Abstract
Disruptions in sleep/wake cycles, including decreased amplitude of rhythmic behaviors and fragmentation of the sleep episodes, are commonly associated with aging in humans and other mammals. While there are undoubtedly many factors contributing to these changes, a body of literature is emerging, suggesting that an age-related decline in the central circadian clock in the suprachiasmatic nucleus (SCN) may be a key element responsible. To explore age-related changes in the SCN, we have performed in vivo multiunit neural activity (MUA) recordings from the SCN of freely moving young (3–5 months) and middle-aged (13–18 months) mice. Importantly, the amplitude of day–night difference in MUA was significantly reduced in the older mice. We also found that the neural activity rhythms are clearly degraded in the subparaventricular zone, one of the main neural outputs of the SCN. Surprisingly, parallel studies indicate that the molecular clockwork in the SCN as measured by PER2 exhibited only minor deficits at this same age. Thus, the circadian output measured at the level of neural activity rhythms in the SCN is degraded by aging, and this decline occurs before the disruption of key components of the molecular clockwork.
Introduction
Disruptions in sleep/wake cycles, including decreased amplitude of rhythmic behaviors and fragmentation of the sleep episodes, are commonly associated with aging in humans and other mammals (Bliwise, 1993; Turek et al., 1995; Dijk et al., 1999; Van Someren, 2000). The mechanisms underlying these changes are unknown; however, there is growing evidence that age-related changes within the central circadian clock (SCN) may be responsible. The aged SCN exhibits altered expression of the key neuropeptides (Kawakami et al., 1997; Krajnak et al., 1998; Duncan et al., 2001) that would be expected to disrupt the synchrony of the SCN population and decreases in glucose utilization (Wise et al., 1988) consistent with such a decrease in synchrony. The observation that some of the age-related decline in behavioral rhythmicity can be reversed by implanting fetal SCN tissue into the third ventricle (Van Reeth et al., 1994; Viswanathan and Davis, 1995; Cai et al., 1997) leads additional support to the hypothesis that age-related changes in the SCN play an important role in driving the age-related behavioral changes. Previous work has shown the electrophysiological activity of aged SCN neurons in vitro is altered (Satinoff et al., 1993; Watanabe et al., 1995; Aujard et al., 2001; Biello, 2009), findings that raise the possibility that electrical activity rhythms that are essential for circadian output may be impacted early in the aging process. In the present study, we sought to determine whether circadian rhythms in spontaneous electrical firing in the SCN and subparaventricular zone (SPZ) are reduced in freely behaving, middle-age mice.
Materials and Methods
Animals.
Male C57BL/6J mice and PER2::LUC knock-in mice (C57BL/6J background) (Yoo et al., 2004) from our breeding colony were used for the experiment. Animals were maintained under controlled environmental conditions (temperature, 22 ± 2°C; light:dark [LD] 12:12 h, light ∼300 lux) with food and water available ad libitum. All procedures and standards of care were approved by the University of California, Los Angeles Division of Laboratory Animals and were conducted according to the National Institutes of Health guidelines for the use of experimental animals.
Locomotor activity recording.
Male C57BL/6J mice were classified into two groups: young adult (average age, 3.19 ± 0.12 months) and middle-aged (12.75 ± 0.21 months). Animals were then housed individually in cages with a running wheel. Locomotor activity was measured as running wheel revolutions recorded in 1 min bins and analyzed with CLOCKLAB software (Actimetrics).
In vivo multiunit neural activity recording from SCN and SPZ.
