Abstract
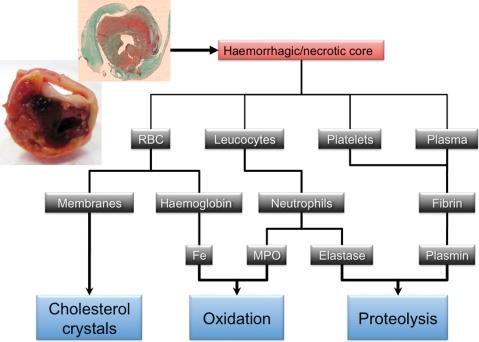
Atherothrombosis remains one of the main causes of morbidity and mortality in the western countries. Human atherothrombotic disease begins early in life in relation to circulating lipid retention in the inner vascular wall. Risk factors enhance the progression towards clinical expression: dyslipidaemia, diabetes, smoking, hypertension, ageing, etc. The evolution from the initial lipid retention in the arterial wall to clinical events is a continuum of increasingly complex biological processes. Current strategies to fight the consequences of atherothrombosis are orientated either towards the promotion of a healthy life style1 and preventive treatment of risk factors, or towards late interventional strategies.2 Despite this therapeutic arsenal, the incidence of clinical events remains dramatically high,3 dependent, at least in part, on the increasing frequency of type 2 diabetes and ageing. But some medical treatments, focusing only on prevention of the metabolic risk, have failed to reduce cardiovascular mortality, thus illustrating that our understanding of the pathophysiology of human atherothrombosis leading to clinical events remain incomplete. New paradigms are now emerging which may give rise to novel experimental strategies to improve therapeutic efficacy and prediction of disease progression. Recent studies strengthen the concept that the intraplaque neovascularization and bleeding (Figure 1, upper panel) are events that could play a major role in plaque progression and leucocyte infiltration, and may also serve as a measure of risk for the development of future events. The recent advances in our understanding of IntraPlaque Hemorrhage as a critical event in triggering acute clinical events have important implications for clinical research and possibly future clinical practice.
Keywords: Angiogenesis, Cholesterol crystals, Haemoglobin, Protease, Adventitial tertiary lymphoid organs, Diabetes, Atherothrombosis
Early observational studies
Vulnerable plaques are characterized by the retention of highly modified, heterogeneous biomaterials within the core of the lesion. This so-called ‘necrotic core’ is encapsulated between the luminal fibrous cap and the outer intima and remaining media (‘intramural atheromatous abscess’ described by T. Leary in 1934).4,5 The core includes components of different ages, cholesterol crystals, frequent calcific nodules, and a more or less identifiable fibrin-rich haemorrhages, highlighting the discontinuous evolution of the plaque from the initial esterified lipid retention to the formation of a more complex necrotic core, potentially responsible for plaque instability and complications.5
The complex nature of the necrotic core in plaques was first suggested by Galien (131 to 201 CE) in his initial description of human atheroma (αθηρωμα, atheroma = gruel). The involvement of the repeated accumulation of haemoglobin-rich intraplaque thrombi in the evolution of the lesions toward complications was proposed as early as 1936.6 In these initial observational studies of human pathology, Paterson7 and Wartman8 described intraplaque haemorrhages caused by neo-capillary rupture (Figure 2, left panel) and claimed that fibrin-rich intraplaque haemorrhages are the common precipitating cause of arterial lumen thrombosis. After this initial period, the clinical and biological importance of intraplaque haemorrhages became rather neglected, and the majority of biological studies focused on lipid metabolism and on the inflammatory response mainly represented by leucocyte extravasation observed within the complicated plaque.9
Figure 2.
Histology of intraplaque haemorrhage (A: Masson's trichrome, mosaic reconstituted section, ×2.5) showing the presence of intact RBCs and free haemoglobin (inset, ×20). (B) Iron visualized by Prussian blue (Perl's) stain with nuclear red counterstaining, showing the haemoglobin-dependent presence of iron, mainly localized in the vicinity of neocapillaries and within phagocytic cells (insets, ×20 and ×40, arrow).
Histopathological description
Histopathological studies are limited by the difficulties of precisely dating fibrin-rich intraplaque haemorrhages by histological examination due to the detersion of haemorrhagic products by phagocytes and progressive oxidative and proteolytic transformation. Virmani and Roberts10 reported that the frequency of intraplaque, erythrocyte extravasation, and the presence of iron and fibrin were proportional to the number of atherothrombotic plaques present. Moreover, intraplaque iron and fibrin were mainly present in association with extravasated erythrocytes, suggesting that all the blood components of haemorrhages are present within the plaque, including plasma and cellular components. Recently, intraplaque haemorrhages have been observed macroscopically by the presence of red liquid in plaques (Figure 1, upper panel), identified microscopically by the presence of more or less intact erythrocytes (Figure 3) in the core of the plaque, or indirectly characterized by the presence of free haemoglobin, and iron staining with Prussian blue, observed as haemosiderin present in phagocytic cells (Figure 2, right panel). In the absence of intact erythrocytes, the presence of a large amount of iron provides evidence of older haemorrhages. Erythrocyte ‘ghosts’ can also be identified by the presence of glycophorin, an abundant antigenic protein of the erythrocyte membrane, detectable by immunohistochemistry11 (Figure 3D). A recent autopsy study observed a higher density of neovessels in non-stenotic, human coronary plaques, which correlated with the presence of iron and glycophorin.12
Figure 1.
Macroscopic view and schematic representation of the detrimental consequences of intraplaque haemorrhages on plaque biology and stability.
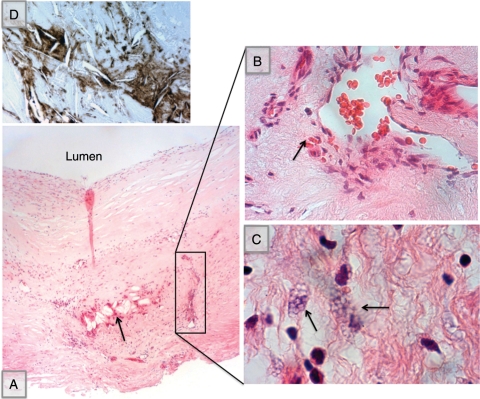
Figure 3.
Centripetal angiogenesis and erythrodiapedesis in early stages of human atheroma. (A) General view of an asymptomatic lesion in human common carotid artery showing both cholesterol crystal clefts (arrow) and centripetal angiogenesis (square) (haematoxylin/eosin, HE ×4); (B) enlarged view of the neovessel showing the presence of RBCs within the circulating lumen, and erythrodiapedesis outside the vessel (arrow, HE ×40); (C) erythrophagocytosis (entosis) evidenced by the presence of RBC skeletons within a phagocyte (arrow, HE ×40); (D) enlargement of cholesterol crystal clefts surrounded by immuno-staining of glycophorin A (brown, ×40).
Usually, intraplaque haemorrhages are associated with a high density of phagocytic cells (CD 68+) involved in RBC and iron phagocytosis (Figure 3C and Figure 4, left panel). The clotting process takes place rapidly following intraplaque haemorrhage, involving platelet and thrombin activation and fibrin formation (Figure 4, right panel).
Figure 4.
