Abstract
A higher body mass index is associated with better outcomes in hemodialysis patients; however, this index does not differentiate between fat and muscle mass. In order to clarify this, we examined the relationship between measures of fat and muscle mass and mortality in 1709 patients from the Hemodialysis Study. Triceps skin-fold thickness was used to assess body fat and mid-arm muscle circumference was used to assess muscle mass. Cox regression was used to evaluate the relationship between measures of body composition with all-cause mortality after adjustments for demographic, cardiovascular, dialysis, and nutrition-related risk factors. During a median follow-up of 2.5 years, there were 802 deaths. In adjusted models with continuous covariates, higher triceps skin-fold thickness and higher body mass index were significantly associated with decreased hazards of mortality, while higher mid-arm muscle circumference showed a trend toward decreased mortality. In adjusted models, lower quartiles of triceps skin-fold thickness, mid-arm muscle circumference, and body mass index were all significantly associated with higher all-cause mortality. These studies show that body composition in end-stage renal disease bears a complex relationship to all-cause mortality.
Keywords: body mass index, dialysis, mortality, obesity
Although obesity is associated with higher mortality in the general population,1 studies of dialysis patients suggest that higher body mass index (BMI) may be associated with lower all-cause and cardiovascular mortality.2–6 The reason for this difference is unknown, but one potential explanation is that BMI does not differentiate between fat and muscle mass.
Higher fat mass is associated with inflammation and adverse outcomes in the general population,7 although there are fewer and inconsistent data regarding this relationship in patients with end-stage renal disease.8,9 Higher muscle mass on the other hand may be protective in dialysis patients because it is a proxy for better nutritional status. Most of the previous studies examining the importance of obesity in dialysis patients have however used BMI as a measure of obesity, therefore the interpretation remain uncertain.
The purpose of this study was to evaluate the separate associations of fat and muscle mass with all-cause mortality in hemodialysis (HD) patients. We also evaluated whether fat and muscle mass confounded or modified the association of one another and compared their associations with BMI.
RESULTS
Baseline characteristics
The demographics and other clinical characteristics of patients at baseline are shown in Table 1. The average age was 58 years, 64% were African Americans, and more than half were females. The most common cause of end-stage renal disease was diabetes (38%), followed by hypertension (34%) and glomerular disease (14%). Forty percent of patients had a history of congestive heart failure (HF) 39% had ischemic heart disease, and 44% had diabetes. Mean triceps skin-fold thickness, mid-arm muscle circumference (MAMC), and BMI were 16.3 mm, 24.8 cm, and 25.2 kg/m2, respectively.
Table 1.
Patient characteristics by quartile of triceps skin-fold thickness
| Overall (n=1709) | Triceps skin-fold thickness Q1 (n=427) | Triceps skin-fold thickness Q2 (n=430) | Triceps skin-fold thickness Q3 (n=422) | Triceps skin-fold thickness Q4 (n=430) | P | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 57.7 (14.0) | 54.4 (15.7) | 59.1 (14.2) | 58.2 (13.6) | 59.2 (11.5) | <0.001 |
| Women | 56 | 26 | 45 | 71 | 80 | <0.001 |
| Black | 64 | 60 | 60 | 68 | 69 | 0.002 |
| Comorbid conditions | ||||||
| Diabetes | 44 | 22 | 41 | 53 | 60 | <0.001 |
| Peripheral vascular disease | 16 | 14 | 16 | 17 | 18 | 0.36 |
| Ischemic heart disease | 39 | 33 | 43 | 39 | 41 | 0.01 |
| Congestive heart failure | 40 | 42 | 40 | 37 | 40 | 0.44 |
| Other heart disease | 63 | 66 | 65 | 62 | 59 | 0.12 |
| Cerebral vascular disease | 20 | 17 | 23 | 22 | 17 | 0.06 |
| Smoking status | ||||||
| Never | 49 | 38 | 49 | 52 | 58 | <0.001 |
| Past | 33 | 36 | 31 | 33 | 32 | |
| Current | 18 | 26 | 20 | 15 | 10 | |
| Physical exam | ||||||
| Height (cm) | 164.8 (9.4) | 167.9 (9.5) | 165.5 (9.3) | 163.4 (9.4) | 162.4 (8.3) | <0.001 |
| Weight (kg) | 68.8 (14.4) | 61.3 (11.0) | 65.9 (12.4) | 69.9 (13.8) | 78.1 (14.4) | <0.001 |
| Triceps skin-fold thickness (mm) | 16.3 (7.9) | 7.1 (1.8) | 12.6 (1.5) | 18.2 (1.8) | 27.4 (4.2) | |
| MAMC (cm) | 24.8 (3.8) | 24.1 (3.9) | 24.4 (3.6) | 25.0 (3.8) | 25.7 (3.9) | <0.001 |
| BMI (kg/m2) | 25.2 (5.1) | 21.5 (3.0) | 23.9 (3.5) | 26.0 (4.4) | 29.6 (5.1) | <0.001 |
| SBP (mm Hg) | 152.1 (22.1) | 150.2 (20.9) | 150.1 (22.3) | 152.4 (22.5) | 155.5 (22.2) | 0.001 |
| Laboratory variables | ||||||
| Creatinine (mg/dl) | 10.3 (2.9) | 10.9 (3.3) | 10.3 (2.9) | 10.1 (2.7) | 9.9 (2.5) | <0.001 |
| Albumin (g/dl) | 3.6 (0.4) | 3.7 (0.4) | 3.6 (0.4) | 3.6 (0.3) | 3.6 (0.3) | 0.86 |
| Calcium (mg/dl) | 9.3 (1.0) | 9.3 (1.0) | 9.3 (1.0) | 9.3 (1.0) | 9.2 (0.9) | 0.47 |
| Phosphorous (mg/dl) | 5.8 (1.9) | 5.8 (2.1) | 5.6 (1.8) | 5.8 (1.9) | 5.9 (1.6) | 0.18 |
| Dialysis-related parameters | ||||||
| Duration of HD (years) | 3.7 (4.4) | 4.8 (5.3) | 3.8 (4.3) | 3.5 (4.0) | 2.8 (3.4) | <0.001 |
Abbreviations: BMI, body mass index; HD, hemodialysis; MAMC, mid-arm muscle circumference; SBP, systolic blood pressure.
