Abstract
The role of the small Rho GTPase Rac2 in mature osteoclasts has not been extensively studied. Rac2−/− mice are of normal size and have normal tooth eruption. However, femoral cortical thickness was significantly greater in Rac2−/− compared to wild-type mice, while percent cortical porosity was lower. As assessed by histomorphometry, trabecular bone mass was significantly higher in male Rac2−/− than wild-type animals, although trabecular bone mass was similar when data from male and female animals were combined. There were no significant differences in the number of osteoblasts per bone surface; however, the number of osteoclasts per total bone area tended to be higher in Rac2−/− mice and was significantly higher in male Rac2−/− mice. In the aggregate, these data suggested a defect in osteoclast function and, consistent with that, rates of bone resorption were significantly reduced in Rac2−/− osteoclasts. In addition, Rac2−/− osteoclasts had a significantly delayed spreading response to treatment with CSF1 for 15 min. Phalloidin staining showed areas of abnormal actin accumulation and impaired actin ring formation in Rac2−/− osteoclasts. Finally, Rac2−/− osteoclasts showed a marked defect in chemotaxis toward a point source of CSF1, with a dramatic reduction in migratory rate. Together, these findings indicate an important role for Rac2 in mature osteoclasts.
Keywords: Osteoclast, Rac2, Chemotaxis, CSF1, Bone resorption
Bone remodeling is a complex, tightly regulated process that is initiated by a cycle of bone resorption mediated at the cellular level by osteoclasts [1]. A distinguishing feature of the resorbing activity of osteoclasts is the ability to move along the bone surface. After excavating a resorbing pit, the sealing zone of an osteoclast is disassembled and the cell moves to a new site of resorption. The signals that attract osteoclasts to new sites of resorption are still unclear. One possible signal may be colony-stimulating factor 1 (CSF1).
Lea et al. [2] demonstrated a central role for CSF1 in bone remodeling. They showed that estrogen withdrawal (a major cause of skeletal loss) is associated with upregulation in the expression of the cell-surface form of CSF1. This is consistent with the idea that estrogen withdrawal leads to local upregulation of CSF1 in bone, causing increased osteoclast formation and activity. Indeed, interdicting the ability of estrogen to upregulate CSF1 expression has been shown to prevent estrogen-deficiency bone loss [3, 4].
CSF1 clearly affects osteoclastogenesis and must therefore have osteoclast progenitors as one of its targets in bone. However, the CSF1 receptor c-fms is most highly expressed on mature osteoclasts [5]. We and others have reported that CSF1 induces cell spreading, motility, and actin reorganization in mature osteoclasts [6–9].
It is generally agreed that motility involves actin remodeling at the leading edge of the cells, resulting in broad skirt-like cytoplasmic extensions called “lamellipodia.” Integrin-mediated adhesion anchors the leading portion of the cell, while cytoplasmic retraction and release of integrin-mediated adhesion characterizes the trailing edge of the cell [10, 11]. In several cell systems, the Rho family small GTPases—Rac, Rho, and Cdc42—are required intermediaries for growth factor–dependent actin remodeling and motility [12]. Activated Rac mediates lamellipodia formation, while Cdc42 stimulates filopodia formation and Rho mediates retraction fiber assembly [12]. Several groups, including our own, have established that Rac plays a central role in mediating cytoskeletal reorganization in osteoclasts [9, 13, 14].
There are three Rac isoforms, Rac1, Rac2, and Rac3. Despite extensive sequence identities, work in knockout mice has highlighted nonredundant roles for these isoforms in several tissues including the immune system, skin, and central nervous system. The roles of the Rac isoforms in osteoclasts have not been extensively studied, and in particular, a distinct function, if any, for Rac2 in these cells has not been well described. Recently, Wang et al. [15] studied the relative contributions of Rac1 and Rac2 to osteoclastogenesis and reported that, although both isoforms contributed to this process, Rac1 had a more important role. Here, we report findings in osteoclasts isolated from Rac2-deficient mice. The genetic absence of Rac2 does not affect cell survival, but these cells have an abnormal actin cytoskeleton, reduced basal rates of bone resorption, and impaired cytoskeletal remodeling and chemotaxis in response to CSF1.
Materials and Methods
Rac2 Knockout Mice
Rac2−/− mice were kindly provided by Dr. David A. Williams (Children’s Hospital Medical Center, Cincinnati OH) [16]. All animal studies were conducted with approval of the Yale Animal Care and Use Committee.
Materials
Recombinant human CSF1 was a generous gift from Genetics Institute (Cambridge, MA). α-MEM cell culture medium was purchased from Sigma-Aldrich (St. Louis, MO), and fetal bovine serum (FBS) was from either Sigma-Aldrich or Atlanta Biologicals (Lawrenceville, GA). Etched gridded coverslips were purchased from Bellco Glass (Vineland, NJ). Matrigel was purchased from BD Biosciences Discovery Labware (Bedford, MA). Type I collagen was purchased from Nitta Gelatin (Osaka, Japan). The Rac 1 antibody, purchased from Cytoskeleton (Denver, CO; catalog ARC 03), is a mouse monoclonal antibody specific for Rac1 that does not recognize Rac2. Rac2 antibody was purchased from Upstate Biotechnologies (Charlottesville, VA; catalog 07–604). This is a polyclonal rabbit antibody raised against residues 1–192 of human Rac2. Akt and phospho-Akt antibodies were purchased from Cell Signaling (Danvers, MA; catalog 9272 and 9271, respectively). The Akt antibody is a polyclonal rabbit antibody raised against the C-terminal sequence of mouse Akt. The phosphoAkt antibody is a polyclonal rabbit antibody raised against the sequences surrounding Ser473 in mouse Akt. This antibody only recognizes Akt when the molecule is phosphorylated at Ser473. The antibody to actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA; catalog sc-47778).
Histomorphometry and micro-CT Analyses
Histomorphometric analyses of proximal tibiae from wild-type and Rac2−/− mice, 8–9 weeks old, were performed as previously described [17, 18]. Micro-CT analysis was performed on femora of wild-type and Rac2−/− mice using a conebeam micro-CT instrument (µCT40; Scanco Medical, Bassersdorf, Switzerland) at the University of Connecticut Health Center Micro-CT Imaging Facility under the direction of Douglas J. Adams, PhD, Associate Professor of Orthopedics, as previously described [19, 20].
Isolation of Mature Osteoclasts
Mature osteoclasts were isolated from neonatal murine long bones by mechanical disaggregation as previously reported [21]. Freshly isolated mature osteoclasts were used in cell-spreading and migration assays. Osteoclast-like cells (OCLs) were generated in vitro by coculturing murine osteoblasts and bone marrow cells in the presence of calcitriol and prostaglandin E2 as previously described [21]. To prepare Rac2−/− OCLs, bone marrow isolated from Rac2−/− animals was cocultured with primary osteoblast cultures prepared from neonatal Rac2−/− mice. OCLs from wild-type and Rac2−/− animals were used to isolate RNA and to prepare cell lysates for Western blotting. OCLs were generated on collagen-coated dishes as described by Suda and colleagues [22]. After 6 days, OCLs were released from the collagen gels by collagenase digestion. They were then replated and used in survival, attachment, and pit-formation assays.
Survival Assay
After purification, mature OCLs prepared from either wild-type or Rac2−/− animals were replated into a 96-well plate and cultured in α-MEM cell culture medium containing 2% FBS and either vehicle or 2.5 nM CSF1. In this assay α-MEM containing 10% FBS served as a positive control. Cells were fixed with 3.7% formaldehyde after 0, 9, and 24 h in culture and stained for tartrate-resistant acid phosphatase (TRAP). Only intact, TRAP-positive, multinuclear (three or more nuclei/cell) cells were counted.
