Abstract
Introduction
Fatigue is a common symptom in RA and used as an outcome measure in RA clinical trials. We studied a large academic clinical practice to estimate the minimally important difference (MID) for a fatigue visual analog scale using patient-reported anchors (fatigue, pain and overall health).
Methods
RA patients (N=307) had clinic visits at 2 time points at a median of 5.9 months apart. They completed fatigue visual analog scale (VAS; 0–10) and retrospective anchor items, “How would you describe your overall fatigue/pain/overall health since the last visit?” Much worsened, Somewhat worsened, Same, Somewhat better, or Much better. The fatigue anchor was used for primary analysis and the pain/ overall health anchors for sensitivity analyses. The minimally changed group was defined by those reporting they were somewhat better or somewhat worsened.
Results
The mean (SD) age was 59.4 (13.2) years, disease duration was 14.1 (11.5) years, and 83% of patients were women. The baseline mean (SD) HAQ-DI score was 0.84 (0.75). The baseline fatigue VAS score was 4.2 (2.9) and at follow up was 4.3 (2.8) (mean change of −0.07 [2.5], p=NS). The fatigue change score (0–10 scale) for somewhat better and somewhat worsened for fatigue anchor averaged −1.12 and 1.26, respectively. Using pain anchor, the fatigue changed score for somewhat better and somewhat worsened averaged −0.87 and 1.13 and using global anchor, the fatigue changed score for somewhat better and somewhat worsened averaged −0.82 and 1.17, respectively.
Effect size (ES) estimates using 3 anchors were small for somewhat better (range: 0.27 to 0.39) and somewhat worsened (range: 0.40 to 0.44) groups but larger than the no-change group (range: 0.03 to 0.08).
Conclusions
The MID for fatigue VAS is between −0.82 to −1.12 for improvement and 1.13 to 1.26 for worsening on 0–10 scale in a large RA clinical practice and similar to that seen in RA clinical trials. This information can aid in interpreting fatigue VAS in day-to-day care in clinical practice.
Keywords: Minimally important differences, Minimal clinically important differences, rheumatoid arthritis, fatigue, visual analog scale, reliable change index, clinical practice
Fatigue is very common in patients with rheumatoid arthritis (RA) and is associated with poor day-to-day functioning and overall sense of well-being (1). Fatigue is related to the RA disease activity and other non-RA factors (poor sleep, stress, etc). The prevalence of fatigue in RA, defined as rating of ≥ 2 on a 0 to 10 visual analogue scale (VAS), is approximately 41%(2). Fatigue remains an important concern for patients with RA (3, 4) and Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) recommends inclusion of fatigue as a patient-centered outcome in RA studies (4).
Recent clinical trials have used fatigue as an outcome measure(5) and fatigue VAS has been found to be sensitive to change in RA-related clinical outcomes(6). In addition, Wolfe recently compared fatigue VAS scale to multi-item fatigue instruments in a large observational study and showed that VAS scale and multi-item scales had comparable responsiveness statistics and similar correlations coefficients with clinical outcomes in RA(7). As future clinical trials and observational studies in RA are likely to include a measure of fatigue, it is important to estimate the minimal clinically important difference (MCID) or minimally important difference (MID)— the smallest improvement in score that patients perceive as beneficial and that may lead to a change in the patient's management of fatigue(8). MID can help clinicians understand whether fatigue score differences between two treatment groups are meaningful and if changes within one group over time are clinically meaningful(9). The MID estimates were assessed using an anchor-based approach (10). An “anchor” is a clinically relevant indicator or pointer to which a patient-reported outcome change can be tied(10, 11). These measures are of clinical relevance and can be “subjective,” such as self-reports of change, or “objective,” such as clinical indicators of response to treatment (joint count or disease severity). Subjective anchors rely on an individual’s (subject’s or his/her physician’s) assessment of the patient’s condition. A global rating of change is a well-accepted subjective anchor in patient-reported outcome research(12). It is a retrospective assessment of change where a person thinks back to a previous time point and states whether s/he has experienced change in a domain of health from that time point to present(13).