(Left panel) Immunostaining of phagocytes (CD 68) in the border area of the necrotic core (mosaic reconstituted section, ×2.5) showing CD 68+ smooth muscle-like cells (left inset, ×20) and phagocytosed RBCs (right inset, ×40). (Right panel) Immunostaining of platelets (C41) showing an important enrichment of the core by the platelet membrane marker (mosaic reconstituted section, ×2.5), particularly in the shoulder area, near the neovessels (×20). Thrombin immunostaining and phosphotungstic acid haematoxylin (PTAH) staining of fibrin are diffuse showing the fibrin-rich nature of intraplaque haemorrhages (×20).
From intraplaque haemorrhages to clinical expression
Numerous clinical histopathological studies have been published since 1979, exploring the relationship between intraplaque haemorrhages in carotid endarteriectomy samples and clinical symptoms. In 1979, Imparato et al.13 established a relationship between the presence of intraplaque haemorrhages in carotid endarteriectomy samples and neurological symptoms in a series of 50 patients. Following this initial study, there have been reports of a strong or weak association between macroscopic or microscopic intraplaque haemorrhages14 and clinical events, which have been recently summarized.15 Other studies focused on the relationship between intraplaque haemorrhages and neovessel density,16,17 or on the prognostic value of carotid intraplaque haemorrhage as a predictor of global cardiovascular morbidity and mortality.18 This prognostic value of neovascularization and intraplaque haemorrhages has been recently emphasized through the Athero-Express biobank evaluation showing that local plaque haemorrhages and increased intraplaque neovessel density were independently related to cardiovascular outcome.19 This study is of specific interest since it raised the concept that locally observed increased plaque neovascularization or haemorrhages are associated with an increased risk of secondary events in other vascular territories, suggesting that the degree of plaque vascularization and bleeding in one site may reflect the situation in other vascular sites.
From centripetal neo-angiogenesis to intraplaque haemorrhages
The mechanisms of plaque enrichment by blood-borne components has been a matter of debate between those favouring repeated plaque fissuring and associated formation of a non-occlusive luminal thrombus which is then incorporated into the plaque20 and proponents of intraplaque haemorrhages being related to leakage from intra-plaque neo-capillaries.21 The fact that erythrocyte extravasation and intraplaque haemorrhages could be observed in relation to a high density of neocapillaries in the absence of plaque fissure provides evidence of the predominant role of the former. Nevertheless, incorporation of luminal thrombi cannot be entirely excluded, particularly in large arteries. For example, it has been reported that atheroma development in pulmonary artery hypertension is directly linked to the migration, adhesion, and subsequent incorporation of mobilized venous thrombi within pulmonary artery wall.22 Similarly, some incorporated luminal thrombi, unrelated to intraplaque haemorrhages, but resulting from plaque fissuring, can be observed in human aorta.
Intraplaque haemorrhages are mainly related to centripetal angiogenesis from the adventitia towards the plaque.23 This neo-angiogenesis takes place early in atheroma development and is related to lipid overload. Heistad et al.24 described an increased perfusion in the outer layer of the aorta of hypercholesterolaemic monkeys. Hypercholesterolaemia promotes the development of adventitial coronary vasa vasorum in a porcine model.25 In this model, coronary neovascularization development preceded hypercholesterolaemia-induced endothelial dysfunction,26 and may promote plaque progression.27 Conversely, hypercholesterolaemia is associated with an elevated plasma level of VEGF in humans and statins reduced both hypercholesterolaemia and plasma VEGF concentration.28 Centripetal neo-angiogenesis, erythrocyte extravasation, and erythrophagocytosis are early events in atheroma progression, usually not observed in association with fatty streaks, but constantly associated with the fibro-atheroma stage29 (Figure 4).
These neo-capillaries could allow diffusion of plasma-borne molecules30 and diapedesis of erythrocytes and leucocytes. Indeed, the density of intimal neo-capillaries correlated with the extent of necrotic core formation, intraplaque haemorrhage, haemosiderin deposits, and inflammatory infiltrates,31 suggesting that centripetal angiogenesis is a determinant of atherothrombotic evolution. Neo-vascularization has been reported in human plaques, whatever their localization: carotid arteries,17,32,33 coronary arteries,21 and aorta,34 all correlating with plaque evolution. Therefore, adventitial neo-angiogenesis appears to be linked to the evolution of atherosclerosis from the early stages towards complicated lesions. The driving force of centripetal angiogenesis is the transmural convection, from inside to outside, due to orthogonal hydraulic conductance, of soluble mediators, such as VEGF, from the wall to the adventitia.23
The immaturity of neo-vessels is the cause of intraplaque haemorrhages occurring in their vicinity.21 Indeed, neo-vessels in plaques appear to be leaky.35,36 When localized in plaques, microvessels are dysmorphic and lack surrounding α-actin-positive mural cells.31,37 It was recently observed that endothelial cells lining microvessels were abnormal, consisting of membrane blebs, vacuoles, open intercellular junctions, with a tendency to detachment.38 These studies suggest that neovessel immaturity is directly involved in leucocyte and erythrocyte extravasation and therefore in intraplaque haemorrhages. Since the angiopoietin system plays a role in vessel maturation/destabilization, the balance between angiopoietin-1 and angiopoietin-2 expression was explored in human plaques.39 A negative correlation was observed between angiopoietin-1 and microvascular density within the plaque, whereas the ratio angiopoietin-2/angiopoietin-1 was positively correlated to microvascular density. This imbalance has been linked to the proteolytic environment of the plaque, involved in the degradation of angiopoietin-1 and in Tie-2 receptor shedding.40 Angiopoietin-1 promotes endothelial cell barrier integrity, and imbalance between angiopoietin-1 and angiopoietin-2 is thought to play a role in brain arteriovenous malformations,41 which lead to recurrent cerebral haemorrhages. Also absence of pericyte recruitment and impaired vessel maturation is reported in myocardial ischaemia in diabetic mice with attenuation of Tie-2 and increase in angiopoietin-2 expressions.42 Lastly, haem/iron, released by free haemoglobin, is directly toxic for endothelial cells.43 Therefore, immaturity and fragility of vessels arising by intraplaque neo-angiogenesis may explain the commonly observed presence of erythrocyte extravasation and haemorrhages of different ages in one evolutive plaque14 and the recurrence of intraplaque haemorrhages after a first haemorrhagic event.44 Nevertheless, the further understandings the cellular and molecular events involved in centripetal angiogenesis and neovessel leakages remain an important scientific and medical challenge.
Biological consequences of intraplaque haemorrhages
Neoangiogenesis and its associated intraplaque haemorrhages convey into the lesion all the blood components, including red blood cells, leucocytes, platelets, and plasma proteins (Figure 1, lower panel). These different blood-borne components are implicated, to different degrees, in the biological processes involved in atherothrombosis progression, influencing mainly three predominant pathological aspects: cholesterol crystal production and retention, oxidant activities, and proteolytic activities within the lesion core.
