Abstract
Background
Women with congenital long-QT syndrome (LQTS) experience increased risk for cardiac events after the onset of adolescence, that is more pronounced among carriers of the LQT2 genotype. We hypothesized that the hormonal changes associated with menopause may affect clinical risk in this population.
Methods and Results
We used a repeated events analysis to evaluate the risk for recurrent syncope during the menopause-transition and post-menopausal periods (5-years before and after the age at onset of menopause, respectively) among 282 LQT1 (n=151) and LQT2 (n=131) women enrolled in the LQTS Registry. Multivariate analysis showed that the risk for recurrent syncope (n=150) among LQT2 women was significantly increased during both menopause-transition (HR = 3.38 [p = 0.005]) and the post-menopausal period (HR = 8.10 [p < 0.001]) as compared with the reproductive period. The risk increase was evident among women who did or did not receive estrogen therapy. In contrast, among LQT1 women the onset of menopause was associated with a reduction in the risk for recurrent syncope (HR = 0.19 [p = 0.05]; p-value for genotype-by-menopause interaction = 0.02). Only 22 women (8%) experienced aborted cardiac arrest (ACA) or sudden cardiac death (SCD) during follow-up. The frequency of ACA/SCD showed a similar genotype-specific association with the onset of menopause.
Conclusions
The onset of menopause is associated with a significant increase in the risk of cardiac events (dominated by recurrent episodes of syncope) in LQT2 women, suggesting that careful follow-up and continued long-term therapy are warranted in this population.
Keywords: long-QT syndrome, women, estrogen, testosterone
Congenital long-QT syndrome (LQTS) is the most common inherited arrhythmogenic disorder that predisposes to sudden cardiac death (SCD) in young individuals without structural heart disease.1 Cardiac events in LQTS patients are attributed to episodes of torsades de pointes, believed to be initiated by after-depolarization events during the prolonged refractory period secondary to a mutation in the affected ion channels.2 We have previously shown that LQTS women experience a significant increase in the risk of cardiac events after the onset of adolescence and during the postpartum period,3-5 with a more pronounced risk increase among carriers of the LQT2 genotype.4,5 Furthermore, female sex was shown to be associated with a baseline longer QTc and is also an independent risk factor for development of torsades de-pointes in acquired (drug-induced) LQTS,6-10 suggesting that the effect of sex hormones on arrhythmic risk may be related to cardiac ion channel mechanisms.6,7 Estrogen and progesterone were shown to exert opposite effects on cardiac potassium channel activity.11-12 Thus, LQTS women who harbor mutations that impair the potassium channel may be sensitive to the changes in the levels of sex hormones that occur during the peri-menopausal period. Currently, however, there are no data regarding the effect of menopause on the clinical course of women with this inherited cardiac disorder.
The aims of the present study were: 1) to evaluate the effect of menopause on the risk for cardiac events among women with genetically-confirmed mutations in the KCNQ1 and KCNH2 potassium channels; and 2) to examine a possible association between treatment with hormonal therapy and the risk of cardiac events in this population.
Methods
Study population
The study population was drawn from the US portion of the International LQTS Registry. Women ≥ 30 years of age were included in the study if they had: 1) genetically-confirmed mutations in the LQT1 and LQT2 genes; and 2) completed a prospectively designed follow-up questionnaire regarding menses, hormonal therapy, and acquired comorbidities (see data collection below). The final study sample comprised 282 LQT1 (n=151) and LQT2 (n=131) women from 104 proband identified families. The LQTS genotype was determined using standard mutational analytic techniques involving 5 established genetic laboratories associated with the International LQTS Registry.
Data collection
Clinical data were recorded on prospectively designed forms and included individual and family history and demographic, ECG, therapeutic, and cardiac event information. Upon enrollment in the Registry, a 12-lead ECG was obtained from each subject as described previously.13 From this first recorded ECG, the duration of the QT interval was assessed from lead II (or lead I or III if the QT interval could not be measured from lead II) and corrected for heart rate (QTc) by use of Bazett’s formula.14 Additional serial QTc recordings were obtained from ECGs recorded during follow-up contacts, usually at yearly intervals. Routine clinical and electrocardiographic (ECG) parameters were acquired at the time of enrollment.
All study patients were also prospectively followed-up for data regarding non-LQTS comorbidities (shown in Table 1), date of last menstrual period, and usage and type of hormonal therapy (i.e, estrogen or progesterone containing hormonal therapy). Data were collected through a pre-formed questionnaire that is sent to enrolled adult subjects at the yearly follow-up assessment.
Table 1.
Clinical characteristics of study patients by genotype.