We prospectively studied a large clinical practice at a university hospital to determine the MID estimates for fatigue VAS (0–10; for improvement and worsening) using 3 patient-reported anchors—fatigue, pain, and overall health. Previous studies have shown an inherent uncertainty around the MID estimates(5, 14, 15) and experts have recommended using several anchors. Our primary anchor was fatigue scale but we also performed our analyses using overall and pain anchors as previous study has shown an association between pain, overall health, and fatigue(2).
Methods
Multiple data are collected routinely on patients seen at St. Joseph’s Hospital Rheumatology clinic, which is affiliated with the University of Western Ontario and services a referral region of approximately one million. The data are from patients (N=307) with RA(16) who had at least 2 consecutive visits within 12 months (median duration of 5.9 months) with their rheumatologists. As patients with low disease activity may not have a frequent follow-up with rheumatologists, we chose the 12-month duration so we can include all eligible subjects. Data were extracted from medical charts by trained data-extraction persons and entered into a database. Patients completed the fatigue VAS (0–10), which stated: “How much problem has unusual fatigue or tiredness been over you over the past week” and was anchored from 0 (fatigue is no problem) to 10 (fatigue is a major problem). In addition, patients completed the health assessment questionnaire-disability index (HAQ-DI)(17) and a retrospective anchor item at visit# 2, “How would you describe your overall fatigue since the last visit?” Much worsened, Somewhat worsened, Same, Somewhat better, or Much better. Patients who reported somewhat better or somewhat worsened at visit # 2 were defined as the minimally changed subgroups. The changes in the fatigue VAS scores (time2 visit –time1 visit) for the group that reported somewhat better and somewhat worsened were determined in order to estimate the MID. This was compared to change scores for the group that reported same, much better, and much worsened. The normality of change in fatigue scores was assessed using the Shapiro-Wilk test. The test was non-significant (p=0.06) and we report the MID estimates as mean and 95% confidence intervals.
The fatigue change scores were calculated (fatigue from time2 visit – fatigue from time1 visit). To assess the usefulness of an anchor, experts recommend reporting correlation between the anchor and changed score; for example, a correlation of zero will make the anchor useless so a correlation coefficient of 0.30–0.35 has been suggested (for details see (9, 18)). We assessed the association between anchor and fatigue change score using the Spearman Correlation Coefficient (as the anchor is an ordinal variable). The MID was estimated by examining change in the fatigue VAS scores in subjects who were slightly better and slightly worsened. These estimates were compared to those who improved or worsened more than slightly. Responsiveness to change was evaluated using the effect size (ES)(19). ES is ratio of observed change to a measure of variance (also known as signal to noise). For ES, the numerator is the mean change in the fatigue VAS from the baseline to follow up and the denominator is the standard deviation of fatigue VAS at baseline (SD=2.87). Cohen’s rule-of-thumb for interpreting ES is that a value of 0.20–0.49 represents a small change, 0.50–0.79 a medium change, and 0.80 or greater a large change (19) and, in general, an ES of 0.2 to 0.5 is usually considered relevant for MID(20).
We also estimated MID scores using pain and overall health assessments as anchors. The patients were asked, “How would you describe your pain/ overall health since the last visit?” on a scale labeled: much worsened, somewhat worsened, same, somewhat better, much better.
In addition, the effect of baseline fatigue VAS score on the MID estimates was estimated. In other words, people with different baseline VAS fatigue scores may require different amounts of improvement or worsening to consider a change to represent an MID(15). We divided the baseline VAS fatigue scores into 2 groups at the median score—“less severe” group (fatigue VAS ≤4.5) and ”more severe” group (fatigue VAS > 4.5).
We also assessed the significance of change at the individual level. The reliable change index (RCI) is a measure to assess the magnitude of change score necessary to be considered statistically reliable and not due to random measurement error and is a function of the standard deviation and reliability coefficient of an instrument(21, 22). When RCI is greater than 1.96, it is unlikely that the posttest score is due to random measurement error and the change is reliable (p<.05). RCI is calculated by the following formula:
We did not assess test-retest reliability of our fatigue VAS. However, reliability (test-retest) coefficient of 0.70 for the fatigue VAS was found in patients with RA(23). Therefore, we based our primary analysis on the reliability coefficient of 0.70 and performed sensitivity analyses with 0.80 and 0.90. We report the scores that are statistically reliable and proportion of patients who had individual change greater the statistical reliable change (improved or worsened) and proportion that had individual changes less than the statistical reliable change.