Cholesterol crystal formation and retention
All stages of atherothrombosis are impacted by cholesterol, including cholesterol esters in the form of droplets, mainly conveyed by lipoproteins, and cholesterol crystals, composed of unesterified cholesterol, which usually remain localized within vessel wall (Figure 3). These crystals form the intracellular and extracellular clefts observed in histological section of fixed, paraffin-embedded tissues. The mobilization of cholesterol crystals from tissue into circulation is an infrequent but highly pathogenic phenomenon.45 Free cholesterol retention in cells and tissues, leading to monohydrate crystal formation, can originate from endocytosed cholesterol esters, hydrolyzed in phagolysosomes,46 or directly from free cholesterol of cell membranes. Membranes of circulating cells, including activated platelets,47,48 and probably dead leucocytes, can release free cholesterol. But the cholesterol content of erythrocyte membranes exceeds that of all other cells in the body, with lipids constituting 40% of their weight.49 Acyl coenzyme A:cholesterol acyltransferase inhibitors highly enhance cholesterol crystal formation by blocking cholesterol esterification and solubilization.50
The relationship between cholesterol crystals and erythrocyte membranes was first suggested by Arbustini et al.22 who demonstrated that atheroma observed in pulmonary hypertension is only observed in thromboembolic pulmonary hypertension, and that pultaceous cores with cholesterol clefts are co-localized with glycophorin A immunostaining. This observation was rapidly extended to coronary atherothrombotic plaques.51 The authors observed that cholesterol crystal clefts co-localized with glycophorin A surrounded by iron deposits in human coronary plaques and were correlated with the complexity of the plaque. They also demonstrated experimentally that injection of erythrocytes into the arterial wall of hypercholesterolaemic rabbits induced cholesterol crystal formation and iron deposits. This experimental model has now been reproduced for exploring the relationship between erythrocyte accumulation and oxidative stress.52,53 The pathogenicity of cholesterol crystals is linked to their ability to rupture biological membranes,54 to erode the thin cap,55 and to protrude within the lumen inducing luminal thrombosis56 and possibly embolism. Lastly, cholesterol crystals within vascular cells at the early stage of atheroma could trigger the inflammatory response. Duewell et al.57 and Rajamaki et al.58 recently reported that intracellular cholesterol crystals, as other forms of crystals (uric acid, silica crystals, etc.), are able to activate NLRP3 inflammasomes through phagolysosome damage, cytosolic cathepsin release, and to induce pro-IL-1β cleavage and active Il-1β release into the extracellular space. Therefore, arterial wall cholesterol content is associated with arterial thrombosis.59
Haemoglobin and oxidative enzymes
Erythrocytes of intraplaque haemorrhages convey and rapidly release large amounts of haemoglobin (Figure 2, left panel). It has been reported that hydroperoxydes,60 oxidized LDLs, and lipids extracted from human atheromatous plaques61 are able to provoke RBC lysis. Haemoglobin is composed of a globin protein core and iron-containing haem (red pigment). Lipids extracted from atheromatous plaques can also oxidize Fe++ haemoglobin to the more reactive Fe+++ haemoglobin.61 Fe+++ haem dissociates more easily from globin than Fe++, releasing highly deleterious, hydrophobic-free haem/iron.62 Haem/iron can mediate oxidative modification of lipids and cause endothelial cytotoxicity.43,63 Therefore, haem/iron considerably amplifies the oxidative capacity of the biological systems, including the formation of protein complexes of high molecular mass, participating in the formation of the ‘gruel’ in the necrotic core. The haemorrhage-dependent colocalization of CD163 (haemoglobin scavenger receptor) and 4-hydroxy-2-nonenal, a marker of oxidation in human unstable coronary plaques, has recently been reported.64 Histologically, Prussian blue (Perl's) staining usually reveals the presence of haemosiderin associated with phagocytes in the close vicinity of neocapillaries (Figure 2, right panel).
In parallel, haemorrhages convey neutrophils and mononuclear cells into the plaque, and leucocytes also extravasate from intraplaque neo-capillaries. Neutrophils are powerful pro-oxidative cells, due to their oxidative enzymes, NADPH oxidases, and myeloperoxidase (MPO), a haem protein abundantly expressed in neutrophils.65 However, these enzymes are not totally specific: macrophages66 also express MPO, but to a lesser extent than neutrophils.67
Conversely, in response to this iron-dependent oxidative environment, cells can counteract this oxidative injury through different anti-oxidative mechanisms operating at different stages of the process: RBC capture and entosis (cell dying as a result of becoming engulfed by a neighbouring cell) through a mechanism dependent on exposed phosphatidylserine,68 ligation of free haemoglobin by haptoglobin and phagocytosis by CD163,69 free haem and iron binding by haemopexin and endocytosis of the complex, and iron transport by ferritin. All these molecules limit the ability of free haemoglobin to generate oxidative stress. In particular, the haptoglobin genotype is a determinant of oxidative activity of free haemoglobin,70 and of iron content71 in human plaques.
Similarly, numerous cellular enzymatic or chelating molecules are present in complicated plaques and are involved in limiting the potential injury caused by the oxidative radicals generated. For example, thioredoxin has been reported to be more highly expressed in culprit coronary plaques than in stable plaques, in relation to intraplaque haemorrhages and iron deposits.72 Their deficits are involved in atherothrombosis acceleration.73
Blood-borne proteolytic activity
The role of the mural haemoglobin-rich thrombus as an important source of proteolytic activity in atherothrombosis was first documented in human abdominal aortic aneurysm (AAA), an atherothrombotic pathology in which proteolysis plays a predominant role.74 Similar blood-borne protease activities are generated during the evolution of intraplaque haemorrhages and play an important role in fibrous cap thinning and final rupture. Necrotic/haemorrhagic cores, associated with the risk of rupture, are characterized by fibrin deposits.75 Neutrophil density is also a hallmark of plaque complexity, linked to both intraplaque haemorrhages and microvessel density76 (Figure 5).
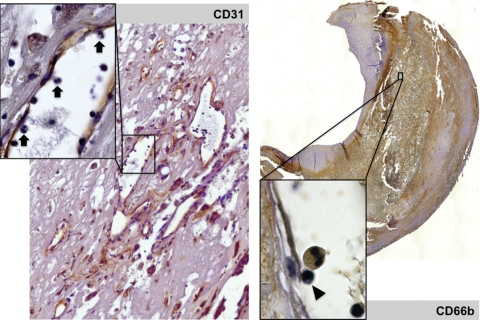
Figure 5.
(Left panel) Immunostaining of endothelium (CD 31, ×20) showing the abundance of neovessels, and the presence of rolling polymorphonuclear cells within these neo-venules (inset, ×40, arrows). (Right panel) Diffuse immunostaining of neutrophil membranes (CD 66b, mosaic reconstituted section, ×2.5) and presence of a rolling CD 66+ polymorphonuclear cell and a mononucleate cell (arrow head) in a neovessel (inset, ×100).
All the fibrinolytic activities, including t-PA, u-PA, and plasmin, are proportional to plaque complexity and are concentrated in the core of the lesion.77 Similarly, neutrophil gelatinase and serine protease activities are mainly conveyed by bleeding within culprit plaques.78 Beside their ability to degrade the extracellular matrix, these serine proteases can also degrade atheroprotective proteins secreted by smooth muscle cells, such as HSP.79,80 Therefore, proteases conveyed by intraplaque haemorrhages could participate in the formation of the necrotic core gruel, and be a determinant of fibrous plaque fissuring and rupture.
However, platelets and angiogenesis-driven macrophage extravasation could also convey factors of resistance to proteolysis, such as PAI-1 and protease nexin-1.81 Indeed, the role played by extravasated macrophages from neo-microvessels at the end-stage of plaque evolution is ambiguous, possibly involved more in detersion and healing the process, through M2 differentiation,82,83 than in plaque rupture.