The experiments were performed as previously described (Nakamura et al., 2008). Young adult (4.28 ± 0.50 months) and middle-aged (15.30 ± 1.89 months) male C57BL/6J mice were anesthetized under isoflurane (2–5%; Phoenix Pharmaceutical) and placed on a stereotaxic instrument (David Kopf Instruments). Electrodes were inserted into the brain, aimed at the SCN (0.1 mm posterior and 0.2 mm lateral to the bregma, 5.9 mm depth from the skull surface) or SPZ (0.2 mm posterior, 0.2 mm lateral to the bregma, and 5.7 mm depth), and attached directly to the skull. After surgery, each mouse was transferred to an open-top cage with a running wheel mounted on one side in a light-tight box with a 12:12 h LD cycle. Output signals were processed by differential input integration amplifiers (Burr-Brown) and then fed into AC amplifiers (bandpass, 500 Hz to 5 kHZ; gain, ×10,000). Spikes were discriminated by amplitude and counted in 1 min bins by using a computer-based window discrimination system. Simultaneously with neural activity monitoring, the locomotor activity in individual mice also was detected as running wheel revolutions recorded in 1 min bins and stored on a computer. After study completion, each animal was anesthetized, and a positive current (2 μA; 60 s) was passed through the recording electrodes. The brain was removed and fixed in 4% paraformaldehyde in 0.1 m phosphate buffer containing 5% potassium ferrocyanide (Sigma–Aldrich). Serial coronal sections (40 μm) were stained with neutral-red, and blue spots of deposited iron were identified with the recording site.
Immunohistochemistry.
The methods were similar to those previously described (Kudo et al., 2011). Young adult (2–4 months; average age, 2.90 ± 0.19 months) and middle-aged (12 months; 11.96 ± 0.07 months) male C57BL/6J mice were used. Rabbit anti-PER2 (1:1000; Alpha Diagnostics) was used. All immunopositive cells within the SCN of these regions were counted manually at 400× with the aid of a grid (200 × 400 μm). All immunopositive cells within the grid were counted equally without regard to the intensity of the staining. Counts were performed by two observers blinded to the treatment protocol, and the results were averaged. Control experiments in which the primary antibody was not added did not exhibit any positive staining.
Real-time monitoring of bioluminescence.
The methods were similar to those previously described (Nakamura et al., 2010). Ex vivo explants of the SCN from young adult (3–6 months) and middle-aged (13–16 months) PER2::LUC mice were prepared between Zeitgeber time 10–11, with the explants entering the Lumicycle photometer (Actimetris). The bioluminescence signal was counted in 1 min bins for at least 7 d without changing media, and data were normalized by subtraction of the 24 h running average from the raw data and then smoothed with a 2 h running average (Origin Lab software). The amplitude was summed by the highest point and the lowest point of the each cycle.
Statistics.
Statistical significances between two groups were determined by Student's unpaired t test. Statistical significances for three or more groups were determined by one-way ANOVA followed by Tukey's post hoc analyses. All results are presented as the mean ± SEM and were considered significant at p < 0.05.
Results
Reduced circadian behavioral rhythm in middle-aged mice
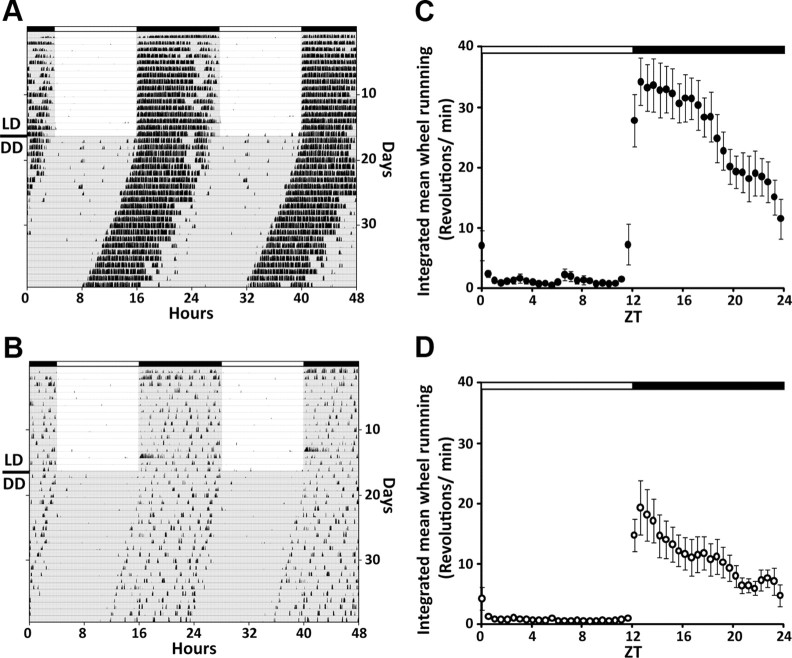
We observed clear reduction in the amplitude of the circadian locomotor rhythm in the 12–14 month, “middle-aged” mice when compared with younger animals (Fig. 1). Both young and middle-aged mice expressed clear rhythms in wheel-running activity in both LD and constant darkness (DD) conditions; however, the amplitudes are markedly different. Along with the reduction in overall activity, middle-aged mice exhibited more fragmented activity (young, 3.79 ± 0.5 bouts/d; middle aged, 6.28 ± 0.8; p < 0.05). Most of the circadian parameters that we could measure continue to decline with age.