| PARAMETER | GENOTYPE | P-VALUE | |
|---|---|---|---|
| LQT1 (n=151) | LQT2 (n=131) | ||
| Family history of SCD, n (%) | 26 (17) | 25 (19) | 0.69 |
| Baseline ECG after age 30 years | |||
| QTc, msec (mean±SD) | 490 ± 49 | 486 ± 54 | 0.19 |
| RR, msec (mean±SD) | 895 ± 155 | 940 ± 183 | 0.05 |
| QRS, msec (mean±SD) | 83 ± 13 | 81 ± 16 | 0.05 |
| PR, msec (mean±SD) | 160 ± 24 | 156 ± 23 | 0.18 |
| LQTS-related therapies | |||
| Beta-blockers, n (%) | 85 (56) | 89 (68) | 0.05 |
| LCSD, n (%) | 1 (1) | 6 (5) | 0.05 |
| Pacemaker, n (%) | 4 (3) | 19 (15) | <.001 |
| ICD, n (%) | 23 (15) | 33 (25) | 0.04 |
| Menopause data and comorbidities | |||
| Menopause, n (%) | 102 (68) | 85 (65) | 0.64 |
| Age at menopause, yrs | 48 ± 6 | 49 ± 6 | 0.54 |
| Estrogen therapy, n (%) | 59 (39) | 43 (33) | 0.28 |
| Angina, n (%) | 7 (5) | 5 (4) | 0.74 |
| MI, n (%) | 4 (3) | 0 (0) | 0.13 |
| Stroke, n (%) | 3 (2) | 1 (1) | 0.63 |
| Smoking, n (%) | 65 (43) | 42 (32) | 0.06 |
| Diabetes mellitus, n (%) | 4 (3) | 7 (5) | 0.24 |
| Hypertention, n(%) | 23 (15) | 13 (10) | 0.18 |
| Asthma, n(%) | 16 (11) | 10 (8) | 0.39 |
| Emphysema, n (%) | 2 (1) | 0 (0) | 0.50 |
| COPD, n (%) | 18 (12) | 10 (8) | 0.23 |
| CABG, n (%) | 0 (0) | 1 (1) | 0.47 |
| Coronary angiography, n(%) | 5 (3) | 3(2) | 0.73 |
| Cardiac Events During Follow-Up | |||
| First syncope, n (%) | 38 (25) | 42 (32) | NA† |
| Total number of syncope events | 63 | 87 | |
| ACA/SCD, n (%) | 11 (7) | 11 (8) | |
| First cardiac event of any-type‡ | 46 (30) | 51 (39) | |
| Follow-up time, yrs (mean±SD) | 24 ± 6 | 24 ± 7 | |
Data on hormone treatment and age of menopause were obtained from a questionnaire that was filled out by study subjects
Event numbers are presented for descriptive purposes only, since a statistical comparison of differences in the crude number of events (that does not take into account the relative time in which the events occurred) has limited interpretability.
Comprises the first occurrence of syncope, ACA, or SCD, during follow-up (i.e. ACA/SCD that occurred after a first syncope was is not considered a first event).
ACA denotes aborted cardiac arrest, CABG coronary artery bypass grafting, COPD chronic obstructive pulmonary disease, ICD implantable cardioverter defibrillator, LCSD left cervical sympathetic denervation, MI myocardial infarction, SCD sudden cardiac death.
Subjects provided informed consent agreeing to inclusion in the registry and subsequent genetic and clinical studies. The study was approved by the University of Rochester Medical Center Research Subjects Review Board.
Definitions
The age at onset of menopause was defined as one one-year after the reported age of the last menstrual period.15 To evaluate the effect of hormonal status on the risk of cardiac events, follow-up time was pre-specified into the following 3 periods based on a model developed at the Stages of Reproductive Aging Workshop (STRAW):15 1) the menopause-transition period, characterized by variable menstrual cycles and high follicular stimulating hormone (FSH) values, most commonly lasting 4–5 years16 (defined in the present study as follow-up starting 5-years prior to the onset of menopause and ending at the age at onset of menopause); 2) the reproductive period, characterized by regular menstrual cycles (defined in the present study as follow-up time beginning at age 30 years and ending at the onset of the menopausal-transition period); and 3) the post-menopause period (defined in the present study as the 5-year period following the age at onset of menopause). The post-menopausal period was further sub-divided by treatment with hormonal therapy during this time period. Since only a minority (2.1%) of study subjects was using a progesterone containing hormonal regimen, only women who received estrogen therapy were included in this sub-analysis.
Outcome measures
To facilitate an evaluation of the effect of the menopausal periods on the risk of recurrent cardiac events, we employed statistical methodology that allowed for the inclusion of repeated non-fatal syncopal episodes in the multivariate models. Therefore, the primary end point of the present study was the occurrence of recurrent syncope during follow-up.
Since modeling of recurrent events does not allow for inclusion of lethal or near-lethal events, the consistency of the results was also assessed by analyzing secondary end points that included 1) the rates of cardiac events of any type (comprising syncope, aborted cardiac arrest [ACA], and SCD), which were adjusted for follow-up during the pre-specified peri-menopausal periods; and 2) the number of life-threatening cardiac events (comprising ACA or SCD) during the peri-menopausal periods.
LQTS-related syncope was defined as a transient loss of consciousness with abrupt onset and offset; ACA was defined as an event requiring defibrillation as part of the resuscitation; and sudden cardiac death was defined as abrupt in onset without evident cause, if witnessed, or death that was not explained by any other cause if it occurred in a non-witnessed setting. The circumstances of the events (including data on onset, prodromal symptoms, and seizures) were corroborated by the study coordinators through the patient’s medical files and interviews with individuals about themselves or about family members, and categorized by the study specialists using pre-specified codes.
Statistical analysis
The clinical characteristics of study subjects were compared between the 2 LQTS genotypes with the χ2 test and Fisher exact test for categorical variables and the t test or Mann-Whitney-Wilcoxon test for continuous variables.