Adventitial immune response
The role of inflammatory cells, including lymphocytes, and various molecules involved in atherothrombosis have been recently reviewed.84 The post-capillary venules are probably the predominant site of leucocyte diapedesis and subsequent movement towards the plaque, as they are specifically equipped for leucocyte rolling and transendothelial migration. Most of the intraplaque leucocytes observed in association with haemorrhages are macrophage-like phagocytic cells (Figure 4, left panel).
Intraplaque haemorrhages also impact the adventitial immune response. As in aortitis85 and AAA,23 complicated vulnerable plaques are characterized by the presence of an adventitial immune reaction, involving the formation of variable lymphoid nodules, possibly evolving towards adventitial tertiary lymphoid organs (ATLO). As early as 1985, Kohchi et al.86 observed in autopsies that coronary lesions responsible for fatal unstable angina exhibited more adventitial lymphoid infiltrate, often associated with autonomic nerve fibres. Further details of this phenomenon have been reported, including the presence of undefined adventitial inflammation87 and mast cells,88 and the ratio between T and B lymphocytes.89,90 These observations have been recently confirmed by reports of a spatial relationship between adventitial lymphoid infiltrate, plaque complexity, intraplaque haemorrhage, and luminal thrombus, and their association with hypertension,91 suggesting a direct relationship between neo-mediators generated within the plaque, their orthogonal convection towards the adventitia, and their involvement in the adventitial immune response.23 The formation of these lymphoid structures requires, and is associated with, an intense angiogenic process. Furthermore, the relationship, if any, between immune cell effectors recruited in ATLOs and those extravasating through intimal neocapillaries remains to be studied.
Impact of risk factors on intraplaque haemorrhages
Risk factors, downstream to their impact on metabolism, could influence intraplaque haemorrhage and its consequences. As previously stated, cholesterol-rich diet-induced adventitial and intramural neovascularization is attenuated by statin treatment, suggesting that cholesterol overload and lowering may affect vessel growth in atherosclerotic lesions. However, the influence of cholesterol on intraplaque bleeding needs to be established. Erythrocyte membranes are particularly rich in cholesterol, and hypercholesterolemia modifies RBC membrane content,92 particularly in diabetes.93 Therefore, membrane cholesterol levels of circulating erythrocytes have been recently proposed as biomarkers of atherothrombosis.94 Similar results were reported for cholesterol density in neutrophil membranes and for neutrophil sensitivity to angiotensin II-induced free radical release.95 In contrast, circulating HDLs could have beneficial effects via their capacity to convey α1-antitrypsin96 into the diseased tissue, whereas tobacco consumption has an inverse impact.
When compared with data on intraplaque haemorrhages and neovessels in atherothrombosis, reports concerning the direct impact of diabetes and hyperglycaemia on this pathological process remain scarce. Drielsma et al.97 reported that carotid plaques in diabetic patients are more highly vascularized than in non-diabetic patients and that control of hyperglycaemia reduced intraplaque neovascularization.98 In apoE-deficient mice, induction of type 1 diabetes promotes more inflammatory and haemorrhagic plaques99 but not neovascularization. Therefore, diabetes promotes microangiopathic neovessels in the plaque as it does in the retina,100 whereas it impairs macroarteriogenesis in peripheral arterial disease.101 Hyperglycaemia induces abnormal angiogenesis and micro-angiopathy in the retina via a VEGF-dependent mechanism.102 Similar mechanisms could take place in the vascular wall at initial stages of atheroma, causing enhanced angiogenesis, extravasation, and capillary fragility. At the stage of intraplaque haemorrhages, the impact of diabetes is mostly dependent on free haemoglobin-induced oxidative stress.
In remarkable studies, Levy et al.69 demonstrated that the Hp1-1 genotype/phenotype of haptoglobin protects diabetic patients from cardiovascular complications. This beneficial effect is due to the greater ability of Hp1-1 haptoglobin to clear free haemoglobin via CD163. In contrast, the homozygous Hp2-2 genotype/phenotype does not facilitate haemoglobin clearance, and therefore the oxidative potential of free haemoglobin is reinforced in such patients.
Application to diagnostic imaging
Imaging atherothrombotic plaques is a large field of experimental and clinical investigation, widely reviewed in the international literature. The use of magnetic resonance imaging (MRI) for intraplaque haemorrhage has been recently reviewed.103 In particular, MRI studies have documented that intraplaque haemorrhages are associated with plaque enlargement within 18 months, whereas without haemorrhage, atheromatous plaques did not progress.44,104
It may become also possible to visualize the vasa vasorum in atherosclerotic plaques and to follow their progression105 using contrast-enhanced intra-vascular ultrasound imaging.106 This could become of interest since vaso vasorum may be altered in response to plaque stabilizing compounds107 and also neovascularization density of plaques has been correlated with adverse cardiovascular outcome in a longitudinal study.19
Circulating biomarkers that may reflect the risk of intraplaque bleeding
Today, there are more indirect than direct biomarkers of intraplaque microbleedings. The cholesterol concentration in erythrocyte membranes probably reflects the lipid profile over a long period of time. Tziakas et al.94 reported that the non-esterified cholesterol content of circulating RBC membranes was strongly associated with clinical instability in patients with coronary artery disease.108,109 This increase in cholesterol content was associated with a parallel increase in IL-8 RBC membrane (Duffy Antigen/Receptor for Chemokines, DARC) retention in patients with acute coronary syndrome.109
Conversely, plasma levels of anti-oxidant proteins could be used as biomarkers of RBC and free haemoglobin-dependent oxidative stress.110
Haptoglobin genotype/phenotype are also considered identifiable genetic risk factors of acute atherothrombotic clinical events in diabetic patients.111 The homozygous Hp1-1 haplotype is protective, whereas the homozygous Hp2-2 or heterozygous Hp1-2 haplotypes are permissive.69 Nevertheless, this powerful effect is restricted to diabetic patients.
In the same way, CD163, the Hb/Hp complex receptor, which is a transmembrane protein highly sensitive to proteolytic shedding and release, has been reported to be increased in the plasma of patients with peripheral artery disease.112 A decrease in plasma levels of proteins secreted by smooth muscle cells113 but degraded by blood-borne intra-tissue proteolytic enzymes,80 or an increase in proteolytically generated peptides, could also be of interest as haemorrhage-dependent biomarkers. Nevertheless, the sensitivity and the specificity of these circulating markers with respect of intraplaque haemorrhages remain to be defined.