Figure 1.
Reduced circadian behavioral rhythm in middle-aged mice. Representative double-plotted actograms of wheel-running activity in C57BL/6J mice showing the effect of aging on circadian rhythms of locomotor activity of young (A) and middle-aged (B) mice. The mice were maintained in LD cycle for 2 weeks and then transferred into DD. Lighting conditions are indicated at the top of the figure; open bars are light phases and closed are dark. C, D, Integrated mean wheel-running activities were plotted along 24 h for the LD condition (C: young, n = 8; D: middle-aged, n = 10).
Reduced multiunit neural activity rhythms in the SCN and SPZ of middle-aged mice
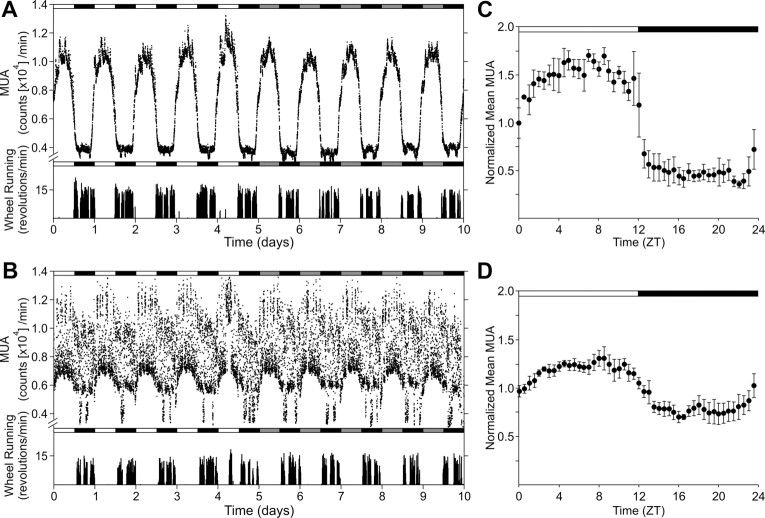
We implanted single bipolar electrodes into either the SCN (n = 8) or SPZ (n = 10) to monitor the long-term multiunit neural activity (MUA) (Fig. 2). All of the young mice (n = 4) exhibited clear daily rhythms in MUA in the SCN when they were exposed to LD cycles as well as robust free-running circadian rhythms when were placed in DD. The peak in MUA occurred in the middle of the day or subjective day, in approximate antiphase with locomotor activity. In contrast, all of the middle-aged SCN (n = 4) showed continually fluctuating levels of MUA leading to degraded “noisy” circadian rhythms on LD cycles and in DD when compared with the records from young animals. The MUA of SCN negatively correlated to the locomotor activity. The correlation coefficients between locomotor activity and MUA in the SCN of young and middle-aged mice were −0.42 ± 0.04 and −0.30 ± 0.09, respectively. The day–night ratio of MUA in the middle-aged SCN (1.50 ± 0.37-fold) was significantly lower (p < 0.05) compared with the young mice (3.00 ± 0.83-fold). Interestingly, the accumulated MUA count per day in the middle-aged SCN (1.62 × 106 MUA counts/d) was significantly larger (p < 0.05) than young SCN (1.15 × 106 MUA counts/d).