Data are presented as mean (s.d.) for continuous variables, or as percentages for binary and categorical variables. The P-values are derived from two-sided hypothesis testing using χ2-test for categorical variables and analysis of variance for continuous variables.
Participants in the highest quartile of triceps skin-fold thickness were older, predominantly women, more likely to be African American, with shorter dialysis vintage, higher prevalence of diabetes and co-morbid conditions, as well as higher BMI, higher MAMC, and lower serum creatinine levels (Table 1).
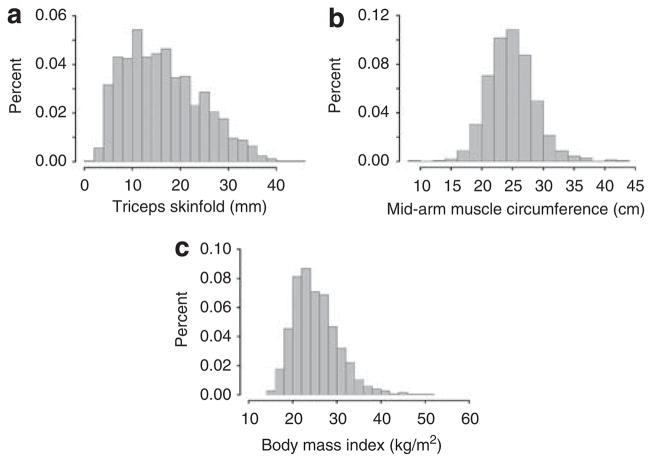
The Pearson correlation coefficients were 0.62, 0.63, and 0.17 between triceps skin-fold thickness and BMI, MAMC and BMI, and triceps skin-fold thickness and MAMC, respectively. MAMC was normally distributed, while triceps skin-fold thickness and BMI were slightly skewed to the right (Figure 1). During a median follow-up of 2.5 years there were 802 deaths.
Figure 1.
Histogram of anthropometric measures.
Univariate analyses
In univariate analysis, higher measures of all three parameters of body composition measures were associated with lower all-cause mortality (hazards ratio (HR) 0.93 (95% confidence interval (CI) 0.86–1.00, P = 0.04), 0.87 (95% CI 0.81–0.94, P<0.001), and 0.92 (95% CI 0.85–0.99, P = 0.02), respectively, for triceps skin-fold thickness, MAMC, and BMI). For triceps skin-fold thickness and BMI, the highest quartile was associated with lower risk compared with the lowest quartile, while for MAMC the three highest quartiles were associated with lower risk compared with the lowest quartile (Table 2).
Table 2.