The data were analyzed using STATA 9.2. P< 0.05 was deemed to be indicative of statistical significance.
Results
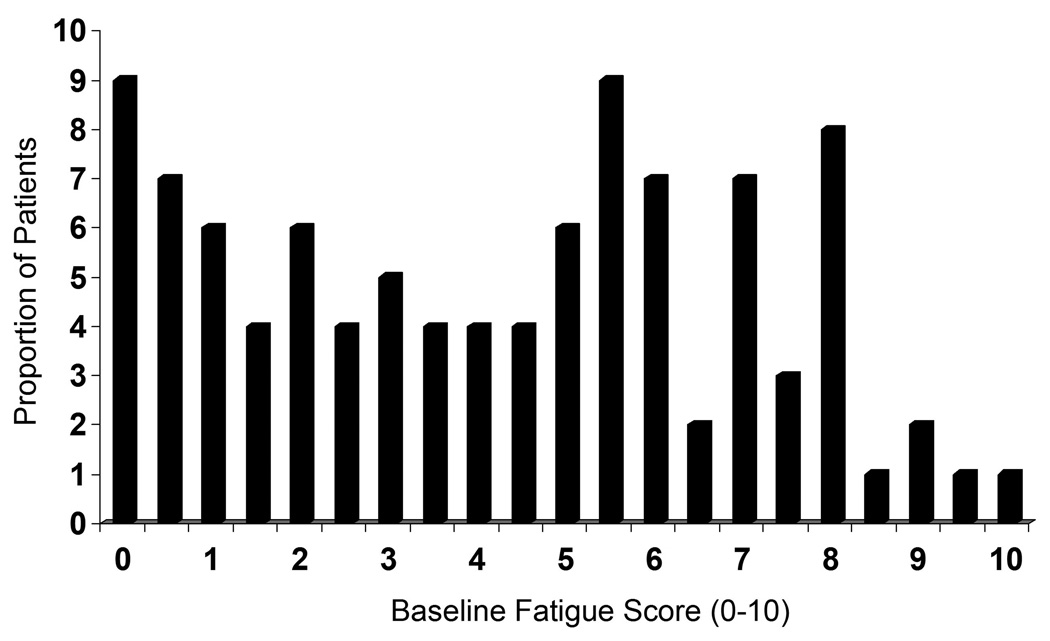
The mean (SD) age of the patients was 59.4 (13.2) years, the mean (SD) disease duration was 14.1 (11.5) years, and 256 (83%) were women; the median (25th, 75th percentile) follow-up between 2 visits was 5.9 (3.9, 7.6) months. Twenty-eight patients (9%) reported no fatigue at baseline and 5 (2%) reported maximum fatigue at baseline (Figure 1). The majority of patients (92%) were using DMARD therapy, 16% prednisone, 50% non-steroidal anti-inflammatory drugs (NSAIDs) and 17% were prescribed biologics. The baseline HAQ-DI was 0.84 (SD = 0.75), suggesting mild functional disability. The baseline fatigue VAS was 4.2 (SD = 2.9) and at follow up was 4.3 (SD = 2.8) (mean change of −0.07 ± 2.5, p=NS).
Histogram illustrating the distribution of baseline fatigue scores on a 0–10 scale.
The Spearman correlation between the patient assessment of fatigue and change in the fatigue VAS was 0.37 (p< 0.001). Of 307 patients, 171 patients (56%) reported no change at follow up visit, 40 (13%) reported being somewhat improved, and 17 (5%) reported being much better. In contrast, 65 (21%) reported being somewhat worsened and 14 (5%) reported being much worsened.
The fatigue change scores for somewhat better (N=40) and somewhat worsened (N=65) groups averaged −1.12 and 1.26 with 95% CI’s of −1.86 to −0.37 and 0.68 to 1.86, respectively (Table 1). The change in fatigue scores for those who were much better (N=17) was −1.44 (95% CI= −2.78, −0.10) and +3.35 (95% CI=1.94, 4.78) for much worse (N=14). All the change scores were statistically different at p< 0.05 than no change group (N=171, −0.23, 95% CI= −0.57, 0.11). ES estimates were small for somewhat better (0.39) and somewhat worse (0.44) groups but larger than the no-change group (ES=0.08).