Figure 2.
Reduced MUA rhythm in the SCN of middle-aged mice in vivo. Representative serial-plotted actograms of neural and locomotor activity showing diurnal and circadian rhythms of MUA in the SCN of young (A) and middle-aged (B) mice. Lighting conditions are indicated at the top of the figure; open bars are light phases and closed are dark. Bottom trace represents simultaneous recorded locomotor activity. The number of spikes for MUA or activity counts for locomotor activity was counted every minute. C, D, Integrated mean activities were plotted for the LD condition. Each of four individual recordings was normalized with 24 h moving average and integrated for each group. Data are shown mean ± SEM, n = 4 per group.
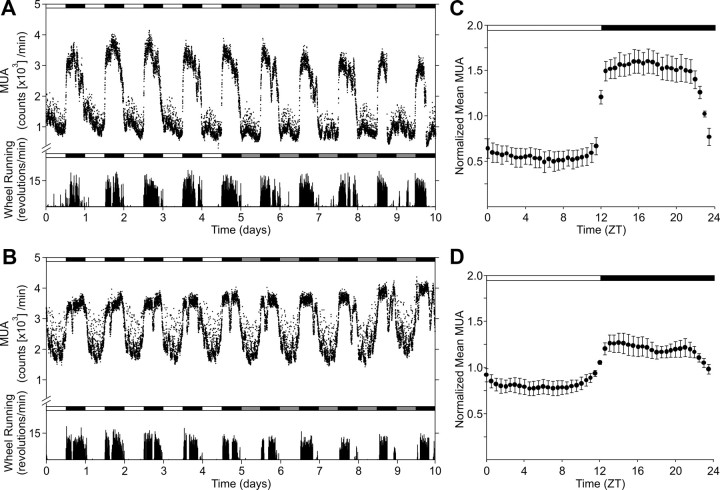
The SPZ of young mice (n = 5) also showed clear daily and circadian rhythms (Fig. 3). In contrast to the SCN, the SPZ exhibited circadian rhythms with peak activity occurring at night or during the subjective night, closely mirroring locomotor activity. In young adult SPZ, the MUA exhibited clear rhythms with day–night ratio (night/day) reached 3.23 ± 1.50-fold. Although the middle-aged SPZ (n = 5) exhibited relatively clear MUA rhythm in both LD and DD, the day–night ratio (night/day) was significantly lower (p < 0.05) than young SPZ (1.53 ± 0.51-fold). The accumulated MUA in the middle-aged SPZ (2.70 × 106 MUA counts/d) was also larger than young SPZ (1.16 × 106 MUA counts/d), but it did not reach to the significant level (p = 0.08). In addition, the MUA of SPZ positively correlated to the locomotor activity, and aging significantly reduced the correlation coefficients (0.60 ± 0.03 for young and 0.36 ± 0.07 for middle-aged; p < 0.05).
Figure 3.
Reduced MUA rhythm in the SPZ of middle-aged mice in vivo. Representative serial-plotted actograms of neural and locomotor activity showing diurnal rhythms of MUA in the SPZ of young (A) and middle-aged (B) mice. Lighting conditions are indicated at the top of the figure; open bars are light phases and closed are dark. Bottom trace represents simultaneous recorded locomotor activity. The number of spikes for MUA or activity counts for locomotor activity was counted every minute. C, D, Integrated mean activities were plotted for the LD condition. Each of four individual recordings was normalized with 24 h moving average and integrated for each group. Data are shown mean ± SEM, n = 5 per group.
