxford University Press; 1993. [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin. 2007;133(6):1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Volokhov RN, Demaree HA. Spontaneous emotion regulation to positive and negative stimuli. Brain and Cognition. 2010;73(1):1–6. doi: 10.1016/j.bandc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- von Hippel W, Gonsalkorale K. “That is bloody revolting!” inhibitory control of thoughts better left unsaid. Psychological Science. 2005;16(7):497–500. doi: 10.1111/j.0956-7976.2005.01563.x. [DOI] [PubMed] [Google Scholar]
- von Hippel W, Silver LA, Lynch ME. Stereotyping against your will: The role of inhibitory ability in stereotyping and prejudice among the elderly. Personality and Social Psychology Bulletin. 2000;26(5):523–532. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, Frye D. Early development of executive function: A problem-solving framework. Review of General Psychology. 1997;1(2):198–226. [Google Scholar]
- Zelazo PD, Cunningham WA. Executive function: Mechanisms underlying emotion regulation. In: Gross James J., editor. Handbook of emotion regulation. Vol. 2007. New York, NY, US: Guilford Press; 2007. pp. 135–158.pp. xvii [Google Scholar]