RT-PCR for Rac1 and Rac2
RNA was isolated from mature, wild-type, and Rac2−/− OCLs using an RNeasy kit (Qiagen, Valencia, CA) and used in RT-PCRs for Rac1 and Rac2. The forward primer for Rac-1 is 5′-ctgaagtgcgacaccactgt-3′, and the reverse primer is 5′-cttgagtcctcgctgtgtga-3′. The expected PCR fragment size is 203 bp. The forward primer for Rac-2 is 5′-catcagctacaccaccaacg-3′, and the reverse primer is 5′-ttggtacccaccaggatgat-3′. The expected PCR fragment size is 288 bp. The protocol employed SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, CA). cDNA synthesis was carried out at 50°C for 30 min, followed by denaturation at 94°C for 2 min. Amplification of DNA was carried out for 37 cycles of 94°C denaturation for 15 s, 55°C annealing for 30 s, and 72°C extension for 30 s.
Protein Assay
The concentration of protein in cell lysates was measured using a commercially available kit (BCA Protein Assay Kit; Pierce, Rockford, IL) and the manufacturer’s recommended protocol. Absorbance was measured at 560 nm using a VICTOR3 1420 multilabel plate reader (Perkin-Elmer, Waltham, MA).
Immunoblotting
OCLs cultured on plastic were used for immunoblotting assay. After 6 days in coculture, osteoblasts were removed by flushing with PBS containing 5.5 µM EDTA. Cells were then treated with α-MEM plus 2% FBS containing either vehicle or 2.5 nM CSF1 for 10 min. After 10 min, cells were rinsed with ice-cold PBS and lysed in HNTG lysis buffer (50 mM HEPES, 150 mM NaCl, 1% Triton X-100, and 10% glycerol containing 1 µM PMSF, 10 µg/mL aprotinin, 10 µg/mL leupeptin, and 1 mM sodium vanadate). Equal amounts of clarified cell lysates were subjected to SDS polyacrylamide gel electrophoresis and transferred onto nitrocellulose paper (Trans-Blot Transfer Medium; Bio-Rad, Hercules, CA). After transfer, blots were blocked in Tris-buffered saline containing 0.05% Tween-20 and either 5% bovine serum albumin or 5% milk and then probed with the relevant primary antibody. Blots were developed using HRP-conjugated secondary antibodies following by enhanced chemiluminescence detection (ECL detection kit; Amersham, Piscataway, NJ).
To determine expression of Rac1, whole-cell lysates were prepared from untreated mature, wild-type, and Rac2−/− OCLs and analyzed by Western blotting for expression of Rac1 using an antibody specific for that isoform that does not recognize Rac2. Blots were then stripped and reprobed for actin.
Phalloidin Staining of Osteoclasts
Freshly isolated osteoclasts from wild-type and Rac2−/− mice were plated on FBS glass coverslips; treated with 2.5 nM CSF1 at 37°C for 0, 15, or 30 min; and fixed and stained with fluorescein-conjugated phalloidin (Molecular Probes, Eugene OR) using our previously published protocol [23]. Cells were imaged using a laser-scanning confocal microscope with an argon laser (LSM 510 Meta; Carl Zeiss Microimaging, Thornwood, NY).
To assess actin ring formation, freshly isolated osteoclasts from wild-type and Rac2−/− mice were plated on FBS-coated glass coverslips, left untreated at 37°C. and fixed, stained, and imaged 6 h later.
Spreading Assay
Freshly isolated osteoclasts were plated onto gridded coverslips for 1.5 h in α-MEM containing 10% FBS. The FBS concentration was then reduced to 2% for an additional 1.5 h. Coverslips were washed, and the concentration of FBS was reduced to 1%. Images of osteoclasts were recorded before and after 15 and 30 min of treatment with either 2.5 nM CSF1 or vehicle. Changes in cell area were quantified using NIH Image software, version 1.34 s (NIH, Bethesda, MD), and expressed as a percent change from baseline cell area.
Osteoclast Migration Assay
Authentic osteoclasts were plated on gridded glass coverslips (Bellco Glass) in α-MEM containing 10% FBS for 1.5 h. The FBS concentration was then reduced to 2% for an additional 1.5 h. Coverslips were washed and the concentration of FBS reduced to 1%. A point source of CSF1 was prepared by adding CSF1 to Matrigel at a final concentration of 2.5 nM. The Matrigel containing CSF1 was placed into a 22-gauge needle, and the tip of the needle (with the bevel facing the grid) was placed 200 µm from the midpoint of the front of the grid. This results in the generation of a 1% gradient of CSF1. Osteoclasts were tracked by serial photographs using a CCD camera. The distance migrated toward the point source was calculated as the total distance each cell traveled toward the point source of CSF1. Cells were considered to be moving toward the point source if they migrated up the CSF1 gradient at an angle greater than 0°. Measurements were made using Adobe Photoshop 7.0 software (Adobe, San Jose, CA).
In Vitro Resorption Assays
To quantify resorptive activity, wild-type or Rac2−/− OCLs, prepared on collagen gels, were replated onto Osteologic™ discs (BD Biosciences Discovery Labware) and cultured in α-MEM with 10% FBS. After 5 days, cells were removed using bleach and the resorptive surface was quantified using Adobe Photoshop 7.0 and NIH Image J software. Data are presented as mean percent of surface resorbed per field. Resorbed area was quantified in 205 separate fields for each determination. Fields were identified at 100× magnification in six separate discs. To confirm the findings using Osteologic discs, OCLs were also plated on dentine slices (OsteoSite Dentine Disc; Immunodiagnostic Systems, Fountain Hills, AZ) for 3 days and the concentration of the crosslinked C-teleopeptide of type I collagen (CTX) was quantified in the media.
Determining the Osteoclastogenic Potential of Rac2−/− Bone Marrow
To determine the effect of the genetic absence of Rac2 on osteoclastogenesis, bone marrow was isolated from wild-type and knockout mice. Red blood cells were lysed on ice for 5 min. Mononuclear cells were centrifuged, washed with PBS, and seeded in phenol red-free α-MEM containing 10% FBS and CSF1 at a final concentration of 100 ng/mL. Twenty-four hours later, nonadherent cells were harvested, layered onto an equivalent volume of Phycol-Hypaque, and centrifuged at 700×g for 20 min. Cells at the interface were collected, washed in PBS twice, and plated at a final concentration of 5 × 105 cells/gridded 60-mm dish. Cells were cultured in phenol red-free α-MEM containing 10% FBS, 75 ng/mL CSF1, 75 ng/mL RANKL, 10 ng/mL TNF-α, and 50 µg/mL AICAR, which has been previously reported to induce osteoclastogenesis [24]. The medium was changed every 3 days. After 7 days in culture, cells were fixed and TRAP-positive cells with three or more nuclei were counted as osteoclasts. The grid of the 60-mm dish was comprised of 62 squares, each 24 mm2 in area. Twenty-five squares were counted, and the mean number of cells/600 mm2 was calculated.