PER2 expression rhythms of middle-aged SCN in vivo and ex vivo
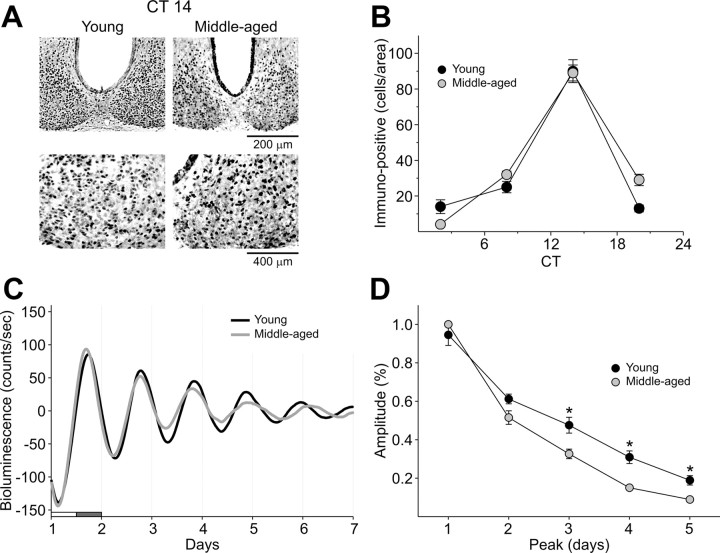
To determine if the molecular machinery necessary to generate circadian oscillations was affected by aging, we used immunohistochemistry (IHC) and bioluminescence to examine PER2 expression rhythms within the SCN (Fig. 4). For IHC experiments, the wheel-running activity of young and middle-aged mice in LD was monitored, and the mice were placed in DD for 3 d. Mice (n = 3–9 per time point) from each age group were sampled at four different points in the daily cycle (CT 2, 8, 14, and 20). PER2 was strongly expressed in the SCN of both ages with immunoreactivity seen throughout the SCN. As measured by the PER2 rhythm, we do not see any evidence that the molecular clockwork is disrupted in the aging mice. We also examined bioluminescence rhythms from young and middle-aged SCN from PER2::LUC knock-in mice to follow the amplitude and phase of each tissue over time. Both young and aged SCN clearly showed circadian rhythms of PER2::LUC expression. While most parameters were the same, there was a significant difference in the amplitudes between young and middle-aged group after the first couple of cycles in culture. Although the free-running periods did not differ between the groups, there was also a significant increase in cycle-to-cycle variability between young (1.17 ± 0.1) and middle-aged mice (2.74 ± 0.2; p = 0.005). Overall, the bioluminescence records from the SCN were very similar between the two ages.
Figure 4.
Unaltered PER2 expression rhythms in SCN of middle-aged mice. A, Photomicrographs of SCN tissue of each group in middle (200×) and high (400×) magnification. IHC was used to measure PER2 immunoreactivity in the SCN (n = 3–9 per time point) of young and middle-aged mice. Tissue was collected in four phases of the daily cycle (CT 2, 8, 14, 20). B, Numbers of PER2 immunopositive cells in the SCN varied as a function of time of day with highest count in early night (CT 14). C, PER2::LUC rhythms as measured by photomultiplier tube in the SCN. Typical examples of bioluminescence of young and middle-aged SCN explants. D, Amplitude of PER2::LUC expression in the SCN over days in culture. Data are shown mean ± SEM, n = 16 for young group and n = 8 for middle-aged group, *p < 0.05.