To model recurrent syncope over time, a hybridization of the conditional model, proposed by Prentice, Williams and Peterson (PWP),17 combined with Andersen-Gill model,18 was used. Complex statistical methodology was employed to assess the effect of the menopausal periods on the risk of recurrent cardiac events since this type of analysis provides important incremental data to sole assessment of crude event rates over those time-periods (shown in Figures 1 and 2), including: 1) adjustment for important covariates in a multivariate model (such as QTc duration, medical therapy with beta-blockers, and acquired comorbities), thereby facilitating an assessment of the independent effect of each menopause stage on the clinical risk; 2) assessment of the statistical significance of the findings; 3) comparison of risk among women in a similar age-range (i.e. the PWP Anderson-Gill multivariate model compares the risk of post-menopausal women to that of women in the reproductive period with a similar age, whereas data regarding crude event rates do not take age into account); and 4) incorporation of data regarding the effect of the timing and frequency (intensity) of events during each menopausal stage on the clinical risk.
Figure 1.
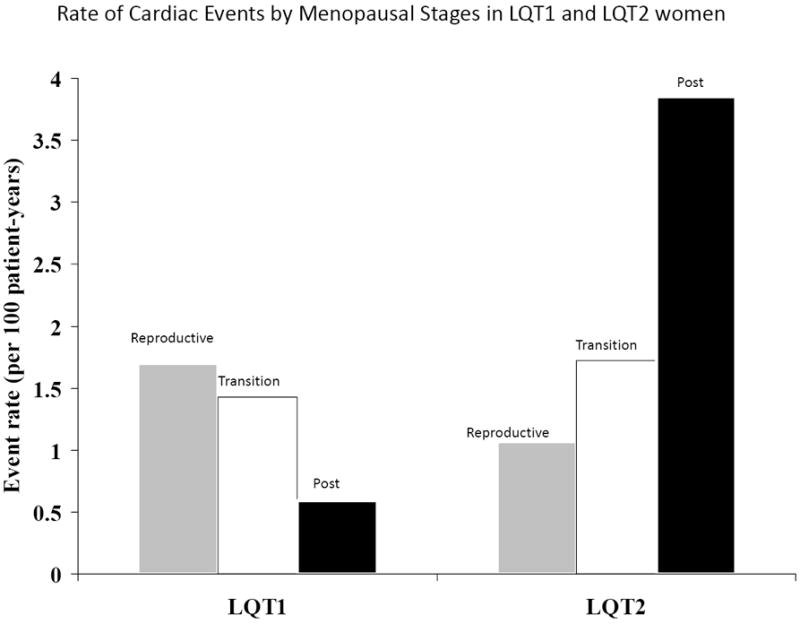
Rate (per 100 patient-years) of cardiac events of any type (comprising syncope, aborted cardiac arrest, or sudden cardiac death) during follow-up among LQT1 and LQT2 study subjects by menopausal period.
Event rates per 100 person-years among LQT1 and LQT2 women were calculated by dividing the number of events during each menopausal period by the follow-up time in the same period, and multiplying the result by 100.
Figure 2.
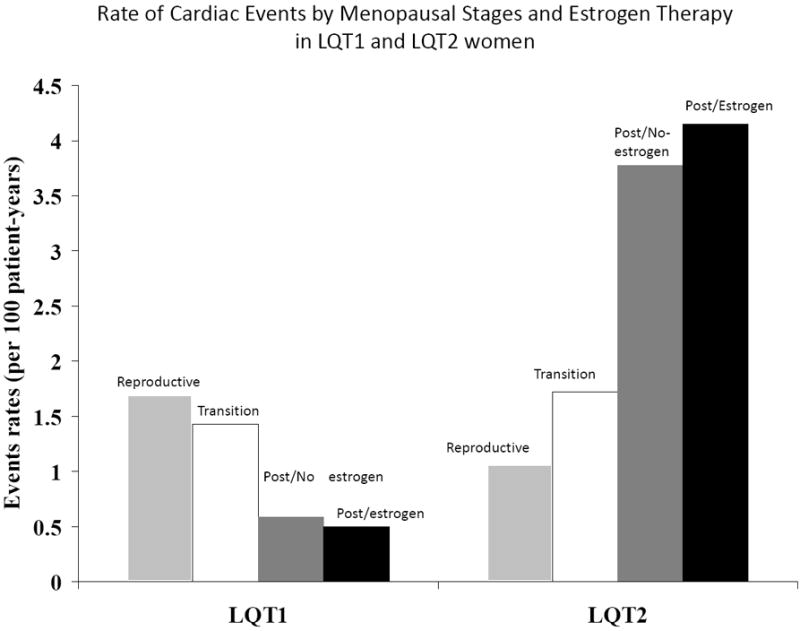
Rate (per 100 patient-years) of cardiac events of any type (comprising syncope, aborted cardiac arrest, or sudden cardiac death) during follow-up among LQT1 and LQT2 study subjects by menopausal period and by estrogen therapy during the postmenopausal period.
Event rates per 100 person-years among LQT1 and LQT2 women were calculated by dividing the number of events each category by the follow-up time in the same category, and multiplying the result by 100.