Table 1.
Minimal Important Difference (MID) Estimates for the Fatigue VAS (0–10)
| Patient-Rated Overall Status | Mean Fatigue VAS Change | 95% CI | Effect Size | P value |
|---|---|---|---|---|
| Much Better (N=17) | −1.44 | −2.78, –0.10 | −0.50 | 0.04 |
| Somewhat Better (N=40) | −1.12 | −1.86, –0.37 | −0.39 | 0.03 |
| Same (N=171) | −0.23 | −0.57, 0.11 | −0.08 | - |
| Somewhat Worse (N=65) | 1.26 | 0.68, 1.86 | 0.44 | <0.001 |
| Much Worse (N=14) | 3.35 | 1.94, 4.78 | 1.17 | <0.001 |
Negative score signify improvement and positive scores signify worsening
For global and pain anchors, the Spearman correlations between the patient assessment of overall symptoms and pain versus change in the fatigue VAS were both 0.33 (p< 0.001). Using pain anchor, the fatigue changed score for somewhat better (N=50) and somewhat worsened (N=70) averaged −0.87 and 1.13 with 95% CI’s of −1.45 to −0.28 and 0.70 to 1.77, respectively. Using global anchor, the fatigue changed score for somewhat better (N=52) and somewhat worsened (N=65) averaged −0.82 and 1.17 with 95% CI’s of -1.40 to −0.24 and 0.48 to 1.86, respectively.
Table 2 provides the mean (95% CI) MID scores for improvement in the fatigue scores stratified by “less severe” fatigue (fatigue VAS< =4.5) vs. ”more severe” (fatigue VAS> 4.5) groups at baseline. The data suggest that patients with ”more severe” baseline fatigue scores require a larger change to be minimally improved (MID for the minimally improved group for the low score group = −0.15, high score group = −3.38). In contrast, the change required for the slightly worsened group with ”more severe” fatigue (0.21) was smaller than the ”less severe” (3.08) fatigue group.
Table 2.
Mean MID scores for improvement in the fatigue scores stratified by “less severe” fatigue vs. “more severe” fatigue at baseline
| Patient-Rated Overall Status | N | Fatigue VAS <=4.5 Mean Score | 95% CI | N | Fatigue VAS >4.5 Mean Score | 95% CI |
|---|---|---|---|---|---|---|
| Much Better | 11 | −0.41 | −1.16, 0.34 | 6 | −3.33 | −7.05, 0.39 |
| Somewhat Better | 28 | −0.15 | −0.86, 0.55 | 12 | −3.38 | −4.50, −2.25 |
| Same | 102 | 0.61 | 0.24, 0.98 | 69 | −1.47 | −2.01, −0.94 |
| Somewhat Worse | 24 | 3.08 | 3.08, 0.45 | 41 | 0.21 | −0.34, 0.76 |
| Much Worse | 5 | 5.70 | 3.66, 7.74 | 9 | 2.06 | 0.69, 3.42 |
Negative score signify improvement and positive scores signify worsening
Reliable change index
The amount of change on the fatigue VAS needed to have the RCI to be 1.96 was 3.47 when the reliability coefficient was 0.70. In other words, a change of score from time1 to time2 (improvement or worsening) of > 3.47 (on a 0–10 scale) was a real change and not due to measurement error of the instrument. A change less than 3.47 may be real but a random measurement error cannot be ruled out. Out of 307 patients, 26 (8%) had worsening and 30 (10%) had improvement of scores of greater than 3.47. The RCI was 3.14 and 2.64 when the test-retest reliability coefficients were 0.80 and 0.90, respectively.
Discussion
Fatigue is very common in patients with RA(1, 24), is likely to be multi-factorial(3), and was recommended as a patient-centered outcome during the OMERACT 8 meeting(4). Work by Kirwan and Hewlett(1) and Wolfe and colleagues(3) have shown that fatigue is an important concern for patients with RA.