Discussion
The most important observation of the present study is that the amplitudes of electrical activity rhythms recorded from the SCN in vivo are already in decline by middle age (Fig. 2). This observation of reduced circadian output complements prior recordings made in SCN brain slice preparations (Satinoff et al., 1993; Watanabe et al., 1995; Nygard et al., 2005; Biello, 2009), as well as cultured SCN neurons (Aujard et al., 2001) that indicate the in vitro MUA rhythms are reduced in amplitude. The results we have obtained using in vivo recording gives us assurance that the age-related changes observed in the brain slice preparation are due to bona fide alterations in neuronal rhythmicity associated with aging. One apparent difference between our in vivo recording and prior in vitro studies in aged animals is the irregularity of the SCN electrical rhythms. Unlike the electrical rhythms of young animals, middle-aged animals showed high variability between adjacent measurement intervals. Such irregularity is not evident in slice SCN recordings; however, some of the difference with prior studies may be due to their larger collection intervals (1 h vs 1 min bins used in the present study). In addition, as there is evidence that the SCN is influenced by activity in other brain regions (Deboer et al., 2003; Vansteensel et al., 2003), the increased bin-to-bin variability could reflect degradation of regulation by other brain regions rather than reflecting a noisy aged SCN. Our in vivo data are consistent with an earlier study on glucose utilization comparing young and middle-aged female rats (Wise et al., 1988). Such metabolic differences might be expected if spontaneous electrical activity is reduced in aged animals in vivo. The middle-aged mice showed a reduced correlation between MUA and locomotor activity, a finding that is consistent with a reduced circadian output.
We were also able to extend our analysis to a major output pathway of the SCN, the SPZ. The aged SPZ exhibits a reduced amplitude electrical rhythm that is expressed in antiphase to the SCN (Fig. 3). An obvious interpretation of this result is that the age-related reduction in amplitude of the SCN electrical rhythm leads to a reduced drive on the SPZ. Such an interpretation is consistent with the results from experiments in rat in which when the SCN was surgically isolated, circadian rhythms were lost in regions surrounding the SCN (Inouye and Kawamura, 1979). In this model, the SCN is the source of neural circadian rhythms, and other brain regions are made rhythmic through their connection with the SCN. One has to be cautious about this interpretation, however, as we now recognize that many brain regions exhibit rhythms in molecular expression (Abe et al., 2002), and it is possible that the electrical rhythm in the SPZ, although synchronized by the SCN, is of intrinsic origin. The fact that middle-aged SPZ recordings are more regular (although still less regular than in young animals) than middle-aged SCN rhythms suggests that the SCN may not simply drive a rhythm on the SPZ, at least in its high-frequency detail.
Interacting molecular feedback loops driving rhythmic transcription and translation of key clock genes such as Period (Hastings et al., 2003) are at the core of the oscillatory mechanism responsible for driving circadian oscillations. As a first screen for possible deficits in this molecular clockwork, we looked at PER2 expression (Fig. 4A,B). We did not see any evidence for an age-related disruption with this in vivo assay. Using ex vivo bioluminescence monitoring of PER2::LUC-driven luciferase activity in transgenic mice, we found both the phase and the amplitude on the first cycle to be indistinguishable between young and middle-aged mice (Fig. 4C). There was a significant impact on the amplitude after the third cycle that may indicate an age-related increase in damping of these molecular oscillations. Other studies looking at the impact of aging on clock gene expression within the SCN have found robust rhythms in the expression of Period genes (Asai et al., 2001; Yamazaki et al., 2002; Kolker et al. 2004; Davidson et al., 2008) while others have found evidence for an age-related disruption in Per2 (Weinert et al., 2001) and other clock genes (Kolker et al., 2003; Wyse and Coogan, 2010). Our observation that the electrical rhythms are already disrupted at middle age at a point in which we did not see much evidence for disruption of the PER2 rhythm suggests that circadian MUA output from the SCN may be particularly sensitive to the impact of aging. The weakening of the electrical output of the SCN provides a possible physiological explanation for changes in the phase relationships between the central clock and peripheral organs that are associated with aging in the circadian system (Yamazaki et al., 2002).