A separate stratum was used for the first and second syncope, as consistent with the conditional approach, while subsequent events were modeled using the independence approach of Andersen-Gill. The combined approach was employed due to the relatively low number of events after the second syncope stratum, as illustrated by Therneau and Grambsch in similar cases.19 Pre-specified covariates in the multivariate models included the menopausal periods (assessed as time-dependent covariates, with the reproductive period as the reference group for the transition- and post-menopausal- periods), QTc (assessed from the first ECG recorded after age 30 years), and beta-blocker therapy (modeled as a time-dependent covariate). The results were also validated in an additional analysis that included further adjustment for acquired comorbidties (including diabetes mellitus, smoking status, myocardial infarction, and the presence of angina pectoris, all assessed as time-dependent covariates). Due to a relatively short follow-up time and number of events (n=5) after age 52 years among women who did not develop menopause by this age (upper quartile of menopause age among study patients), this patient subset was not included in the primary multivariate models. To validate the consistency of our findings across all menopause ages, we carried out a secondary analysis in the total population which was further stratified by the age at onset of menopause (<52 years vs. ≥52 years).
The risk for recurrent syncope during the post-menopausal period among women who did or did not receive estrogen therapy was evaluated by including post-menopausal estrogen therapy as a time-dependent covariate in the multivariate models. The benefit of beta-blocker therapy in reducing the risk of recurrent syncope within each menopausal period was assessed using interaction-term analysis.
The main analyses reported in this study were carried out in separate models for LQT1 and LQT2 women. A total population model, employing a menopause period-by-genotype interaction-term, was used to assess the difference in menopause-related risk between the 2 genotypes.
The effect of lack of independence, both because of multiple events being contributed by subjects and due to the inherited nature of menopause age, was adjusted for by using the robust sandwich covariance estimate of Lin and Weil.19 The statistical software used for the analyses was SAS version 9.20 (SAS Institute Inc, Cary, NC). A 2-sided 0.05 significance level was used for hypothesis testing.
Results
The clinical characteristics of the study subjects by genotype are presented in Table 1. No statistically significant differences between LQT1 and LQT2 women existed regarding baseline QTc, age at onset of menopause, usage of estrogen therapy after menopause, or non-LQTS-related comorbidties. Cardiac events during follow-up were dominated by recurrent episodes of syncope (n=150), whereas only 22 study patients (8%) experienced ACA or SCD during follow-up. The overall number of syncope episodes during follow-up was somewhat higher among LQT2 as compared with LQT1 women (Table 1).
Menopause onset and recurrent syncope
Multivariate analysis showed that the onset of menopause was associated with a significant increase in the risk of recurrent syncope in LQT2 women (Table 2). Women with the LQT2 genotype had more than a 3-fold (HR = 3.38 [p = 0.005]) increase in the risk of recurrent syncope during the menopause-transition period and an 8-fold (HR = 8.10 [p < 0.001]) risk increase during the post-menopausal period as compared with women in the same age-group who were in the reproductive period (Table 2). In contrast, among LQT1 women, the risk of recurrent syncope was not significantly changed during the menopause-transition period, and was reduced (HR = 0.19 [p = 0.05]) after the onset of menopause (Table 2). Notably, interaction-term analysis in a total population model showed a statistically significant difference in the risk related to the onset of menopause between LQT1 and LQT2 women (p-value for post-menopause-by-genotype interaction = 0.02). Due to the relatively short follow-up time and a corresponding low event rate after age 52 year among women who not develop menopause by this age, this patient subset was not included in the models of the primary analyses. However, a secondary analysis in the total population that was further stratified by the age at onset of menopause, yielded consistent results in both LQT1 patients (menopause-transition vs. reproductive period: HR = 0.82 [95% CI 0.29-2.33]; post-menopause vs. reproductive period: HR = 0.22 [95% CI 0.04-1.01]) and LQT2 patients (menopause-transition vs. reproductive period: HR = 3.45 [95%CI 1.75-6.85]; post-menopause vs. reproductive period: HR = 5.38 [95% CI 2.02-16.78]).
Table 2.
Multivariate analysis: effect of menopause phase on the risk of recurrent syncope in LQT1 and LQT2 women*
| MENOPAUSAL PERIOD† | LQT1 WOMEN | LQT2 WOMEN | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P value | Hazard Ratio (95% Confidence Interval) | P value | |
| Menopause-transition period vs. reproductive period | 0.66 (0.19 - 2.21) | 0.50 | 3.38 (1.44 - 7.94) | 0.005 |
| Post-menopause period vs. reproductive period | 0.19 (0.03 - 1.02) | 0.05 | 8.10 (2.45 - 26.80) | <0.001 |
Multivariate analysis was carried out using combined PWP and Anderson-Gill modeling, with the menopausal periods assessed as time-dependent covariates in the multivariate models; findings are adjusted time-dependent beta-blocker therapy and QTc (assessed as a continuous measure); similar results were obtained in a secondary analysis that included further adjustment for time-dependent comorbidities (including diabetes mellitus, smoking status, prior myocardial infarction, and the presence of angina pectoris).
Menopause-transition is defined as the 5-year time period prior to the reported age at onset of menopause; the post-menopausal period is defined as 5-year time period following the reported age at onset of menopause; the reproductive period is defined as the follow time beginning at age 30 years and ending at the onset of the menopause-transition period.
The risk increase associated with the post-menopausal period among LQT2 women was evident among those who either were or were not treated with estrogen therapy (Table 3). Thus, LQT2 women who were not treated with estrogen therapy after menopause experienced nearly an 8-fold (HR = 7.73 [p < 0.001]) increase in the risk of recurrent syncope during the post-menopausal period compared with the reproductive period, and women who received estrogen therapy experienced more than a 5-fold risk increase (HR = 5.10 [p < 0.001]). In contrast, LQT1 women showed a non-significant reduction in the risk of recurrent syncope after the onset of menopause regardless of treatment with estrogen therapy (Table 3).