Results
Rac2−/− Mice Have Significantly Increased Bone Mass
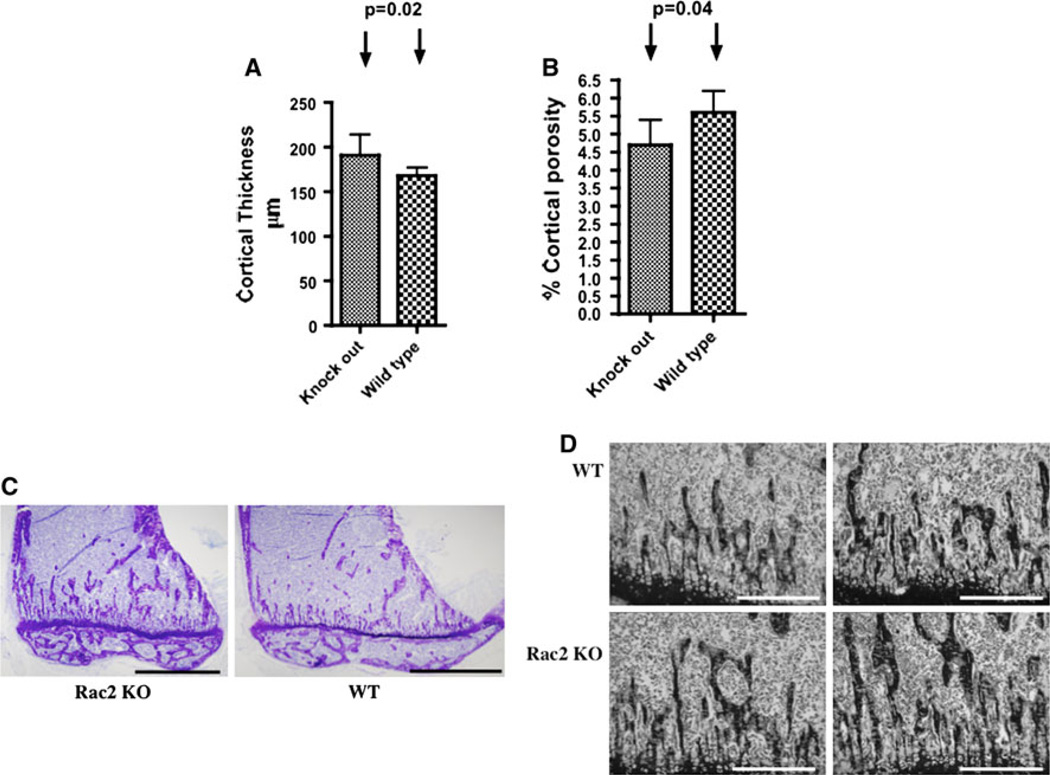
Rac2−/− mice were weaned without difficulty, had normal tooth eruption, and grew at a rate comparable to wild-type littermates. When quantified by micro-CT analysis, femoral cortical thickness was significantly higher in Rac2−/− compared to wild-type mice (191 ± 0.02 vs. 168 ± 0.1 µm, P = 0.02), while percent cortical porosity was lower (4.7 ± 0.7 vs. 5.6 ± 0.6%, P = 0.04) (Fig. 1a, b). As assessed by histomorphometry, trabecular bone mass was significantly higher in male Rac2−/− than male wild-type animals (Fig. 1c, d; Table 1), although trabecular bone mass was similar when data from male and female animals were combined (Table 2). There were no significant differences in the number of osteoblasts per bone surface; however, the number of osteoclasts per total bone area tended to be higher in Rac2−/− mice (Table 2), and in male Rac2−/− mice the difference was at the level of significance (P = 0.05) (Table 1). Increased bone density in the setting of increased numbers of osteoclasts suggests a possible defect in osteoclast function as the basis for this skeletal abnormality. Since Rac has been reported to be important for osteoclast function while effects in osteoblasts have not been reported, we focused on osteoclast function in these mice.
Fig. 1.
Both cortical parameters and trabecular bone mass are increased in Rac2−/− mice. a Mean cortical thickness (in micrometers) in 10 Rac2−/− mice and 10 wild-type controls. b Percent cortical porosity in 10 Rac2−/− mice and 10 wild-type controls. c Representative low-power view (×4) of proximal tibiae from 8-week-old male Rac2−/− (left panel) and wild-type (right panel) mice. Bar = 2 mm. d Representative sections of proximal tibial metaphyseal bone demonstrating increased trabecular bone volume in Rac2−/− male mice 8–9 weeks old (lower two panels) in comparison to age-matched wild-type mice (upper two panels, ×10 magnification). Bar = 500 µm
Table 1.
Bone histomorphometry in Rac2−/− and wild-type male mice
| n | BV/TV | Noc/TAR | Nob/TAR | ObS/BS | OS/BS | |
|---|---|---|---|---|---|---|
| Rac2 KO | 8 | 25.2 ± 1.8 | 102 ± 18.3 | 782 ± 95.1 | 20.3 ± 2.01 | 31.5 ± 3.13 |
| WT | 9 | 20.0 ± 0.9 | 63.1 ± 13.0 | 548 ± 83.1 | 20.5 ± 1.34 | 31.3 ± 1.97 |
| P | 0.02 | 0.05 | NS | NS | NS |
BV/TV bone volume as a percent of total tissue volume, Noc/TAR number of osteoclasts per total bone area, Nob/TAR number of osteoblasts per total bone area, ObS/BS osteoblast surface as a percentage of bone surface, OS/BS osteoblast surface as a percentage of bone surface, KO knockout, WT wild type
Table 2.
Bone histomorphometry in a combined analysis of male and female Rac2−/− and wild-type mice
| n | BV/TV | Noc/TAR | Nob/TAR | ObS/BS | OS/BS | |
|---|---|---|---|---|---|---|
| Rac2 KO | 17 | 20.2 ± 1.6 | 70.4 ± 11.7 | 589 ± 68.8 | 23.2 ± 1.6 | 35.2 ± 2.1 |
| WT | 21 | 19.2 ± 0.6 | 52.3 ± 7.2 | 465 ± 46.6 | 24.9 ± 1.3 | 36.1 ± 1.7 |
| P | NS | NS | NS | NS | NS |
See legend for Table 1
The Genetic Absence of Rac2 Results in Increased Levels of Expression of Rac1 in Osteoclasts
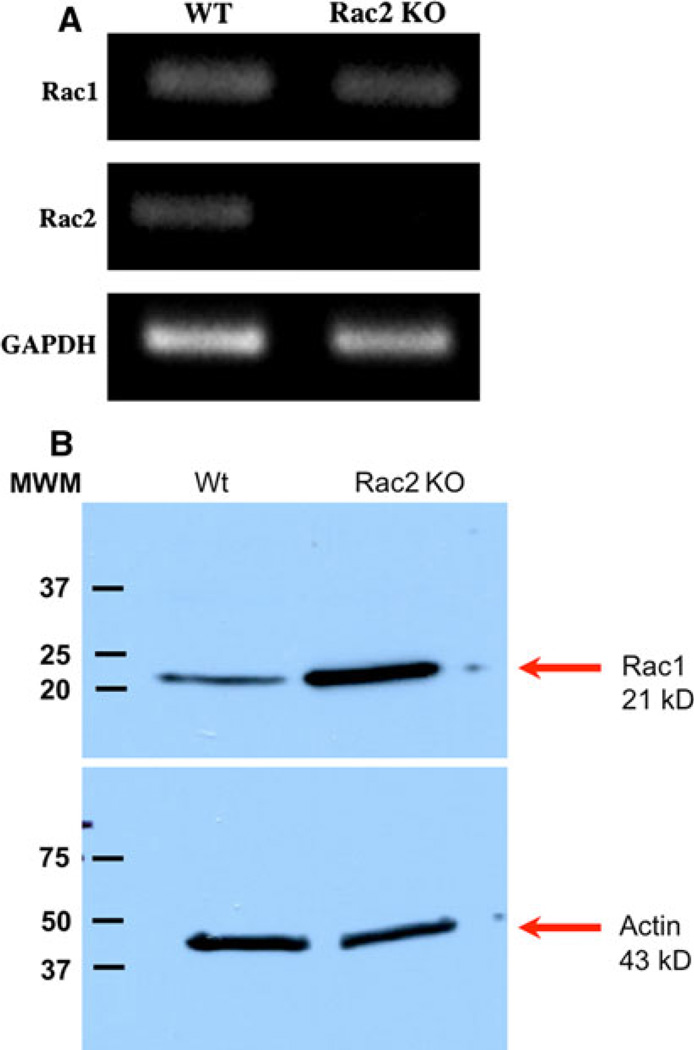
We first sought to determine if the genetic absence of Rac2 affected the level of expression of Rac1 in osteoclasts. We did not examine levels of Rac3 since its expression is largely confined to the central nervous system and levels of its transcript in osteoclasts are very low (T. I., unpublished observation). As demonstrated in Fig. 2a, the levels of Rac1 transcript were equivalent in wild-type osteoclasts and cells prepared from Rac2−/− mice. As expected, transcripts for Rac2 were not detected in Rac2−/− osteoclasts. Western analysis demonstrated a significant increase in Rac1 expression in Rac2−/− cells (Fig. 2b). The mean increase in Rac 1 expression in three separate cell preparations analyzed by Western blotting was 3.7-fold (P < 0.05).