Therapeutic consequences
There is experimental evidence that statins preserve the adventitial vasa vasorum architecture and prevent neovascularization development in hypercholesterolaemic pigs, independently of cholesterol lowering.114 Statins could also influence the consequences of microbleeding due to their ability to limit the cholesterol content of RBC membranes.115 In particular, statins change the profile of cellular phospholipids by reducing the sphingomyelin content of cell membranes.116 In parallel, statins are also able to limit neutrophil transendothelial migration.117 In contrast, coumarin-type anticoagulation is associated with a higher occurrence of intraplaque haemorrhages.118
The interest in intraplaque angiogenesis has been spurred by the potential to target plaque neovascularization with angiogenesis inhibitors, including gene therapy and theranostic methods, approaches that have been associated with reductions in plaque progression in animal models. For example, angiostatin has been shown to limit plaque progression in mice.119 More recently, it was shown experimentally that thalidomide, which impacts neo-microvessel formation, could prevent plaque progression in pigs.107 Therefore, antiangiogenic therapy has been proposed in atherosclerosis.120 Nevertheless, as described above, intraplaque angiogenesis has a dual role: neo-angiogenesis is responsible for the intrapalque haemorrhage itself, but also conveys leucocytes capable of detersion of haemorrhagic products, a necessary step towards healing. Indeed, there are now some recent clinical reports showing that antiangiogenic therapy for cancer121 or age-related macular degeneration122 could increase the risk of cardiovascular diseases. Conversely, rosiglitazone, a PPAR agonist, despite its beneficial effects on glucose metabolism, significantly increases the risk of atherothrombotic events.123 It has been reported that PPAR agonists increase the expression of VEGF in vascular smooth muscle cells124 and macrophages.125 These data suggest that the pro-angiogenic effects of PPAR-γ agonists could be one of the limitations to their clinical use. Therefore, the impact of new compounds developed for atherothrombosis therapy or as antiangiogenic therapy in other diseases should be tested on IPH risk before clinical use.
Due to the prominent role of oxidative stress in the intraplaque haemorrhage-dependent clinical expression of atherothrombosis in diabetic patients, vitamin E supplementation has been proposed in Hp2-2 diabetic patients for the prevention of atherothrombotic complications.126 In the same way, anti-oxidant interventions have demonstrated their ability to prevent neovascularization in hypercholesterolaemic pigs.127
Conclusion
The newly established impact of intraplaque haemorrhages on the evolution of atherothrombotic plaques towards clinical expression provides an innovative conceptual framework for future research and development in human atherothrombotic diseases. New biological challenges are to increase the understanding of how neo-angiogenesis is initiated in the early stages of human atheroma, why neo-vessels do not mature in the plaques, how intraplaque haemorrhages lead to plaque rupture and clinical expression, how cholesterol crystals impact plaque progression towards rupture, and how diabetes and other risk factors directly influence these phenomena These new concepts also reinforce the interest of exploiting human tissue and cell biobanks for research in cardiovascular diseases in general and in atherothrombosis in particular. They also underline the importance in tissue collection and diversification of sample preparation; i.e. chemical fixation for histology, direct freezing, preparation of conditioned media, smooth muscle and endothelial cell primary culture, leucocyte extraction, etc. These new paradigms will also impact translational research by promoting innovation in diagnostic tools (biomarkers, molecular imaging) and therapeutics in human atherothrombotic disease.
Funding
INSERM U698 is supported by the European FP7 through the FAD integrated project (www.fighting-aneurysm.org). Funding to pay the Open Access publication charges for this article was provided by the FP7, EU integrated project FAD (Fighting Aneurysmal Disease, HEALTH-F2-2008-200647).
Conflict of interest: none declared.
References
- 1.Kahn R, Robertson RM, Smith R, Eddy D. The impact of prevention on reducing the burden of cardiovascular disease. Circulation. 2008;118:576–585. doi: 10.1161/CIRCULATIONAHA.108.190186. [DOI] [PubMed] [Google Scholar]
- 2.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group Members, Wayne R, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy M, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee, and Stroke Statistics Subcommittee. Circulation 2008. 117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 5.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 6.Paterson JC. Vascularization and hemorrhage of the intima of coronary atherosclerotic arteries. Arch Pathol. 1936;22:312–324. [Google Scholar]
- 7.Paterson J. Capillary rupture with intimal hemorrhage as a causative factor in coronary thrombus. Arch Pathol Lab Med. 1938;25:474–487. [Google Scholar]
- 8.Wartman WB. Occlusion of the coronary arteries by hemorrhage into their walls. Am Heart J. 1938;5:459–470. [Google Scholar]
- 9.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 10.Virmani R, Roberts WC. Extravasated erythrocytes, iron, and fibrin in atherosclerotic plaques of coronary arteries in fatal coronary heart disease and their relation to luminal thrombus: frequency and significance in 57 necropsy patients and in 2958 five mm segments of 224 major epicardial coronary arteries. Am Heart J. 1983;105:788–797. doi: 10.1016/0002-8703(83)90242-9. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira S, Saldanha C. An overview about erythrocyte membrane. Clin Hemorheol Microcirc. 2010;44:63–74. doi: 10.3233/CH-2010-1253. [DOI] [PubMed] [Google Scholar]
- 12.Gossl M, Versari D, Hildebrandt HA, Bajanowski T, Sangiorgi G, Erbel R, Ritman EL, Lerman LO, Lerman A. Segmental heterogeneity of vasa vasorum neovascularization in human coronary atherosclerosis. JACC Cardiovasc Imaging. 2010;3:32–40. doi: 10.1016/j.jcmg.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imparato AM, Riles TS, Gorstein F. The carotid bifurcation plaque: pathologic findings associated with cerebral ischemia. Stroke. 1979;10:238–245. doi: 10.1161/01.str.10.3.238. [DOI] [PubMed] [Google Scholar]
- 14.Ammar AD, Ernst RL, Lin JJ, Travers H. The influence of repeated carotid plaque hemorrhages on the production of cerebrovascular symptoms. J Vasc Surg. 1986;3:857–859. doi: 10.1067/mva.1986.avs0030857. [DOI] [PubMed] [Google Scholar]
- 15.Gao P, Chen ZQ, Bao YH, Jiao LQ, Ling F. Correlation between carotid intraplaque hemorrhage and clinical symptoms: systematic review of observational studies. Stroke. 2007;38:2382–2390. doi: 10.1161/STROKEAHA.107.482760. [DOI] [PubMed] [Google Scholar]