We found that our baseline mean (SD) fatigue score of 4.2 (2.9) is very similar to mean score of 4.2 (2.8) seen by Wolfe and colleagues in 7760 patients with RA(7). Our data adds to the literature by comparing various patient-reported outcome anchors and assessing the MID overall in fatigue and in high and low fatigue. Our MID estimates for improvement ranged from −0.82 to −1.12 for improvement and 1.13 to 1.26 for worsening. Previous studies have shown an inherent uncertainty around the MID estimates(5, 14, 15) and experts have recommended using several anchors. Our primary anchor was fatigue scale but we also performed our analyses using overall and pain anchors as previous studies have shown an association between pain, overall health, and fatigue(2). Our results were very similar using the 3 anchors.
Wells et al assessed MID estimates for fatigue VAS scale in 2 randomized controlled studies comparing abatacept vs. placebo in RA and found similar results; their MID estimates for improvement ranged from 6.7 to 17.0 for 2 studies on a 0–100 scale (or 0.67 to 1.70 on a 0–10 scale)(5). Apart from differences in our and Wells et al study design, we used external anchors to determine our MID estimates whereas Wells et al used internal anchors (change in HAQ-DI, pain VAS, and patient global VAS) based on previous studies. They followed their estimates with a Delphi exercise and reached a consensus estimate of 10 (on a 0–100 scale).
Previous literature has shown that an effect size of 0.20 to 0.50 corresponds to MID for a patient-reported outcome measure (14, 20). The effect size of the somewhat better and somewhat worsen group were small (0.39 and 0.44, respectively), supporting the MID results(20). In addition, the MID estimates were in the right direction and of larger magnitude than the no change group.
As previously noted(10, 15), the MID estimates may depend on the baseline scores. This trend was seen in our analysis (Table 2) where people with higher baseline scores required a larger change in their fatigue VAS for improvement to be considered as minimally improved. Conversely, patients with lower baseline scores required a larger change in their fatigue VAS for worsening. This may be related to floor and ceiling effect (where people near to bottom of the scale are limited by how much they can improve or top of the scale are limited by how much they can worsen) or may represent difference in interpretation of the scale along the continuum(25).
We introduce the concept of statistically significant change in individual patient-reported outcome scores rather than group scores. Reliable changes index (RCI) indicates whether change for an individual patient is beyond the measurement error of the instrument; RCI has the advantage of yielding a direct test of the significance of individual change(21). As expected, clinically significant change was larger for individual patients (3.47) compared to group change (−0.82 to −1.12 for improvement and 1.13 to 1.26 for worsening) and RCI decreased as the test-retest reliability of the VAS increased.
Our study is not without limitations. First, the global ratings of change ask people to remember how their health was at last visit and the retrospective self-reports are known to be subject to recall bias (13). Researchers have proposed prospective anchors and they should be studied in future studies(13). When using a prospective anchor, a person rates his/her pain, for example, at time1 and time2: mild, moderate, moderately severe, severe, very severe or unbearable. Those reporting a change over the 2 time points (e.g., “moderate” at time1 and “mild” at time2) constitute the minimal change subgroup. Second, the MID estimates are based on patients followed at one center and may need to be assessed in different clinical settings before the estimates can be generalized. Our patients were older than the average RA disease onset of 50 years and had mild functional disability as assessed by the HAQ-DI(26). The MID estimates may differ in younger patients and in those with moderate-to-high disability.
These limitations notwithstanding, we provide MID estimates for fatigue for improvement and worsening that can aid in interpreting fatigue VAS in day-to-day care in clinical practice and may be relevant for sample size calculations in observational studies.
Acknowledgment
Dr. Dinesh Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-01A1). Dr. Puja P. Khanna was supported by a National Institutes of Health Award (T32 AR 053463). Dr. Hays was supported by the UCLA Center for Health Improvement in Minority Elderly/Resource Centers for Minority Aging Research, NIH/NIA/NCMHD, under Grant 2P30-AG-021684 and a grant from the National Institute on Aging (AG 020679-01). Pfizer Inc. and Bristol-Meyer Squibs Inc. provided unrestricted grants for portions of the study but had no role in data collection, analysis, writing or submission of the manuscript.