Fig. 2.
Genetic absence of Rac2 increases the level of Rac1 protein but not transcript expression. a RT-PCR was performed using RNA isolated from wild-type (left column) or Rac2−/− (right column) OCLs. As shown in the upper panel, the level of Rac1 transcript expression is comparable in cells from wild-type and Rac2−/− animals. As expected, there was no Rac2 transcript seen in Rac2−/− OCLs (middle panel). GAPDH was used as an internal control for RT-PCR (lower panel). b Western blot analysis of whole-cell lysates from wild-type (left) and Rac2−/−(right) OCLs. The blot was initially probed with an antibody specific for Rac1 and then stripped and reprobed for actin as a loading control. The mean increase in Rac1 expression in three separate experiments was 3.7-fold (P < 0.05). Results are representative of findings in three separate experiments
Rac2 Is Not Required for CSF1-Dependent Prosurvival Effects in Osteoclasts
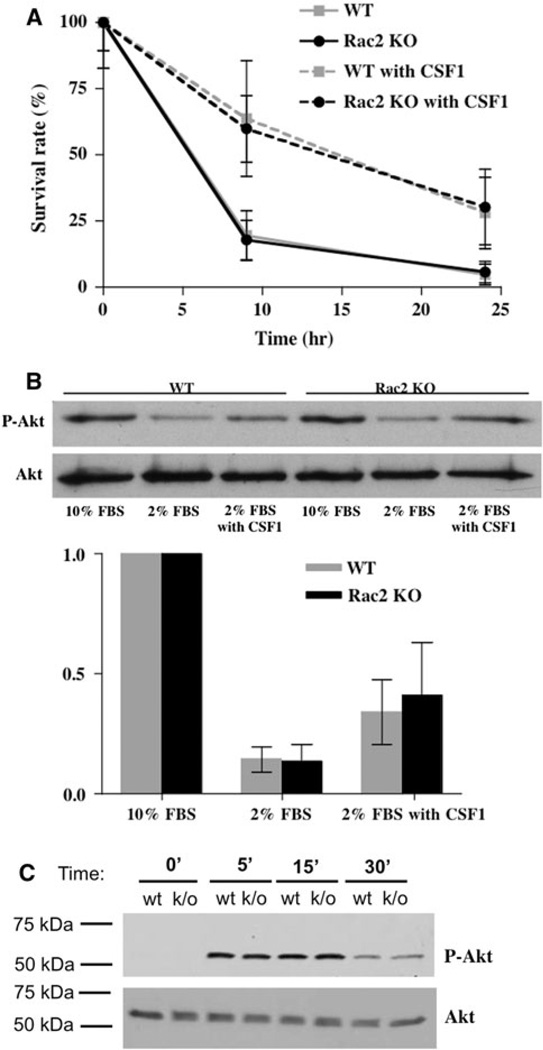
Rac plays an important role in mediating the prosurvival effects of CSF1 in osteoclasts [14, 25, 26]. We therefore next determined if osteoclast survival was affected by the genetic absence of Rac2. As shown in Fig. 3a, osteoclast survival under reduced serum conditions was the same in wild-type and Rac2−/− osteoclasts. Further, CSF1 prolonged cell survival to the same extent in wild-type and Rac2−/− cells. CSF1-dependent activation of Rac1 leads to activation of Akt, and the latter has been reported to be an important mediator of osteoclast survival [27, 28]. The CSF1/Rac1/Akt pathway has been reported to be important for the prosurvival effect of CSF1 in OCLs [14]. To determine if Rac2 contributes to this Akt-mediated survival pathway, the extent of Akt phosphorylation induced by CSF1 was examined in wild-type and Rac2−/− osteoclasts. Phosphorylation of Akt at position Ser473 is associated with Akt activation [29]; therefore, an antibody specific for phospho-Ser473 Akt was used to determine the extent of Akt activation following treatment with CSF1. As shown in Fig. 3b, treatment with CSF1 for 10 min induced comparable degrees of phosphorylation of Akt in wild-type and Rac2−/− OCLs. In addition, the time course of activation of Akt was identical in wild-type and Rac2−/− cells (Fig. 3c). These data indicate that Rac2 is not required for CSF1 activation of Akt and or for the prosurvival effects of the cytokine.
Fig. 3.
Rac2 does not affect apoptosis or Akt activation in OCLs. a Percent surviving wild-type (gray lines) and knockout (black lines) OCLs after 9 and 24 h of culture in the absence (solid lines) or presence (dashed lines) of 2.5 nM CSF1. Survival was the same for both types of cells in the absence of CSF1. CSF1 markedly prolonged the survival of both types of cells to the same extent. Data represent mean results from three separate experiments with an average of ~150 cells at the start of each experiment for each of the starting culture conditions. b Upper panel Representative Western blot of whole-cell lysates from wild-type OCLs (first three lanes) or Rac2−/− OCLs (second three lanes) probed for phospho-Akt (upper panel) or Akt (lower panel), the latter as a loading control. For each cell type, purified OCLs were cultured for 10 min in either 10% FCS, 2% FCS, or 2% FCS plus 2.5 nM CSF1. As expected, culturing cells in a reduced serum concentration reduced the level of Akt phosphorylation, which was partially restored by CSF1. Lower panel Mean data quantifying the extent of Akt phosphorylation (expressed as the ratio of phospho-Akt band intensity to Akt band intensity) from three separate experiments (one of which is shown in the upper panel). There were no significant differences between wild-type and knockout cells. c Time course of activation of Akt by CSF1. Upper panel Western blot for phospho-Akt at the indicated times after treatment with 2.5 nM CSF1. Lower panel The same blot stripped and reprobed with an antibody to Akt
The Actin Cytoskeleton of Rac2−/− Osteoclasts Is Abnormal
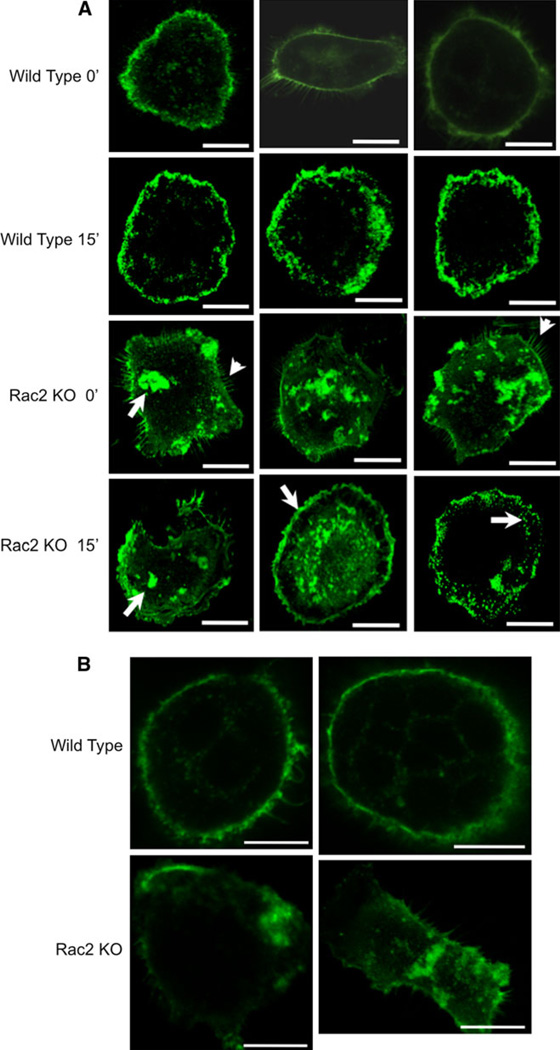
Since Rac plays an important role in cytoskeletal remodeling, we wondered whether Rac2 contributes to normal cytoskeletal architecture both in resting osteoclasts and in cells stimulated with CSF1. Mature osteoclasts were isolated from Rac2−/− and wild-type mice, fixed, and stained with phalloidin before or 15 min after treatment with CSF1. Untreated Rac2−/− osteoclasts evidenced areas of dense actin accumulation that were rarely seen in osteoclasts isolated from wild-type animals (Fig. 4a, first row compared with third row, see arrow in third row, first panel). Eighty-four percent (16/19) of authentic osteoclasts isolated from Rac2−/− osteoclasts exhibited dense actin accumulations. In contrast, only 12% (2/16) of wild-type cells exhibited a similar finding. Cells from knockout animals also demonstrated frequent filopodia formation, which was rarely seen in wild-type osteoclasts (Fig. 4a, third row, arrowheads). Following treatment with CSF1 for 15 min, wild-type cells showed accumulation of actin at the spreading edge of the cell (Fig. 4a, second row). While some Rac2−/− osteoclasts also showed areas of actin accumulation at the spreading edge (Fig. 4, fourth row, second panel, arrow), areas of dense actin accumulation persisted in many cells (Fig. 4a, fourth row, first panel, arrow). Finally, some Rac2−/− cells showed what appeared to be punctate areas of actin accumulation (Fig. 4a, fourth row, third panel, arrow).