Table 3.
Multivariate analysis: effect of therapies during follow-up on the risk of recurrent syncope in LQT1 and LQT2 women*
| MENOPAUSAL PHASE† | LQT1 WOMEN | LQT2 WOMEN | ||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P value | Hazard Ratio (95% Confidence Interval) | P value | |
| Post-menopause vs. pre-menopause risk by treatment with estrogen therapy† | ||||
|
Post-menopause without estrogen HRT vs. reproductive period |
0.20 (0.02 - 2.08) |
0.18 | 7.73 (2.22 - 26.93) |
<0.001 |
|
Post-menopause with estrogen HRT vs. reproductive period |
0.46 (0.07 - 3.07) |
0.42 | 5.10 (1.62-15.97) |
<0.001 |
| Beta-blocker effect† | ||||
| Beta-blocker- vs, no beta-blocker-therapy | 0.40 (0.17 - 0.87) |
0.02 | 0.51 (0.29 - 0.89) |
0.02 |
Multivariate analysis was carried out using combined PWP and Anderson-Gill modeling, with the menopausal periods and therapies assessed as time-dependent covariates in the multivariate models; findings are adjusted time-dependent beta-blocker therapy and QTc (assessed as a continuous measure); similar results were obtained in a secondary analysis that included further adjustment for time-dependent comorbidities (including diabetes mellitus, smoking status, prior myocardial infarction, and the presence of angina pectoris).
Estrogen therapy during the postmenopausal phase was assessed as a time-dependent covariate; the effects of progesterone therapy and of estrogen therapy during the transitional phases were not assessed due to sample size limitations.
Time-dependent beta-blocker therapy was shown to be associated with significant reduction in the risk of recurrent syncope among both LQT1 and LQT2 women (HR = 0.40 and 0.51, respectively [p = 0.02 for both]; Table 3). The benefit of beta-blocker therapy in LQT2 women was not significantly different among the reproductive, menopause-transition, and post-menopausal periods (all p-values for menopause period-by-beta blocker therapy interactions >0.10), suggesting continued efficacy for this mode of medical therapy after the onset of menopause.
Menopause onset and cardiac events of any type
Consistent with the results from the multivariate models that assessed the primary end point of recurrent syncope among study patients, analysis of the rate of cardiac events of any type during follow-up (comprising syncope, ACA or SCD) showed that among LQT2 women cardiac event rate was lowest during the reproductive period (1.1 per 100 patient-years), intermediate during the menopause-transition period (1.7 per 100 patient-years), and highest during the post-menopause period (3.9 per 100 patients years), whereas LQT1 women experienced a reduction in event rates after the reproductive period (Fig. 1). Furthermore, analysis of the effect of estrogen therapy on the rate of cardiac events (Fig. 2) showed that the onset of menopause was associated with an increase in the rate of cardiac events among LQT2 women who did or did not receive estrogen therapy (4.2 and 3.8 events per 100 patient-years, respectively) as compared with the reproductive and menopause-transition periods (1.1 and 1.7 events per 100 patient-years, respectively), whereas LQT1 women experienced a reduction in the rate of cardiac events after the onset of menopause regardless of treatment with estrogen therapy (Fig. 2).
Menopause onset and life-threatening cardiac events
The frequency of life-threatening cardiac events (comprising ACA or SCD) was similar between LQT1 and LQT2 women (Table 1). However, when the number of life-threatening cardiac events was related to the onset of menopause a similar pattern was identified, showing a reduction in the number of life-threatening events among the evaluated time-periods in LQT1 patients (5, 4, and 2 events during the reproductive, menopause-transition, and postmenopausal periods, respectively) and an increase in LQT2 patients (2, 3, and 6 events during the respective time-periods). Due to the relatively small number of life-threatening cardiac events during follow-up (n=22) this end point was not assessed using multivariate modeling.
Discussion
The present study is the first to assess the clinical course of women with congenital long-QT syndrome after the onset of menopause. Our findings suggest several important clinical implications regarding risk assessment and management of women with this inherited cardiac disorder in the older age-group: 1) the peri-menopausal period (comprising the 5-year time periods prior to- and following- the age at onset of menopause) is associated with a pronounced increase in the risk for recurrent episodes of syncope among women with genetically-confirmed LQT2; 2) menopausal status has an opposite effect on the risk of LQT1 women; 3) the effect of menopause on the clinical course of LQTS women is independent of treatment with estrogen therapy; and 4) beta-blocker therapy is associated with a significant reduction in the risk of recurrent syncope in this population, and should therefore be continued in all women with the LQT2 genotype (without contraindications) even after the onset of menopause. It should be noted that syncope accounted for 85% and 89% of the total number of cardiac events experienced by LQT1 and LQT2 women, respectively, suggesting that the risk related to the onset of menopause is dominated by nonfatal syncopal events.