- 16.Fryer JA, Myers PC, Appleberg M. Carotid intraplaque hemorrhage: the significance of neovascularity. J Vasc Surg. 1987;6:341–349. doi: 10.1067/mva.1987.avs0060341. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy MJ, Loftus IM, Thompson MM, Jones L, London NJ, Bell PR, Naylor AR, Brindle NP. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg. 1999;30:261–268. doi: 10.1016/s0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- 18.Falke P, Matzsch T, Sternby NH, Bergqvist D, Stavenow L. Intraplaque haemorrhage at carotid artery surgery—a predictor of cardiovascular mortality. J Intern Med. 1995;238:131–135. doi: 10.1111/j.1365-2796.1995.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 19.Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, de Vries JP, Seldenrijk KA, De Bruin PC, Vink A, Velema E, de Kleijn DP, Pasterkamp G. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–1950. doi: 10.1161/CIRCULATIONAHA.109.887497. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Thomas AC. Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 22.Arbustini E, Morbini P, D'Armini AM, Repetto A, Minzioni G, Piovella F, Vigano M, Tavazzi L. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: the critical role of thrombotic material in pultaceous core formation. Heart. 2002;88:177–182. doi: 10.1136/heart.88.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 24.Heistad DD, Armstrong ML, Marcus ML. Hyperemia of the aortic wall in atherosclerotic monkeys. Circ Res. 1981;48:669–675. doi: 10.1161/01.res.48.5.669. [DOI] [PubMed] [Google Scholar]
- 25.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 27.Gossl M, Versari D, Mannheim D, Ritman EL, Lerman LO, Lerman A. Increased spatial vasa vasorum density in the proximal lad in hypercholesterolemia—implications for vulnerable plaque-development. Atherosclerosis. 2007;192:246–252. doi: 10.1016/j.atherosclerosis.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Trape J, Morales C, Molina R, Filella X, Marcos JM, Salinas R, Franquesa J. Vascular endothelial growth factor serum concentrations in hypercholesterolemic patients. Scand J Clin Lab Invest. 2006;66:261–267. doi: 10.1080/00365510600564949. [DOI] [PubMed] [Google Scholar]
- 29.Jeziorska M, Woolley DE. Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum Pathol. 1999;30:919–925. doi: 10.1016/s0046-8177(99)90245-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Plasma protein insudation as an index of early coronary atherogenesis. Am J Pathol. 1993;143:496–506. [PMC free article] [PubMed] [Google Scholar]
- 31.Kockx MM, Cromheeke KM, Knaapen MW, Bosmans JM, De Meyer GR, Herman AG, Bult H. Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:440–446. doi: 10.1161/01.ATV.0000057807.28754.7F. [DOI] [PubMed] [Google Scholar]
- 32.Milei J, Parodi JC, Alonso GF, Barone A, Grana D, Matturri L. Carotid rupture and intraplaque hemorrhage: immunophenotype and role of cells involved. Am Heart J. 1998;136:1096–1105. doi: 10.1016/s0002-8703(98)70169-3. [DOI] [PubMed] [Google Scholar]
- 33.Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 2001;88:945–950. doi: 10.1046/j.0007-1323.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 35.Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol. 1999;188:189–196. doi: 10.1002/(SICI)1096-9896(199906)188:2<189::AID-PATH336>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol. 1993;143:164–172. [PMC free article] [PubMed] [Google Scholar]
- 37.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–159. doi: 10.1016/j.jvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 38.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, Daemen MJ. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Post S, Peeters W, Busser E, Lamers D, Sluijter JP, Goumans MJ, de Weger RA, Moll FL, Doevendans PA, Pasterkamp G, Vink A. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res. 2008;45:244–250. doi: 10.1159/000112939. [DOI] [PubMed] [Google Scholar]
- 40.Le Dall J, Ho-Tin-Noe B, Louedec L, Meilhac O, Roncal C, Carmeliet P, Germain S, Michel JB, Houard X. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc Res. 2010;85:184–193. doi: 10.1093/cvr/cvp253. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T, Lam T, Boudreau NJ, Bollen AW, Lawton MT, Young WL. Abnormal balance in the angiopoietin-Tie2 system in human brain arteriovenous malformations. Circ Res. 2001;89:111–113. doi: 10.1161/hh1401.094281. [DOI] [PubMed] [Google Scholar]
- 42.Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991;11:1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- 44.Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 45.Meyrier A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int. 2006;69:1308–1312. doi: 10.1038/sj.ki.5000263. [DOI] [PubMed] [Google Scholar]
- 46.Tangirala RK, Jerome WG, Jones NL, Small DM, Johnson WJ, Glick JM, Mahlberg FH, Rothblat GH. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J Lipid Res. 1994;35:93–104. [PubMed] [Google Scholar]
- 47.Chandler AB, Hand RA. Phagocytized platelets: a source of lipids in human thrombi and atherosclerotic plaques. Science. 1961;134:946–947. doi: 10.1126/science.134.3483.946. [DOI] [PubMed] [Google Scholar]
- 48.Kruth HS. Platelet-mediated cholesterol accumulation in cultured aortic smooth muscle cells. Science. 1985;227:1243–1245. doi: 10.1126/science.3975612. [DOI] [PubMed] [Google Scholar]
- 49.Kolodgie FD, Burke AP, Nakazawa G, Cheng Q, Xu X, Virmani R. Free cholesterol in atherosclerotic plaques: where does it come from? Curr Opin Lipidol. 2007;18:500–507. doi: 10.1097/MOL.0b013e3282efa35b. [DOI] [PubMed] [Google Scholar]
- 50.Kellner-Weibel G, Yancey PG, Jerome WG, Walser T, Mason RP, Phillips MC, Rothblat GH. Crystallization of free cholesterol in model macrophage foam cells. Arterioscler Thromb Vasc Biol. 1999;19:1891–1898. doi: 10.1161/01.atv.19.8.1891. [DOI] [PubMed] [Google Scholar]
- 51.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 52.Lin HL, Xu XS, Lu HX, Zhang L, Li CJ, Tang MX, Sun HW, Liu Y, Zhang Y. Pathological mechanisms and dose dependency of erythrocyte-induced vulnerability of atherosclerotic plaques. J Mol Cell Cardiol. 2007;43:272–280. doi: 10.1016/j.yjmcc.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 53.Lin HL, Zhang L, Liu CX, Xu XS, Tang MX, Lv HX, Li CJ, Sun HW, Zhang M, Hong J, Zhang Y. Haemin-enhanced expression of haem oxygenase-1 stabilizes erythrocyte-induced vulnerable atherosclerotic plaques. Br J Pharmacol. 2010;160:1484–1495. doi: 10.1111/j.1476-5381.2010.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abela GS, Aziz K. Cholesterol crystals cause mechanical damage to biological membranes: a proposed mechanism of plaque rupture and erosion leading to arterial thrombosis. Clin Cardiol. 2005;28:413–420. doi: 10.1002/clc.4960280906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events—a novel insight into plaque rupture by scanning electron microscopy. Scanning. 2006;28:1–10. doi: 10.1002/sca.4950280101. [DOI] [PubMed] [Google Scholar]
- 56.Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. 2009;103:959–968. doi: 10.1016/j.amjcard.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, Eklund KK. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma H, Aziz KS, Huang R, Abela GS. Arterial wall cholesterol content is a predictor of development and severity of arterial thrombosis. J Thromb Thrombolysis. 2006;22:5–11. doi: 10.1007/s11239-006-7861-x. [DOI] [PubMed] [Google Scholar]
- 60.van den Berg JJ, Op den Kamp JA, Lubin BH, Roelofsen B, Kuypers FA. Kinetics and site specificity of hydroperoxide-induced oxidative damage in red blood cells. Free Radic Biol Med. 1992;12:487–498. doi: 10.1016/0891-5849(92)90102-m. [DOI] [PubMed] [Google Scholar]
- 61.Nagy E, Eaton JW, Jeney V, Soares MP, Varga Z, Galajda Z, Szentmiklosi J, Mehes G, Csonka T, Smith A, Vercellotti GM, Balla G, Balla J. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller YI, Shaklai N. Oxidative crosslinking of LDL protein induced by hemin: involvement of tyrosines. Biochem Mol Biol Int. 1994;34:1121–1129. [PubMed] [Google Scholar]