Fig. 4.
Phalloidin staining of the actin cytoskeleton in Rac2−/− osteoclasts. a Freshly isolated mature osteoclasts isolated from wild-type and Rac2−/− mice were fixed and stained with phalloidin before or 15 min after treatment with 2.5 nM CSF1. White arrows indicate areas of dense actin accumulation (first column, third panel, arrow) and frequent filopodia formation (first column, third panel and third column, third panel; arrowheads in each panel) in Rac2−/− resting osteoclasts. In CSF1-treated Rac2−/− osteoclasts, areas of normal actin accumulation at the spreading edge of cells are seen (second column, fourth panel, arrow) but areas of dense actin accumulation persist (first column, fourth panel, arrow). Punctate areas of actin accumulation were also seen (third column, fourth panel, arrow). b Actin ring appearance in two wild-type osteoclasts (upper row) and two osteoclasts isolated from Rac2−/− animals (lower row). Cells were plated on FBS-coated glass slides for 6 h and then fixed, stained, and imaged by confocal microscopy. Bar = 20 µm in each panel of a and b
Actin ring formation was assessed in freshly isolated, untreated osteoclasts 6 h after plating on FBS-coated glass coverslips as described in “Materials and Methods” section. Only 23% (3/13) of Rac2−/− osteoclasts were able to form actin rings, while 92% (11/12) of wild-type cells had actin rings (Fig. 4b).
There was no observed difference in the number of nuclei per cell in wild-type and Rac2−/− osteoclasts. The mean number of nuclei in 19 mature osteoclasts isolated from Rac2−/− mice was 5.3 ± 0.8, with 4.9 ± 0.4 in 18 cells isolated from wild-type mice.
Increased Osteoclastogenic Potential of Rac2−/− Bone Marrow
Osteoclast precursors were isolated from the bone marrow of wild-type and Rac2−/− animals and cultured as described in Materials and Methods. After 7 days in culture, cells were fixed and TRAP-positive cells with three or more nuclei were counted as osteoclasts. The mean number of osteoclasts per 600 mm2 was 207 ± 8, when marrow from Rac2−/− animals was cultured under osteoclastogenic conditions, while the mean number of osteoclasts formed from the marrow of control animals was 119 ± 5 per 600 mm2. These values are statistically significantly different (P = 0.03). This finding is consistent with our in vivo findings of a trend toward a higher number of osteoclasts in Rac2−/− animals.
Rac2−/− Osteoclasts Exhibit a Delayed Spreading Response and Reduced Chemotaxis to CSF1
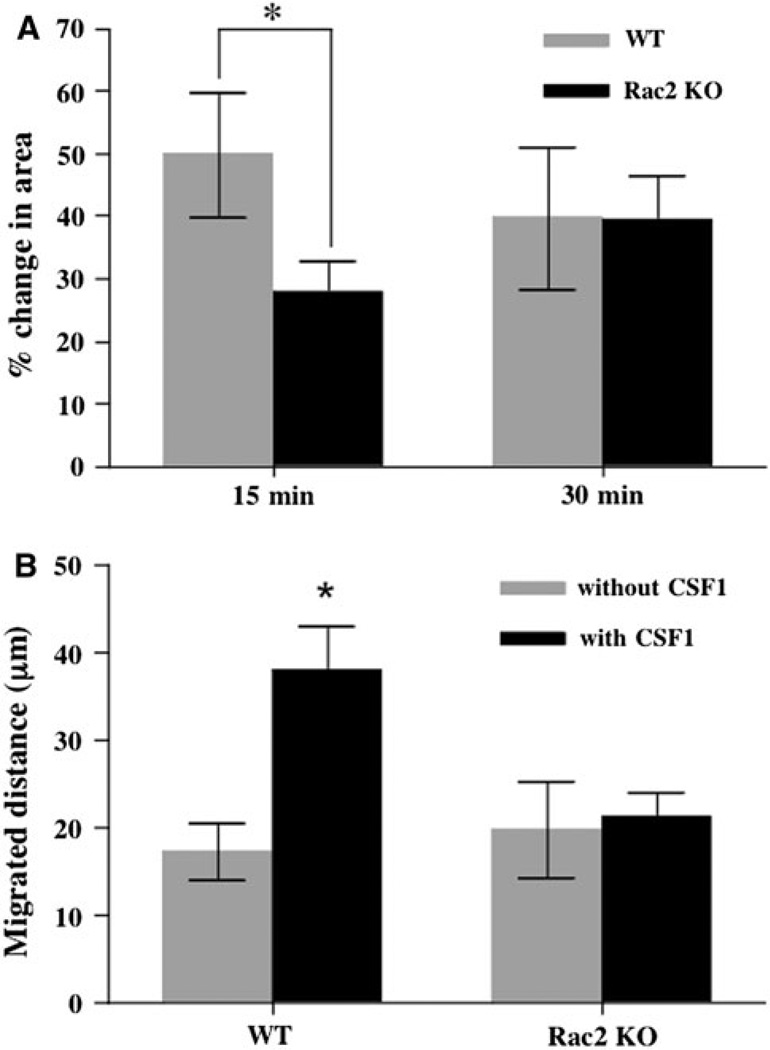
It is well established that CSF1 stimulates osteoclast motility and spreading [7, 21, 23, 30], and it has recently been reported that CSF1-dependent cytoskeletal remodeling requires Rac1 [9, 14]. To determine if Rac2 makes any contribution to these actions of CSF1, mature osteoclasts were isolated from wild-type and Rac2−/− mice and cell spreading and motility were quantified. After 15 min of treatment with 2.5 nM CSF1, the mean area of wild-type osteoclasts increased by 49.9 ± 9.9% (n = 47). In contrast, the mean area of Rac2−/− osteoclasts increased by only 27.8 ± 4.96% (n = 55). The difference in the spreading response to CSF1 was statistically significant (P = 0.05) (Fig. 5a). However, after 30 min of exposure to CSF1, there was no longer any difference in mean spread area between the two cell types and the final absolute mean area for both cell types was similar. This indicates that while cell spreading was significantly delayed in Rac2−/− cells, it eventually reached the same final spread area.
Fig. 5.