Effect of gonadal hormones on cardiac ion channel activity related to LQTS types 1 and 2
Progesterone and estrogen are lipophilic gonadal steroid hormones, whose effects are derived mainly from long acting transcriptional regulation on nuclear receptors referred to as genomic effects. Progesterone was shown to have a short acting (i.e. non-genomic) effect on shortening the action potential duration and to provide protection against arrhythmias through modulation of slowly-activating delayed rectifier potassium currents (IKs) and L-type calcium currents (ICa,L) by a pathway that involves progesterone receptors.11 However, studies that examined the genomic effects of progesterone on the cardiac ion channels did not identify a similar long-term effect.20,21 Compared to progesterone, estrogen was shown to exhibit both acute12 and genomic effects on the Ikr channel, including reduction in channel expression and prolongation of ventricular repolarization.20,21 A large-scale clinical study of post-menopausal women treated with hormone therapy revealed a significant QTc prolongation with unopposed estrogen therapy, compared to no effect on the QTc interval with combined estrogen-progesterone therapy or with no therapy.22 Notably, recent data suggest that the long-term mechanism related to the effect estrogen on IKr regulation by is receptor-independent,23,24 and therefore may not fall into the classic definition of “genomic derived”effect. These long-term estrogen effects provide a possible explanation for the susceptibility of females to drug-induced QT prolongation and arrhythmias25,26 and for the increase in the risk for cardiac events that occurs in LQT2 women after the onset of adolescence.4 Hormonal changes may also explain the increase in the risk that was observed among LQT2 women during the postpartum period, 6 since several studies in which serum measurements of gonadal hormones were obtained at the postpartum era indicate higher estrogen levels at this time period, although this pattern is more evident among lactating mothers.27-29
Testosterone, the male steroid hormone generally derived in women from peripheral intracrine conversion of dehydroepiandrosterone (DHEA), has also been shown to exert a substantial impact on ventricular repolarization and action potential duration through nontranscriptional modulation of the IKr channel.30,31 Chronic testosterone exposure has been shown to shorten action potential duration and ventricular repolarization in several studies32-34 and to diminish the risk of early afterdepolarizations associated with dofetolide treatment in other animal models.35 Accordingly, the QTc interval, while similar in male and female children, has been shown to shorten in males after the onset of adolescence while remaining close to its preadolescent value in women.36 These androgen-related effects may also explain the higher risk observed among LQT2 women as compared with men after the onset of adolescence.4
Peri-menopausal changes in gonadal hormones levels and the risk of cardiac events in LQTS types 1 and 2
As FSH levels start to rise at the 4-5 year period prior to menopause, both estrogen and progesterone levels decline, until reaching a minimum after menopause has occurred.37 While estrogen decline is characterized by a fluctuation pattern, with frequent level changes until reaching its nadir, progesterone decline is steadier.37 A steady and significant decline also takes place in testosterone and dehydrotestosterone (DHT) levels during and after menopause, with smaller changes observed after the age of 60 years.38 After the onset of menopause, adipose tissue becomes quantitatively the most important site of aromatization of androgens to estrogens and thus the main source of extraglandular estrogen synthesis.39,40 These adipose tissue-based estrogens were found to correlate with plasma levels in post-menopausal women.41 Thus, a new and complex hormonal status-quo is established after menopause at which progesterone and androgen levels are almost non-measurable, whereas estrogen levels are low, yet measurable, and more so for women with larger adipose tissues. Based on these physiologic changes, our findings that both the hormonal fluctuation associated with the menopause-transition period and the post-menopausal hormonal decline pose a significant risk in LQT2 women are not surprising. This may occur because low (yet measurable) levels of estrogen prolong ventricular repolarization through inhibition of IKr while at the same time the IKs and Ica,l mediated protective effects of progesterone and the IKr mediated protective effects of testosterone are diminished. In this regard, the possible role of reduced androgen levels in mediating arrhythmic risk should be emphasized, since in the present study the onset of menopause was associated with a pronounced risk increase in LQT2 women regardless of supplemental usage of estrogen therapy.
Our findings are also consistent with prior studies that showed an increase in the risk for drug-induced torsade de pointes (mediated through inhibition of the IKr channel also affected in the LQT2 syndrome carriers), after the onset of menopause,6 further suggesting that changes in sex hormones following the onset of menopause affect arrhythmic risk in LQT2 patients. In contrast, menopause was not associated with an increase in the risk for cardiac events among LQT1 women since carriers of this genotype harbor mutations that affect IKs, whereas the estrogen and diminished testosterone levels in menopause exert their main QT-prolonging effects through suppression of IKr and loss of IKr enhancement, respectively.
It is also possible that differences in the risk related to the onset of menopause between LQT1 and LQT2 women may be related to the different mode of onset of torsade de pointes between the 2 genotypes. The onset of arrhythmic events in LQTS was shown to be genotype-specific, being predominantly pause-dependent in LQT2, and rarely associated with a pause in LQT1.42,43 Thus, the possible increased occurrence of ventricular extra-systolic beats (and the associated extrasystolic pauses) during the peri-menopausal period is likely to trigger arrhythmias more frequently in LQT2 than in LQT1 women.
Study limitations
Although the onset of menopause is known to be associated with declining estrogen, progesterone, and androgen levels, these levels may fluctuate significantly,37,39 and thus the exact hormonal status for each patient could only be established by hormonal blood levels, which were not obtained for this study. Increase in rate of cardiac events after menopause may be related to acquired comorbities in an older population. To reduce possible age-related bias we carried multivariate models that included further adjustments for acquired comorbidties and stratified the analyses by age. Furthermore, menopause-related risk was shown to be significantly different between LQT1 and LQT2 women, suggesting that the association between menopause and the risk of cardiac events among women with LQT2 genotype is independent of an aging effect.