This degradation of circadian output at the level of the SCN is likely to have profound consequences on the individual's health (Takahashi et al., 2008). It is becoming increasing clear that robust daily rhythms of sleep/wake are essential to good health. This brings up the question as to the extent that circadian perturbations exacerbate the age-related alterations in the functions of other physiological systems. In humans, a weakening of the circadian system can account for certain aspects of age-related sleep changes, and treating the circadian system in the elderly offers a potential target for ameliorating age-related changes in sleep and daytime alertness (Bliwise, 1993; Cajochen et al., 2006). In the United States, 40–70% of the elderly population is estimated to experience chronic sleep disturbances (Van Someren, 2000). Prior work on humans found evidence for age-related decline in sleep consolidation and suggests a reduced strength of a circadian signal promoting sleep in the early morning (Czeisler et al., 1999; Dijk et al., 1999). The reduced amplitude of circadian output as measured by electrical rhythm within the rodents SCN may provide the mechanistic underpinning of this reduced circadian signal and may be an important model for understanding similar changes in humans.
Footnotes
This work was supported by National Institutes of Health Grants RO1 MH062517 (G.D.B.), P30-AG028748 (T.J.N.), and CHDI A-2702 (C.S.C.).
The authors declare no competing financial interests.
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neurosci. 2001;106:255–261. doi: 10.1016/s0306-4522(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Biello SM. Circadian clock resetting in the mouse changes with age. Age. 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Cai A, Scarbrough K, Hinkle DA, Wise PM. Fetal grafts containing suprachiasmatic nuclei restore the diurnal rhythm of CRH and POMC mRNA in aging rats. Am J Physiol. 1997;273:1764–1770. doi: 10.1152/ajpregu.1997.273.5.R1764. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Knoblauch V, Blatter K, Wirz-Justice A. Age-related changes in the circadian and homeostatic regulation of human sleep. Chronobiol Int. 2006;23:461–474. doi: 10.1080/07420520500545813. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, Menaker M, Block GD. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Vansteensel MJ, Détári L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MJ, Herron JM, Hill SA. Aging selectively suppresses vasoactive intestinal peptide messenger RNA expression in the suprachiasmatic nucleus of the Syrian hamster. Brain Res Mol Brain Res. 2001;87:196–203. doi: 10.1016/s0169-328x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kolker DE, Vitaterna MH, Fruechte EM, Takahashi JS, Turek FW. Effects of age on circadian rhythms are similar in wild-type and heterozygous Clock mutant mice. Neurobiol Aging. 2004;25:517–523. doi: 10.1016/j.neurobiolaging.2003.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS. Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease. Exp Neurol. 2011;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell CS, Block GD. Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels. Steroids. 2010;75:203–212. doi: 10.1016/j.steroids.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T. In vivo monitoring of circadian timing in freely moving mice. Curr Biol. 2008;18:381–385. doi: 10.1016/j.cub.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Nygård M, Hill RH, Wikström MA, Kristensson K. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res Bull. 2005;65:149–154. doi: 10.1016/j.brainresbull.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, Gillette MU. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Penev P, Zhang Y, van Reeth O, Zee P. Effects of age on the circadian system. Neurosci Biobehav Rev. 1995;19:53–58. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Zhang Y, Zee PC, Turek FW. Grafting fetal suprachiasmatic nuclei in the hypothalamus of old hamsters restores responsiveness of the circadian clock to a phase shifting stimulus. Brain Res. 1994;643:338–342. doi: 10.1016/0006-8993(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ. Circadian rhythms and sleep in human aging. Chronobiol Int. 2000;17:233–243. doi: 10.1081/cbi-100101046. [DOI] [PubMed] [Google Scholar]
- Vansteensel MJ, Yamazaki S, Albus H, Deboer T, Block GD, Meijer JH. Dissociation between circadian Per1 and neuronal and behavioral rhythms following a shifted environmental cycle. Curr Biol. 2003;13:1538–1542. doi: 10.1016/s0960-9822(03)00560-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan N, Davis FC. Suprachiasmatic nucleus grafts restore circadian function in aged hamsters. Brain Res. 1995;686:10–16. doi: 10.1016/0006-8993(95)00423-n. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695:237–239. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18:559–565. doi: 10.1081/cbi-100103976. [DOI] [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED. Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1988;85:5305–5309. doi: 10.1073/pnas.85.14.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci U S A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]