Rac2−/− osteoclasts have a delayed spreading response to CSF1 and do not migrate up a CSF1 gradient. a Mean increase in cell area (as percent of initial cell area) of freshly isolated mature osteoclasts in response to treatment with 2.5 nM CSF1 for 15 min (left two bars) or 30 min (right two bars). Mean data from three separate experiments are summarized. Data for wild-type osteoclasts are represented by the gray bars and those for Rac2−/− osteoclasts, by black bars. The response to CSF1 at 15 min was significantly attenuated in the knockout cells (P = 0.05). b Mean distance migrated by freshly isolated mature wild-type osteoclasts (left two bars) or Rac2−/− osteoclasts (right two bars) in the absence (gray bars) or presence (black bars) of a 1% CSF1 gradient. While the rate of random migration was comparable in the two cell types, Rac2−/− osteoclasts showed virtually no chemotactic response to CSF1 (P < 0.01 when comparing the mean distance traveled up the CSF1 gradient by the two cell types)
These data, together with the finding of an abnormal cytoskeletal response to CSF1 in Rac2−/− osteoclasts, indicate that Rac2 participates in CSF1-induced cytoskeletal remodeling. Since chemotaxis requires normal cytoskeletal remodeling, we next determined if Rac2 plays a role in CSF1-induced osteoclast migration. As shown in Fig. 5b, when Matrigel loaded with vehicle (0.1% BSA in PBS) was used in the chemotaxis assay, the mean distance wild-type cells traveled in the direction of this point source was 17.3 ± 3.4 µm (n = 20), while Rac2−/− osteoclasts migrated a mean distance of 20.2 ± 5.6 µm (n = 33) toward the vehicle-loaded needle over the 3 h of observation. These mean values are not statistically different. In striking contrast, when the needle was loaded with Matrigel containing 2.5 nM CSF1, wild-type osteoclasts migrated a mean distance of 38.7 ± 5.3 µm (n = 39) toward the point source, while Rac2−/− osteoclasts migrated only 21.4 ± 2.8 µm (n = 43), a difference that was highly significant (P < 0.01).
Rac2−/− Osteoclasts Have Impaired Resorptive Capacity
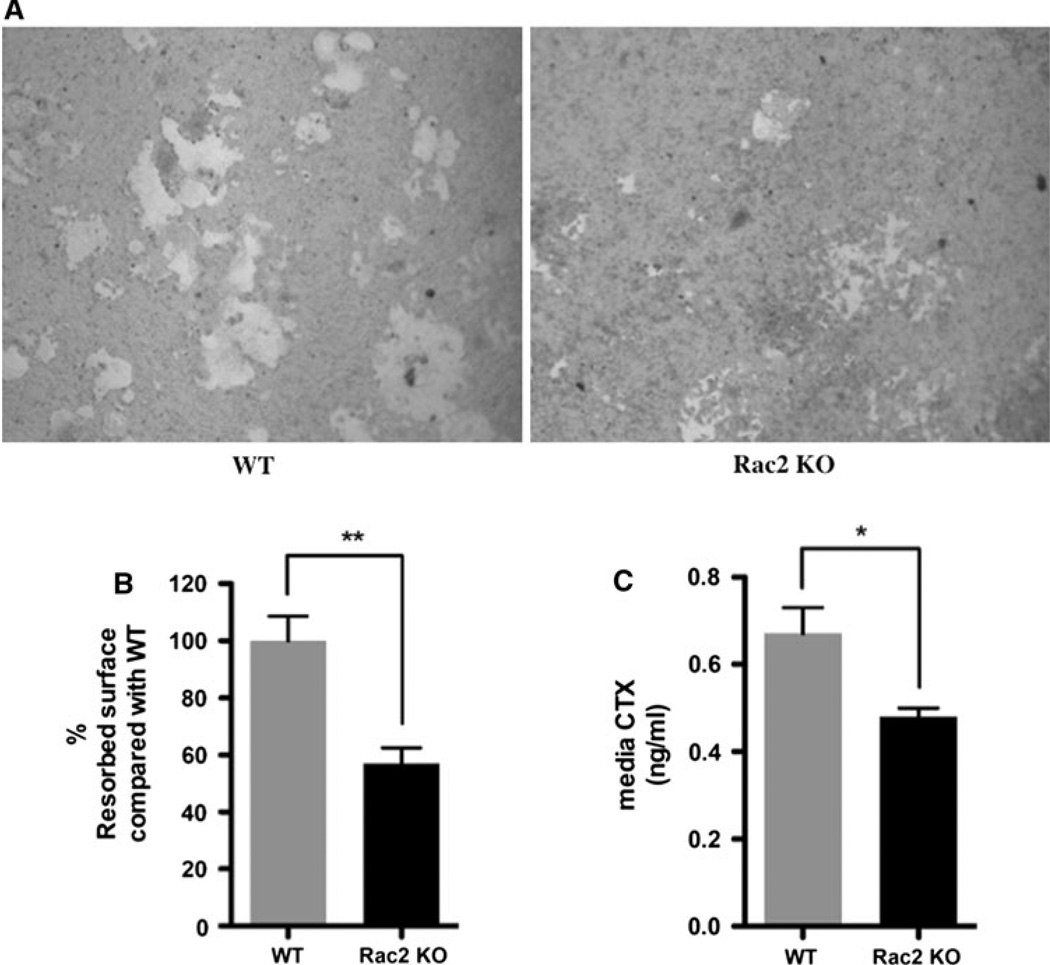
Finally, given the increase in bone mass in Rac2−/− mice, we were interested in determining if Rac2−/− osteoclasts had normal resorptive activity. Pit formation was quantified using Osteologic discs. As shown in Fig. 6a, b, wild-type osteoclasts resorbed a mean 4.03 ± 0.35% of the surface disc surface, while Rac2−/− osteoclasts only resorbed 2.30 ± 0.22% of the surface area, indicating a significant impairment in basal resorptive activity of these cells (P < 0.001). Consistent with the findings on Osteologic discs, when the resorptive activity of Rac2−/− osteoclasts was analyzed on dentine slices, we found that these cells showed significantly less basal resorptive activity. Thus, the mean concentration of CTX in the medium of Rac2−/− osteoclasts plated on dentine slices was significantly lower than that for wild-type cells (0.48 ± 0.02 vs. 0.67 ± 0.06 ng/mL, P = 0.04) (Fig. 6c).
Fig. 6.
Rac2−/− osteoclasts have reduced basal rates of resorption. a Representative appearance of resorption pits generated by wild-type OCLs (left) or Rac2−/− OCLs (right). b Summary of mean resorbed area from three separate experiments. In each experiment the mean area resorbed by wild-type cells was defined as 100%. Rac2−/− cells resorbed 43% less area than did wild-type cells (P < 0.001). c CTX concentration in medium of OCLs prepared from wild-type animals (left) or knockout mice (right) incubated on dentine slices for 72 h. Rac2−/− cells resorbed significantly less bone, as reflected by a significantly lower concentration of CTX in the medium (*P < 0.04). The concentration of CTX in medium alone was below the detection limit of the assay
Discussion
The three Rac isoforms, Rac1, -2, and -3, are highly homologous at the amino acid level. Murine Rac1 and Rac2 show 94% sequence identity. Rac3 shows strongest homology to Rac1 (90%), with divergence at the carboxy terminus, and less but still extensive homology with Rac2 (83%). Rac1 is ubiquitously expressed. Expression of Rac2 is restricted to hematopoietic tissue, while Rac3 is widely expressed, with the highest expression in the developing nervous system. Despite extensive sequence identities, recent work has highlighted the nonredundant roles of these three isoforms. Deletion of Rac1 leads to embryonic lethality, deletion of Rac2 results in abnormalities in neutrophil function and B- and T-cell development, while Rac3 knockout mice are characterized by behavioral abnormalities in adult animals [16, 31–35]. Fukuda et al. [14] and Sakai et al. [9] used dominant negative constructs to provide evidence that Rac1 is important for CSF1-induced cytoskeletal remodeling in osteoclasts.
The findings in the current study establish a nonredundant role for Rac2 in bone. Cortical thickness and density were greater in knockout mice, and male Rac2−/− mice also had higher trabecular bone mass. The cellular basis for these changes appears to be a defect in osteoclast function. While the genetic absence of Rac2 did not affect osteoclast survival, Rac2−/− osteoclasts showed reduced basal resorptive activity and a clear defect in the ability to respond to the chemoattractant CSF1. The finding of increased expression of Rac1 protein in the genetic absence of Rac2 was surprising and unexpected. It raises the interesting possibility that Rac1 to some extent compensates for Rac2. It will be of interest to study the phenotype of animals in which both Rac1 and Rac2 have been deleted in osteoclasts.