- 63.Abraham NG, Lavrovsky Y, Schwartzman ML, Stoltz RA, Levere RD, Gerritsen ME, Shibahara S, Kappas A. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA. 1995;92:6798–6802. doi: 10.1073/pnas.92.15.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yunoki K, Naruko T, Komatsu R, Ehara S, Shirai N, Sugioka K, Nakagawa M, Kitabayashi C, Ikura Y, Itoh A, Kusano K, Ohe T, Haze K, Becker AE, Ueda M. Enhanced expression of haemoglobin scavenger receptor in accumulated macrophages of culprit lesions in acute coronary syndromes. Eur Heart J. 2009;30:1844–1852. doi: 10.1093/eurheartj/ehp257. [DOI] [PubMed] [Google Scholar]
- 65.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 66.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 68.Kolb S, Vranckx R, Huisse MG, Michel JB, Meilhac O. The phosphatidylserine receptor mediates phagocytosis by vascular smooth muscle cells. J Pathol. 2007;212:249–259. doi: 10.1002/path.2190. [DOI] [PubMed] [Google Scholar]
- 69.Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, Anbinder Y, Lache O, Nakhoul FM, Asaf R, Farbstein D, Pollak M, Soloveichik YZ, Strauss M, Alshiek J, Livshits A, Schwartz A, Awad H, Jad K, Goldenstein H. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12:293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 70.Kalet-Litman S, Moreno PR, Levy AP. The haptoglobin 2-2 genotype is associated with increased redox active hemoglobin derived iron in the atherosclerotic plaque. Atherosclerosis. 2010;209:28–31. doi: 10.1016/j.atherosclerosis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreno PR, Purushothaman KR, Purushothaman M, Muntner P, Levy NS, Fuster V, Fallon JT, Lento PA, Winterstern A, Levy AP. Haptoglobin genotype is a major determinant of the amount of iron in the human atherosclerotic plaque. J Am Coll Cardiol. 2008;52:1049–1051. doi: 10.1016/j.jacc.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 72.Nishihira K, Yamashita A, Imamura T, Hatakeyama K, Sato Y, Nakamura H, Yodoi J, Ogawa H, Kitamura K, Asada Y. Thioredoxin in coronary culprit lesions: possible relationship to oxidative stress and intraplaque hemorrhage. Atherosclerosis. 2008;201:360–367. doi: 10.1016/j.atherosclerosis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Kondo T, Hirose M, Kageyama K. Roles of oxidative stress and redox regulation in atherosclerosis. J Atheroscler Thromb. 2009;16:532–538. doi: 10.5551/jat.1255. [DOI] [PubMed] [Google Scholar]
- 74.Michel JB, Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2010 doi: 10.1093/cvr/cvq337. doi:10.1093/cvr/cvq337. Published online ahead of print 28 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tavora F, Cresswell N, Li L, Ripple M, Burke A. Immunolocalisation of fibrin in coronary atherosclerosis: implications for necrotic core development. Pathology. 2010;42:15–22. doi: 10.3109/00313020903434348. [DOI] [PubMed] [Google Scholar]
- 76.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries JP, Pasterkamp G, Vink A, de Kleijn DP. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2010;30:1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 77.Leclercq A, Houard X, Loyau S, Philippe M, Sebbag U, Meilhac O, Michel JB. Topology of protease activities reflects atherothrombotic plaque complexity. Atherosclerosis. 2007;191:1–10. doi: 10.1016/j.atherosclerosis.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Leclercq A, Houard X, Philippe M, Ollivier V, Sebbag U, Meilhac O, Michel JB. Involvement of intraplaque hemorrhage in atherothrombosis evolution via neutrophil protease enrichment. J Leukoc Biol. 2007;82:1420–1429. doi: 10.1189/jlb.1106671. [DOI] [PubMed] [Google Scholar]
- 79.Martin-Ventura JL, Leclercq A, Blanco-Colio LM, Egido J, Rossignol P, Meilhac O, Michel JB. Low plasma levels of hsp70 in patients with carotid atherosclerosis are associated with increased levels of proteolytic markers of neutrophil activation. Atherosclerosis. 2007;194:334–341. doi: 10.1016/j.atherosclerosis.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 80.Martin-Ventura JL, Nicolas V, Houard X, Blanco-Colio LM, Leclercq A, Egido J, Vranckx R, Michel JB, Meilhac O. Biological significance of decreased hsp27 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1337–1343. doi: 10.1161/01.ATV.0000220108.97208.67. [DOI] [PubMed] [Google Scholar]
- 81.Mansilla S, Boulaftali Y, Venisse L, Arocas V, Meilhac O, Michel JB, Jandrot-Perrus M, Bouton MC. Macrophages and platelets are the major source of protease nexin-1 in human atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2008;28:1844–1850. doi: 10.1161/ATVBAHA.108.171389. [DOI] [PubMed] [Google Scholar]
- 82.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stone JR. Aortitis, periaortitis, and retroperitoneal fibrosis, as manifestations of IgG4-related systemic disease. Curr Opin Rheumatol. 2011;23:88–94. doi: 10.1097/BOR.0b013e3283412f7c. [DOI] [PubMed] [Google Scholar]
- 86.Kohchi K, Takebayashi S, Hiroki T, Nobuyoshi M. Significance of adventitial inflammation of the coronary artery in patients with unstable angina: results at autopsy. Circulation. 1985;71:709–716. doi: 10.1161/01.cir.71.4.709. [DOI] [PubMed] [Google Scholar]
- 87.Moreno PR, Purushothaman KR, Fuster V, O'Connor WN. Intimomedial interface damage and adventitial inflammation is increased beneath disrupted atherosclerosis in the aorta: implications for plaque vulnerability. Circulation. 2002;105:2504–2511. doi: 10.1161/01.cir.0000017265.52501.37. [DOI] [PubMed] [Google Scholar]
- 88.Laine P, Kaartinen M, Penttila A, Panula P, Paavonen T, Kovanen PT. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–369. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M, Sangawa A, Sasaki Y, Yamashita M, Tanaka-Shintani M, Shintaku M, Ishikawa Y. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14:325–331. doi: 10.5551/jat.e489. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz CJ, Mitchell JR. Cellular infiltration of the human arterial adventitia associated with atheromatous plaques. Circulation. 1962;26:73–78. doi: 10.1161/01.cir.26.1.73. [DOI] [PubMed] [Google Scholar]
- 91.Tavora F, Kutys R, Li L, Ripple M, Fowler D, Burke A. Adventitial lymphocytic inflammation in human coronary arteries with intimal atherosclerosis. Cardiovasc Pathol. 2010;19:e61–e68. doi: 10.1016/j.carpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Vaya A, Martinez Triguero M, Reganon E, Vila V, Martinez Sales V, Sola E, Hernandez Mijares A, Ricart A. Erythrocyte membrane composition in patients with primary hypercholesterolemia. Clin Hemorheol Microcirc. 2008;40:289–294. [PubMed] [Google Scholar]
- 93.Babu N. Influence of hypercholesterolemia on deformability and shape parameters of erythrocytes in hyperglycemic subjects. Clin Hemorheol Microcirc. 2009;41:169–177. doi: 10.3233/CH-2009-1165. [DOI] [PubMed] [Google Scholar]
- 94.Tziakas DN, Chalikias GK, Stakos D, Tentes IK, Papazoglou D, Thomaidi A, Grapsa A, Gioka G, Kaski JC, Boudoulas H. Independent and additive predictive value of total cholesterol content of erythrocyte membranes with regard to coronary artery disease clinical presentation. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.02.022. Published online ahead of print 9 March. [DOI] [PubMed] [Google Scholar]
- 95.Seres I, Foris G, Varga Z, Kosztaczky B, Kassai A, Balogh Z, Fulop P, Paragh G. The association between angiotensin II-induced free radical generation and membrane fluidity in neutrophils of patients with metabolic syndrome. J Membr Biol. 2006;214:91–98. doi: 10.1007/s00232-006-0020-7. [DOI] [PubMed] [Google Scholar]