The primary analysis in the present study focused on the risk for recurrent syncope, which may also comprise events that are nonarrhythmic in nature. To minimize the bias associated with this limitation, syncopal events were categorized by the study specialists. Thus, non-abrupt events associated with prodromal symptoms of dizziness or lightheadedness (comprising 21% of the total events experienced by women during follow-up) and those associated with seizures (comprising 4% of the total events experienced by women during follow-up) were not included in the present analysis. Despite this, it is possible that some events that were assessed in the present study were non-arrhythmic in nature since 33% of neurocardiogenic syncopal events may occur without prodromal symptoms, especially in older adults 44 Furthermore, in the older age group even more severe cardiac events (including ACA or SCD) may not be LQTS-related.45 It should be noted, however, that for this end point a similar genotype-specific pattern was identified, showing a reduction in the number of life-threatening events among the evaluated time periods in LQT1 women and an increase in the number of life-threatening events in LQT2 women. Thus, the fact that the 3 end points that were assessed in the present study (including recurrent syncope, cardiac events of any type, and ACA/SCD) consistently showed an opposite genotype-specific association with menopause strongly suggests that menopause onset affects the risk for arrhythmic events in this population.
Follow-up time after age 52 years of women who did not experienced the onset of menopause by this age was very limited. Therefore, women with a menopause age of ≥52 years were not included in the primary analyses. The consistent results that were obtained in the secondary total population models suggest that the genotype-specific effect of menopause on the risk of cardiac events in LQTS patients is independent of the age at onset of menopause.
Conclusions and clinical implications
The current study extends prior observation regarding the pivotal effect of sex steroidal hormones on the phenotypic expression of LQTS. We have shown that the clinical course of LQTS women during the peri-menopausal period is related to the type of the affected cardiac ion channel. Thus, LQT2 women (having mutations that affect IKr) were shown to have a pronounced increase in the risk for recurrent episodes of syncope after the onset of menopause, whereas among LQT1 women (having mutations that affect IKs) the risk of recurrent syncope was reduced during the same time period. These findings suggest that a genotype-specific approach should be utilized for risk assessment and management of LQTS women even after the onset of menopause.
Clinical Summary.
Prior studies have shown that women with congenital long-QT syndrome (LQTS) experience increased risk for cardiac events after the onset of adolescence and during the postpartum period. This risk increase was shown to be more pronounced among women with the LQT2 genotype, suggesting that sex hormones may modify the clinical course of patients with this inherited arrhythmic disorder. The present study is the first to assess the clinical course of LQTS women after the onset of menopause. We show a genotype-specific association with the risk for cardiac events during the peri-menopausal period, including a pronounced increase in the risk for cardiac events (dominated by recurrent episodes of syncope) among LQT2 women and an opposite reduction in the rate of cardiac events in LQT1 women. Notably, the pronounced effect of menopause on the clinical course of LQTS women was independent of administration of estrogen therapy. The study also shows that beta-blocker therapy is associated with a significant reduction in the risk of recurrent episodes of syncope in LQTS women during this time-period, supporting the continued usage of this mode of medical therapy in all women with the LQT2 genotype (without contraindications) even after the onset of menopause. Thus, the present findings suggest that a genotype-specific approach should be utilized for risk assessment and management of LQTS women even after the onset of menopause.
Acknowledgments
Funding/Support: This work was supported by research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, Md and by a research grant from GeneDx to the Heart Research Follow-Up Program in support of the LQTS Registry.
Footnotes
Disclosures: None
References
- 1.Schwartz PJ, Crotti L. Long QT and short QT syndromes. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: From Cell to Bedside. 5. Philadelphia, Pa: Elsevier/saunders; 2009. pp. 731–744. [Google Scholar]
- 2.Vincent GM. The long QT and Brugada syndromes: Causes of unexpected syncope and sudden cardiac death in children and young adults. Semin Pediatr Neurol. 2005;1:15–24. doi: 10.1016/j.spen.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, Andrews M, Timothy K, Hall WJ. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 4.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A. International Long QT Syndrome Registry. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 5.Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, Robinson JL, Goldenberg I, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT Syndrome and Pregnancy. J Am Coll Cardiol. 2007;49:1092–1098. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann MH, Hardy S, Archibald D, quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation. 1996;94:2535–2541. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 7.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 8.Drici MD, Knollmann BC, Wang WX, Woosley RL. Cardiac actions of erythromycin: influence of female sex. JAMA. 1998;280:1774–1776. doi: 10.1001/jama.280.20.1774. [DOI] [PubMed] [Google Scholar]
- 9.James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94:265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, Zhu ZI, Clancy CE, Isobe M, Furukawa T. Progesterone regulates cardiac repolarization through a nongenomic pathway: An in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–2922. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa J, Tamagawa M, Harada N, Honda S, Bai CX, Nakaya H, Furukawa T. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586:2961–2973. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A., Jr The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 14.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–67. [Google Scholar]
- 15.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive Summary: stages of reproductive aging workshop (STRAW) Climacteric. 2001;4:267–272. [PubMed] [Google Scholar]
- 16.North American Menopause Society. [July 23rd, 2010];Menopause Guidebook. (6). http://www.menopause.org/MGI.pdf.