Rac2−/− osteoclasts showed a significantly delayed spreading response to CSF1 after 15 min of exposure to the cytokine. The fact that after 30 min Rac2−/− osteoclasts eventually spread to the same extent as did wild-type cells indicates that compensatory mechanisms are operative in the knockout cells. Rac2−/− osteoclasts displayed a normal level of random cellular migration in the absence of a chemoattractant gradient ~7 vs. 6 µm/h (Rac2−/− vs. wild type). Interestingly, it has recently been shown that Rac1 is required for normal rates of random migration in fibroblasts and epithelial cells [36], and as noted, we found that the level of Rac1 expression was actually increased in Rac2−/− osteoclasts. In contrast to the normal rate of random migration, Rac2−/− osteoclasts showed a much reduced rate of migration up a shallow gradient of CSF1. On average, cells isolated from knockout mice migrated only 56% as far as those isolated from wild-type mice. Wang et al. [15] reported on the role of Rac1 and Rac2 in regulating the motility of preosteoclasts. In contrast to our findings, Wang et al. did not observe a defect in CSF1-induced chemotaxis in Rac2−/− preosteoclasts, while Rac1−/− preosteoclasts failed to respond to the growth factor gradient. One reason for these divergent findings may be that Wang and coworkers studied preosteoclasts, while we studied mature osteoclasts. Differences in the cytoskeletal response of preosteoclasts and mature osteoclasts to CSF1 have been noted previously. For example, preosteoclasts prepared from Src−/− mice spread normally in response to CSF1, while mature osteoclasts do not [23, 37].
The contribution of Rac1 and Rac2 to cytoskeletal remodeling and motility in macrophages and leukocytes has also been studied. Rac1−/− macrophages have abnormal morphology and a reduced spreading response to CSF1 but show normal chemotaxis in response to CSF1 [38]. Rac2−/− macrophages have slightly reduced speeds of migration in a Dunn chamber assay using CSF1 as a chemoattractant [39] and significantly reduced rates of migration in a transwell assay using vitronectin [40]. Using a series of extracellular matrix substrates, Pradip et al. [40] concluded that in bone marrow macrophages Rac2 was required for αvβ3 integrin–dependent migration. It is noteworthy that αvβ3 is the principal integrin used by osteoclasts. Roberts et al. [16] and Gu et al. [32] also reported significant defects in chemotaxis of Rac2−/− leukocytes in response to the chemoattractant fMetLeuPhe. Thus, in macrophages, leukocytes, and mature osteoclasts, the genetic absence of Rac2 seems to result in impaired chemotaxis.
Phalloidin staining revealed an abnormal actin cytoskeleton in Rac2−/− osteoclasts both at rest and following CSF1 treatment, indicating a nonredundant function for this small GTPase in actin remodeling in these cells. Our findings are consistent with the studies of Pradip, Roberts, Gu, and coworkers summarized above, indicating that integrin-mediated actin remodeling and motility were impaired in the absence of Rac2. In addition, we found significantly impaired actin ring formation in Rac2−/− osteoclasts. The ability to form actin rings was reduced by 75% in Rac2−/− osteoclasts compared to cells isolated from wild-type animals. Razzouk et al. [41] reported similar cytoskeletal changes in rat osteoclasts microinjected with a neutralizing antibody to Rac2. They found that neutralizing Rac2 inhibited actin ring formation by 48% and led to the appearance of abnormal accumulations of actin that are not unlike those we observed.
We found an increase in the number of osteoclasts present in bone in vivo in Rac2−/− mice. There was also an increase in the osteoclastogenic potential of the marrow from Rac2−/− animals when cultured in vitro. These changes could be interpreted as a compensatory response to the decrease in resorptive efficiency of the mature cells. A similar trend toward increased osteoclast numbers has been reported in Src−/− mice, which have osteoclasts with impaired resorptive activity [42]. However, Wang et al. [15] reported decreased numbers of osteoclasts in Rac2−/− mice. We used histomorphometry to quantify osteoclasts, while Wang and colleagues used TRAP and immunohistochemical staining for cathepsin K to assess osteoclast number. Since they also observed that expression of these markers is delayed during osteoclastogenesis in Rac2−/− cells, it is possible that they underestimated the actual number of osteoclasts present in vivo.
The Rac2−/− osteoclasts showed a normal survival rate when cultured in medium containing reduced concentrations of serum, suggesting that Rac2−/− did not play a role in the response to that apoptotic signal. Consistent with this, the ability of the prosurvival factor CSF1 to induce phosphorylation of Akt was not influenced by the absence of Rac2. Indeed, Fukuda et al. [14] reported that it is Rac1 which is required for CSF1’s prosurvival actions and that inhibiting Rac1 prevented CSF1-induced Akt phosphorylation in osteoclasts.
In the absence of Rac2, basal osteoclast resorptive rates were significantly reduced (Fig. 6). Since we used calcium phosphate-coated discs rather than bone slices, these results may not reflect resorptive activity in vivo. However, our data demonstrating reduced release of collagen fragments by Rac2−/− osteoclasts cultured on bone slices support the relevance of our findings. Further, Razzouk et al. [41] found that, in addition to the cytoskeletal changes noted above, a neutralizing antibody to Rac2 significantly inhibited the resorbing activity of mature rat osteoclasts when these cells were cultured on bone slices. Finally, Wang et al. [15] reported that OCLs prepared from Rac2−/− mice have reduced resorptive activity. The reason for this defect in basal resorptive rates is not clear, but one likely possibility is that it is due to the combined defects in actin ring formation and chemotaxis observed in Rac2−/− osteoclasts. Normal resorption requires that osteoclasts move from one resorption site to the next and form effective sealing zones at each site. The therapeutic implications of defective resorptive activity in Rac2−/− osteoclasts have been explored by Kawano and coworkers [43]. They reported that Rac2−/− mice treated with single daily subcutaneous doses of parathyroid hormone had a significantly greater anabolic response in bone than did wild-type littermates. They speculated that this was due to the fact that the increase in bone resorption, which eventually occurs during anabolic PTH regimens, was attenuated in the Rac2−/− mice, allowing for a greater increase in bone mass.
In summary, our in vivo and in vitro data demonstrate that Rac2 is required for normal bone remodeling by playing a nonredundant role in regulating osteoclast cytoskeletal remodeling, motility, and resorptive activity.
Acknowledgment
This work was supported by NIH grants DE12459 and DK45228 and by the Yale Core Center for Musculoskeletal Disorders, which is supported by a P30 Core Center Award from NIAMS (AR46032).
Footnotes
The authors have stated that they have no conflict of interest.
Contributor Information
Takashi Itokowa, Department of Medicine, Yale School of Medicine, 333 Cedar St., TAC S133, New Haven, CT 06520-8020, USA, itok81@aol.com.
Mei-ling Zhu, Department of Medicine, Yale School of Medicine, 333 Cedar St., TAC S133, New Haven, CT 06520-8020, USA, meiling.zhu@yale.edu.
Nancy Troiano, Department of Orthopedics and Rehabilitation, Yale School of Medicine, 333 Cedar St., New Haven, CT 06520, USA, nancy.troiano@yale.edu.
Jessica Bian, Department of Medicine, Yale School of Medicine, 333 Cedar St., TAC S133, New Haven, CT 06520-8020, USA, jessica.bian@yale.edu.
Tustomu Kawano, Department of Medicine, Yale School of Medicine, 333 Cedar St., TAC S133, New Haven, CT 06520-8020, USA, tsutomu@ortho.med.kyushu-u.ac.jp.
Karl Insogna, Department of Medicine, Yale School of Medicine, 333 Cedar St., TAC S133, New Haven, CT 06520-8020, USA, karl.insogna@yale.edu.