- 96.Ortiz-Munoz G, Houard X, Martin-Ventura JL, Ishida BY, Loyau S, Rossignol P, Moreno JA, Kane JP, Chalkley RJ, Burlingame AL, Michel JB, Meilhac O. HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J. 2009;23:3129–3139. doi: 10.1096/fj.08-127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Drielsma RF, Burnett JR, Gray-Weale AC, Byrne K, Lusby RJ. Carotid artery disease: the influence of diabetes mellitus. J Cardiovasc Surg (Torino) 1988;29:692–696. [PubMed] [Google Scholar]
- 98.Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc Diabetol. 2004;3:1. doi: 10.1186/1475-2840-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci USA. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 101.Ruiter MS, van Golde JM, Schaper NC, Stehouwer CD, Huijberts MS. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci (Lond) 2010;119:225–238. doi: 10.1042/CS20100082. [DOI] [PubMed] [Google Scholar]
- 102.Rajappa M, Saxena P, Kaur J. Ocular angiogenesis: mechanisms and recent advances in therapy. Adv Clin Chem. 2010;50:103–121. [PubMed] [Google Scholar]
- 103.Wasserman BA. Advanced contrast-enhanced MRI for looking beyond the lumen to predict stroke: building a risk profile for carotid plaque. Stroke. 2010;41:S12–S16. doi: 10.1161/STROKEAHA.110.596288. [DOI] [PubMed] [Google Scholar]
- 104.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, Garden GA, Cramer SC, Maravilla KR, Hashimoto B, Hatsukami TS. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 105.Schinkel AF, Krueger CG, Tellez A, Granada JF, Reed JD, Hall A, Zang W, Owens C, Kaluza GL, Staub D, Coll B, Ten Cate FJ, Feinstein SB. Contrast-enhanced ultrasound for imaging vasa vasorum: comparison with histopathology in a swine model of atherosclerosis. Eur J Echocardiogr. 2010;11:659–664. doi: 10.1093/ejechocard/jeq048. [DOI] [PubMed] [Google Scholar]
- 106.Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, Krueger C, Thomenius KE, Adam D, Sijbrands EJ, ten Cate FJ, Feinstein SB. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. 2010;3:761–771. doi: 10.1016/j.jcmg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 107.Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, Rajkumar SV, Lerman LO, Lerman A. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol. 2009;104:695–706. doi: 10.1007/s00395-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tziakas DN, Kaski JC, Chalikias GK, Romero C, Fredericks S, Tentes IK, Kortsaris AX, Hatseras DI, Holt DW. Total cholesterol content of erythrocyte membranes is increased in patients with acute coronary syndrome: a new marker of clinical instability? J Am Coll Cardiol. 2007;49:2081–2089. doi: 10.1016/j.jacc.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 109.Tziakas DN, Chalikias GK, Stakos D, Tentes IK, Chatzikyriakou SV, Mitrousi K, Kortsaris AX, Boudoulas H, Kaski JC. Cholesterol composition of erythrocyte membranes and its association with clinical presentation of coronary artery disease. Coron Artery Dis. 2008;19:583–590. doi: 10.1097/MCA.0b013e328313819b. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Pinna R, Lindholt JS, Blanco-Colio LM, Dejouvencel T, Madrigal-Matute J, Ramos-Mozo P, Vega de Ceniga M, Michel JB, Egido J, Meilhac O, Martin-Ventura JL. Increased levels of thioredoxin in patients with abdominal aortic aneurysms (AAAs). A potential link of oxidative stress with AAA evolution. Atherosclerosis. 2010;212:333–338. doi: 10.1016/j.atherosclerosis.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 111.Farbstein D, Levy AP. The genetics of vascular complications in diabetes mellitus. Cardiol Clin. 2010;28:477–496. doi: 10.1016/j.ccl.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moreno JA, Dejouvencel T, Labreuche J, Smadja DM, Dussiot M, Martin-Ventura JL, Egido J, Gaussem P, Emmerich J, Michel JB, Blanco-Colio LM, Meilhac O. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler Thromb Vasc Biol. 2010;30:1253–1262. doi: 10.1161/ATVBAHA.110.203364. [DOI] [PubMed] [Google Scholar]
- 113.Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tunon J, Vivanco F, Egido J. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- 114.Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr, Napoli C, Ritman EL, Lerman A. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 2002;105:415–418. doi: 10.1161/hc0402.104119. [DOI] [PubMed] [Google Scholar]
- 115.Tziakas DN, Chalikias GK, Stakos D, Tentes IK, Thomaidi A, Chatzikyriakou S, Mitrousi K, Kortsaris AX, Kaski JC, Boudoulas H, Konstantinides S. Statin use is associated with a significant reduction in cholesterol content of erythrocyte membranes. A novel pleiotropic effect? Cardiovasc Drugs Ther. 2009;23:471–480. doi: 10.1007/s10557-009-6202-7. [DOI] [PubMed] [Google Scholar]
- 116.Dietzen DJ, Page KL, Tetzloff TA, Bohrer A, Turk J. Inhibition of 3-hydroxy-3-methylglutaryl coenzyme a (HMG CoA) reductase blunts factor VIIa/tissue factor and prothrombinase activities via effects on membrane phosphatidylserine. Arterioscler Thromb Vasc Biol. 2007;27:690–696. doi: 10.1161/01.ATV.0000255949.51053.ce. [DOI] [PubMed] [Google Scholar]
- 117.Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RW. Statins alter neutrophil migration by modulating cellular rho activity—a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol. 2009;85:186–193. doi: 10.1189/jlb.0608382. [DOI] [PubMed] [Google Scholar]
- 118.Derksen WJ, Peeters W, Tersteeg C, de Vries JP, de Kleijn DP, Moll FL, van der Wal AC, Pasterkamp G, Vink A. Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: a large histopathological study in carotid and femoral plaques. Atherosclerosis. 2011;214:139–143. doi: 10.1016/j.atherosclerosis.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 119.Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doyle B, Caplice N. Plaque neovascularization and antiangiogenic therapy for atherosclerosis. J Am Coll Cardiol. 2007;49:2073–2080. doi: 10.1016/j.jacc.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 121.Vaklavas C, Lenihan D, Kurzrock R, Tsimberidou AM. Anti-vascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist. 2010;15:130–141. doi: 10.1634/theoncologist.2009-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tunon J, Ruiz-Moreno JM, Martin-Ventura JL, Blanco-Colio LM, Lorenzo O, Egido J. Cardiovascular risk and antiangiogenic therapy for age-related macular degeneration. Surv Ophthalmol. 2009;54:339–348. doi: 10.1016/j.survophthal.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 123.Wang AT, McCoy CP, Murad MH, Montori VM. Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ. 2010;340:c1344. doi: 10.1136/bmj.c1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, Nishizawa Y. Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;271:571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 125.Jozkowicz A, Dulak J, Piatkowska E, Placha W, Dembinska-Kiec A. Ligands of peroxisome proliferator-activated receptor-gamma increase the generation of vascular endothelial growth factor in vascular smooth muscle cells and in macrophages. Acta Biochim Pol. 2000;47:1147–1157. [PubMed] [Google Scholar]
- 126.Levy AP, Blum S. Pharmacogenomics in prevention of diabetic cardiovascular disease: utilization of the haptoglobin genotype in determining benefit from vitamin e. Expert Rev Cardiovasc Ther. 2007;5:1105–1111. doi: 10.1586/14779072.5.6.1105. [DOI] [PubMed] [Google Scholar]
- 127.Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]