- 17.Prentice RL, Williams BJ, Peterson AV. On the regression-analysis of multivariate failure time data. Biometrika. 1981;68:373–379. [Google Scholar]
- 18.Andersen PK, Gill RD. Cox’s regression for counting processes: A large sample study. The Annals of Statistics. 1982;10:1100–1120. [Google Scholar]
- 19.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 20.Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- 21.Helguera G, Olcese R, Song M, Toro L, Stefani E. Tissue specific regulation of Ca2+ channel protein expression by sex hormones. Biochim Biophys Acta. 2002;1569:59–66. doi: 10.1016/s0304-4165(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 22.Kadish AH, Greenland P, Limacher MC, Frishman WH, Daugherty SA, Schwartz JB. Estrogen and progestin use and the QT interval in postmenopausal women. Ann Noninvasive Electrocardiol. 2004;9:366–374. doi: 10.1111/j.1542-474X.2004.94580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanguinetti MC, Mitcheson JS. Predicting drug-hERG channel interactions that cause acquired long QT syndrome. Trends Pharmacol Sci. 2005;26:119–124. doi: 10.1016/j.tips.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Clancy CE, Kurokawa J, Tateyama M, Wehrens XH, Kass RS. K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annu Rev Pharmacol Toxicol. 2003;43:441–461. doi: 10.1146/annurev.pharmtox.43.100901.140245. [DOI] [PubMed] [Google Scholar]
- 25.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 26.Hulot JS, Démolis JL, Rivière R, Strabach S, Christin-Maitre S, Funck-Brentano C. Influence of endogenous oestrogens on QT interval duration. Eur Heart J. 2003;24:1663–1667. doi: 10.1016/s0195-668x(03)00436-6. [DOI] [PubMed] [Google Scholar]
- 27.Fan F, Zou Y, Ma A, Yue Y, Mao W, Ma X. Hormonal changes and somatopsycologic manifestations in the first trimester of pregnancy and post partum. International Journal of Gynecology and Obstetrics. 2009;105:46–49. doi: 10.1016/j.ijgo.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Lawrie TA, Hofmeyr GJ, De Jager M, Berk M, Paiker J, Viljoen E. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 1998;105:1082–1090. doi: 10.1111/j.1471-0528.1998.tb09940.x. [DOI] [PubMed] [Google Scholar]
- 29.Delvoye P, Demaegd M, Uwayitu-Nyampeta, Robyn C. Serum prolactin, gonadotropins, and estradiol in menstruating and amenorrheic mothers during two years’ lactation. Am J Obstet Gynecol. 1978;130:635–639. doi: 10.1016/0002-9378(78)90319-8. [DOI] [PubMed] [Google Scholar]
- 30.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, Bertran G, Arini P, Biagetti MO, Quinteiro RA. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 32.Hara M, DaniloM P, Jr, Rosen MR. Effects of gonadal steroids on ventricular repolarization and on the response to E4031. J Pharmacol Exp Ther. 1998;285:1068–1072. [PubMed] [Google Scholar]
- 33.Brouillette J, Trépanier-Boulay C, Fiset C. Effect of androgen deficiency on mouse ventricular repolarization. J Physiol. 2003;546:403–413. doi: 10.1113/jphysiol.2002.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley JM, Shuba YM, James AF, Hancox JC. Modulation by testosterone of an endogenous hERG potassium channel current. J Physiol Pharmacol. 2008;59:395–407. [PubMed] [Google Scholar]
- 35.Pham TV, Sosunov EA, Anyukhovsky EP, Danilo P, Jr, Rosen MR. Testosterone diminishes the proarrhythmic effects of dofetilide in normal female rabbits. Circulation. 2002;106:2132–2136. doi: 10.1161/01.cir.0000033596.21845.d8. [DOI] [PubMed] [Google Scholar]
- 36.Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, Davignon A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–5. [PubMed] [Google Scholar]
- 37.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 38.Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 39.Nevton CJ, Samuel DL, James VHT. Aromatase activity and concentrations of cortisol, progesterone and testosterone in breast and abdominal adipose tissue. J Steroid Biochem. 1986;24:1033–1039. doi: 10.1016/0022-4731(86)90356-0. [DOI] [PubMed] [Google Scholar]
- 40.Folkered EJ, James VHT. Aromatization of steroids in peripheral tissues. J Steroid Biochem. 1983;19:687–690. doi: 10.1016/0022-4731(83)90236-4. [DOI] [PubMed] [Google Scholar]
- 41.Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–321. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 42.Viskin S, Alla SR, Barron HV, Heller K, Saxon L, Kitzis I, Hare GF, Wong MJ, Lesh MD, Scheinman MM. Mode of onset of torsade de pointes in congenital long QT syndrome. J Am Coll Cardiol. 1996;28:1262–1268. doi: 10.1016/s0735-1097(96)00311-7. [DOI] [PubMed] [Google Scholar]
- 43.Tan HL. Genotype-specific onset of arrhythmias in congenital long-QT syndrome: possible therapy implications. Circulation. 2006;114:2096–2103. doi: 10.1161/CIRCULATIONAHA.106.642694. [DOI] [PubMed] [Google Scholar]
- 44.Grubb BP. Neurocardiogenic syncope. N Engl J Med. 2005;352:1004–1010. doi: 10.1056/NEJMcp042601. [DOI] [PubMed] [Google Scholar]
- 45.Goldenberg I, Moss AJ, Peterson DR, Bradley J, Polonski S, Petersom DE, McNitt S, Zareba W, Andrews ML, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Napolitano C, Priori SG, Qi M, Schwartz PJ, Towbin JA, G Vincent M, Zhang L. Long QT syndrome after age 40. Circulation. 2008;117:2192–2201. doi: 10.1161/CIRCULATIONAHA.107.729368. [DOI] [PubMed] [Google Scholar]