References
- 1.Roodman GD. Advances in bone biology: the osteoclast. Endocr Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- 2.Lea CK, Sarma U, Flanagan AM. Macrophage colony stimulating-factor transcripts are differentially regulated in rat bone-marrow by gender hormones. Endocrinology. 1999;140:273–279. doi: 10.1210/endo.140.1.6451. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, Weitzmann MN, Kimble RB, Rizzo M, Zahner M, Milbrandt J, Ross FP, Pacifici R. Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp-1. J Clin Invest. 1998;102:1850–1859. doi: 10.1172/JCI4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J Clin Invest. 2000;105:1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir EC, Horowitz MC, Baron R, Centrella M, Kacinski BM, Insogna KL. Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res. 1993;8:1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- 6.Owens J, Chambers TJ. Macrophage colony-stimulating factor (M-CSF) induces migration in osteoclasts in vitro. Biochem Biophys Res Commun. 1993;195:1401–1407. doi: 10.1006/bbrc.1993.2199. [DOI] [PubMed] [Google Scholar]
- 7.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grey A, Chen Y, Paliwal I, Carlberg K, Insogna K. Evidence for a functional association between phosphatidylinositol 3-kinase and c-src in the spreading response of osteoclasts to colony-stimulating factor-1. Endocrinology. 2000;141:2129–2138. doi: 10.1210/endo.141.6.7480. [DOI] [PubMed] [Google Scholar]
- 9.Sakai H, Chen Y, Itokawa T, Yu KP, Zhu ML, Insogna K. Activated c-Fms recruits Vav and Rac during CSF-1-induced cytoskeletal remodeling and spreading in osteoclasts. Bone. 2006;39:1290–1301. doi: 10.1016/j.bone.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz AR, Parsons JT. Cell migration—movin’ on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 12.Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110(Pt 6):707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 13.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, Ross FP, Swat W. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda A, Hikita A, Wakeyama H, Akiyama T, Oda H, Nakamura K, Tanaka S. Regulation of osteoclast apoptosis and motility by small GTPase binding protein Rac1. J Bone Miner Res. 2005;20:2245–2253. doi: 10.1359/JBMR.050816. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Lebowitz D, Sun C, Thang H, Grynpas MD, Glogauer M. Identifying the relative contributions of rac1 and rac2 to osteoclastogenesis. J Bone Miner Res. 2008;23:260–270. doi: 10.1359/jbmr.071013. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell–specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 17.Knopp E, Troiano N, Bouxsein M, Sun BH, Lostritto K, Gundberg C, Dziura J, Insogna K. The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology. 2005;146:1983–1990. doi: 10.1210/en.2004-0770. [DOI] [PubMed] [Google Scholar]
- 18.Insogna KL, Stewart AF, Vignery AM, Weir EC, Namnum PA, Baron RE, Kirkwood JM, Deftos LM, Broadus AE. Biochemical and histomorphometric characterization of a rat model for humoral hypercalcemia of malignancy. Endocrinology. 1984;114:888–896. doi: 10.1210/endo-114-3-888. [DOI] [PubMed] [Google Scholar]
- 19.Lee S-K, Kadono Y, Okada F, Claire Jacquin C, Koczon-Jaremko B, Gloria Gronowicz G, Adams DJ, Aguila HL, Choi Y, Lorenzo JA. T lymphocyte deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
- 20.Harrison JR, Huang YF, Wilson KA, Kelly PL, Adams DJ, Gronowicz GA, Clark SH. Col1a1 promoter-targeted expression of p20 CCAAT enhancer-binding protein beta (C/EBPbeta), a truncated C/EBPbeta isoform, causes osteopenia in transgenic mice. J Biol Chem. 2005;280:8117–8124. doi: 10.1074/jbc.M410076200. [DOI] [PubMed] [Google Scholar]
- 21.Insogna K, Tanaka S, Neff L, Horne W, Levy J, Baron R. Role of c-Src in cellular events associated with colony-stimulating factor-1-induced spreading in osteoclasts. Mol Reprod Dev. 1997;46:104–108. doi: 10.1002/(SICI)1098-2795(199701)46:1<104::AID-MRD16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Suda T, Jimi E, Nakamura I, Takahashi N. Role of alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 1997;282:223–235. doi: 10.1016/s0076-6879(97)82110-6. [DOI] [PubMed] [Google Scholar]
- 23.Insogna KL, Sahni M, Grey AB, Tanaka S, Horne WC, Neff L, Mitnick M, Levy JB, Baron R. Colony-stimulating factor-induces cytoskeletal reorganization and c-src-dependent tyrosine phosphorylation of selected cellular proteins in rodent osteoclasts. J Clin Invest. 1997;100:2476–2485. doi: 10.1172/JCI119790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn J, Tam S, Sims N, Saleh H, McGregor N, Poulton Walker E, Scott J, Kemp B, Gillespie M. Mice lacking AMP-activated kinase (AMPK) subunits β1 or β2 have low bone mass, while AICAR acts AMPK-independently to increase osteoclast formation. Bone. 2009;44:S136. [Google Scholar]
- 25.Jimi E, Shuto T, Koga T. Macrophage colony-stimulating factor and interleukin-1alpha maintain the survival of osteoclast-like cells. Endocrinology. 1995;136:808–811. doi: 10.1210/endo.136.2.7835314. [DOI] [PubMed] [Google Scholar]
- 26.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 27.Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activation of Akt and ERK. J Biol Chem. 2001;276:49343–49349. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, Nakamura K, Tanaka S. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000;148:333–342. doi: 10.1083/jcb.148.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakamoto K, Arnolds DE, Ekberg I, Thorell A, Goodyear LJ. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem Biophys Res Commun. 2004;319:419–425. doi: 10.1016/j.bbrc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Palacio S, Felix R. The role of phosphoinositide 3-kinase in spreading osteoclasts induced by colony-stimulating factor-1. Eur J Endocrinol. 2001;144:431–440. doi: 10.1530/eje.0.1440431. [DOI] [PubMed] [Google Scholar]
- 31.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, Katsuki M. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 33.Walmsley MJ, Ooi SK, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Yamauchi A, Marchal CC, Molitoris JK, Quilliam LA, Dinauer MC. Chemoattractant-stimulated Rac activation in wild-type and Rac2-deficient murine neutrophils: preferential activation of Rac2 and Rac2 gene dosage effect on neutrophil functions. J Immunol. 2002;169:5043–5051. doi: 10.4049/jimmunol.169.9.5043. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta S, Gualdoni S, Albertinazzi C, Paris S, Croci L, Consalez GG, de Curtis I. Generation and characterization of Rac3 knockout mice. Mol Cell Biol. 2005;25:5763–5776. doi: 10.1128/MCB.25.13.5763-5776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pankov R, Endo Y, Even-Ram S, Araki M, Clark K, Cukierman E, Matsumoto K, Yamada KM. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura I, Lipfert L, Rodan GA, Le TD. Convergence of αvβ3 integrin- and macrophage colony stimulating factor-mediated signals on phospholipase Cγ in prefusion osteoclasts. J Cell Biol. 2001;152:361–373. doi: 10.1083/jcb.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci. 2004;117:1259–1268. doi: 10.1242/jcs.00997. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler AP, Wells CM, Smith SD, Vega FM, Henderson RB, Tybulewicz VL, Ridley AJ. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci. 2006;119:2749–2757. doi: 10.1242/jcs.03024. [DOI] [PubMed] [Google Scholar]
- 40.Pradip D, Peng X, Durden DL. Rac2 specificity in macrophage integrin signaling: potential role for Syk kinase. J Biol Chem. 2003;278:41661–41669. doi: 10.1074/jbc.M306491200. [DOI] [PubMed] [Google Scholar]
- 41.Razzouk S, Lieberherr M, Cournot G. Rac-GTPase, osteoclast cytoskeleton and bone resorption. Eur J Cell Biol. 1999;78:249–255. doi: 10.1016/S0171-9335(99)80058-2. [DOI] [PubMed] [Google Scholar]
- 42.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Invest. 1992;90:1622–1627. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano T, Troiano N, Adams DJ, Wu JJ, Sun BH, Insogna K. The anabolic response to parathyroid hormone is augmented in Rac2 knockout mice. Endocrinology. 2008;149:4009–4015. doi: 10.1210/en.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]