References List
- 1.Kirwan JR, Hewlett S. Patient perspective: reasons and methods for measuring fatigue in rheumatoid arthritis. J Rheumatol. 2007 May;34(5):1171–1173. [PubMed] [Google Scholar]
- 2.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996 Aug;23(8):1407–1417. [PubMed] [Google Scholar]
- 3.Wolfe F, Michaud K. Fatigue, rheumatoid arthritis, and anti-tumor necrosis factor therapy: an investigation in 24,831 patients. J Rheumatol. 2004 Nov;31(11):2115–2120. [PubMed] [Google Scholar]
- 4.Kirwan JR, Minnock P, Adebajo A, Bresnihan B, Choy E, de Wit M, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol. 2007 May;34(5):1174–1177. [PubMed] [Google Scholar]
- 5.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007 Feb;34(2):280–289. [PubMed] [Google Scholar]
- 6.Wells GA, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality and activity limitation and quality of life following treatment with abatacept in patients with rheumatoid arthritis. Ann Rheum Dis. 2007 Sep 25; doi: 10.1136/ard.2007.069690. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F. Fatigue assessments in rheumatoid arthritis: comparative performance of visual analog scales and longer fatigue questionnaires in 7760 patients. J Rheumatol. 2004 Oct;31(10):1896–1902. [PubMed] [Google Scholar]
- 8.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989 Dec;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 9.Hays RD, Farivar S, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD: Journal of chronic obstructive pulmonary disease. 2005;(2):63–67. doi: 10.1081/copd-200050663. [DOI] [PubMed] [Google Scholar]
- 10.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003 May;56(5):395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 11.Khanna D, Tsevat J. Health-related quality of life--an introduction. Am J Manag Care. 2007 Dec;13 Suppl 9:S218–S223. [PubMed] [Google Scholar]
- 12.Guyatt GH, Norman GR, Juniper EF, Griffith LE. A critical look at transition ratings. J Clin Epidemiol. 2002 Sep;55(9):900–908. doi: 10.1016/s0895-4356(02)00435-3. [DOI] [PubMed] [Google Scholar]
- 13.Yost KJ, Sorensen MV, Hahn EA, Glendenning GA, Gnanasakthy A, Cella D. Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the Functional Assessment of Cancer Therapy-Biologic Response Modifiers (FACT-BRM) instrument. Value Health. 2005 Mar;8(2):117–127. doi: 10.1111/j.1524-4733.2005.08202.x. [DOI] [PubMed] [Google Scholar]
- 14.Khanna D, Furst DE, Wong WK, Tsevat J, Clements PJ, Park GS, et al. Reliability, validity, and minimally important differences of the SF-6D in systemic sclerosis. Qual Life Res. 2007 Aug;16(6):1083–1092. doi: 10.1007/s11136-007-9207-3. [DOI] [PubMed] [Google Scholar]
- 15.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally Important Difference in Diffuse Systemic Sclerosis- Results from the D-Penicillamine Study. Ann Rheum Dis. 2006 Mar 15;65(10):1325–1329. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980 Feb;23(2):137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 18.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008 Feb;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 20.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol. 2005 Dec;58(12):1217–1219. doi: 10.1016/j.jclinepi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson RJ, Robinson AB, Splaine M. Use of the reliable change index to evaluate clinical significance in SF-36 outcomes. Qual Life Res. 2002 Sep;11(6):509–516. doi: 10.1023/a:1016350431190. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Brodsky M, Johnston MF, Spritzer KL, Hui KK. Evaluating the statistical significance of health-related quality-of-life change in individual patients. Eval Health Prof. 2005 Jun;28(2):160–171. doi: 10.1177/0163278705275339. [DOI] [PubMed] [Google Scholar]
- 23.Kvien TK, Mowinckel P, Heiberg T, Dammann KL, Dale O, Aanerud GJ, et al. Performance of health status measures with a pen based personal digital assistant. Ann Rheum Dis. 2005 Oct;64(10):1480–1484. doi: 10.1136/ard.2004.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewlett S, Hehir M, Kirwan JR. Measuring fatigue in rheumatoid arthritis: a systematic review of scales in use. Arthritis Rheum. 2007 Apr 15;57(3):429–439. doi: 10.1002/art.22611. [DOI] [PubMed] [Google Scholar]
- 25.Hays RD, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000 Nov;18(5):419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan E, Sokka T, Hakkinen A, Hubert H, Hannonen P. Normative values for the Health Assessment Questionnaire disability index: benchmarking disability in the general population. Arthritis Rheum. 2004 Mar;50(3):953–960. doi: 10.1002/art.20048. [DOI] [PubMed] [Google Scholar]