Associations between anthropometric measurements and all-cause mortality in HD patients
| Mortality n (%) | Unadjusted model
|
Adjusted modela |
Additional adjustmentb |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Triceps skin-fold thickness quartiles | Pc=0.02 | Pc<0.001 | Adjusted for MAMC, Pc<0.001 | ||||
| Q1 (1.7–10.1 mm) | 212 (49.6) | Reference | Reference | Reference | |||
| Q2 (1.0.2–15.3 mm) | 213 (49.5) | 0.92 | 0.76–1.12 | 0.68 | 0.56–0.84 | 0.70 | 0.57–0.86 |
| Q3 (15.4–21.6 mm) | 207 (49.1) | 0.95 | 0.78–1.15 | 0.70 | 0.56–0.88 | 0.75 | 0.60–0.94 |
| Q4 (21.7–44.5 mm) | 170 (39.5) | 0.77 | 0.63–0.95 | 0.55 | 0.43–0.70 | 0.59 | 0.46–0.76 |
| MAMC quartiles | Pc<0.001 | Pc=0.003 | Adjusted for triceps skin-fold thickness, Pc=0.07 | ||||
| Q1 (9.0–22.3 cm) | 240 (56.2) | Reference | Reference | Reference | |||
| Q2 (22.4–24.5 cm) | 202 (47.3) | 0.73 | 0.60–0.88 | 0.68 | 0.55–0.83 | 0.71 | 0.58–0.87 |
| Q3 (24.6–27.0 cm) | 181 (42.3) | 0.64 | 0.52–0.78 | 0.70 | 0.56–0.87 | 0.76 | 0.61–0.95 |
| Q4 (27.0–43.9 cm) | 179 (41.9) | 0.61 | 0.50–0.75 | 0.69 | 0.55–0.86 | 0.77 | 0.62–0.97 |
| BMI quartiles | Pc=0.02 | Pc=0.002 | |||||
| Q1 (15.5–21.6 kg/m2) | 222 (52.1) | Reference | Reference | — | — | ||
| Q2 (21.6–24.4 kg/m2) | 206 (47.9) | 0.90 | 0.74–1.09 | 0.79 | 0.65–0.96 | — | — |
| Q3 (24.5–28.1 kg/m2) | 193 (45.2) | 0.90 | 0.74–1.10 | 0.80 | 0.65–0.99 | — | — |
| Q4 (28.2–52.0 kg/m2) | 181 (42.5) | 0.77 | 0.64–0.95 | 0.64 | 0.52–0.80 | — | — |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; MAMC, mid-arm muscle circumference.
Adjusted for age, sex, race, duration of dialysis, baseline creatinine, albumin, dialysis treatment group, diabetes, peripheral vascular disease, ischemic heart disease, congestive heart failure, other heart disease, smoking, systolic blood pressure (linear and quadratic), and interaction between albumin and follow-up time.
Adjusted for the above variables as well as MAMC in the triceps skin-fold thickness model, and for triceps skin-fold thickness in the MAMC model and triceps skin-fold thickness.
P-values are test for trend.
Functional form of anthropometric measures
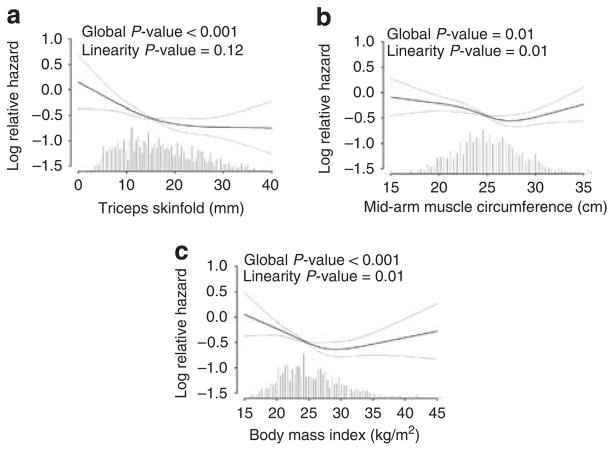
Adjusted restricted cubic spline analysis suggested a linear relationship between all-cause mortality and triceps skin-fold thickness (Figure 2). For MAMC the relationship was not linear, with mortality highest in the lowest quartile and reaching a plateau at 25 cm. The quadratic term for MAMC was not significant (P = 0.14). For BMI the relationship was U-shaped (P = 0.001 for quadratic term), with the lowest risk at 30 kg/m2 (Figure 2).
Figure 2.
Adjusted restricted cubic splines of body composition variables with all-cause mortality.
Multivariate analyses
The negative association between triceps skin-fold thickness and all-cause mortality became stronger after adjusting for demographics, comorbid conditions, nutritional status, laboratory test, and dialysis characteristics. For example, after adjustment for diabetes, the HR for triceps skin-fold thickness for the second, third, and fourth quartile in comparison with the first quartile changed from 0.92, 0.95, and 0.77 to 0.81, 0.77, and 0.60 respectively. The HR further decreased slightly after adjustment for other covariates as mentioned above, including demographics, comorbid conditions, systolic blood pressure, and smoking (Table 2). Higher triceps skin-fold thickness and BMI were significantly associated with lower all-cause mortality in continuous analysis, while MAMC was marginally significant. The HRs per 1 s.d. increase were 0.84 (95% CI 0.76–0.92), 0.93 (95% CI 0.86–1.00), and 0.88 (95% CI 0.81–0.96) for triceps skin-fold thickness, MAMC, and BMI, respectively. The three highest quartiles of triceps skin-fold thickness, MAMC, and BMI were all significantly associated with lower all-cause mortality in comparison with the lowest quartile (Table 2). There was no significant interaction between triceps skin-fold thickness and MAMC (P = 0.45), sex and triceps skin-fold thickness (P = 0.49), and sex and MAMC (P = 0.17). There were also no significant interactions (P>0.05) between any of the body composition measures with either diabetes, ischemic heart disease, HF, other heart disease, or smoking.
Sensitivity analyses
Higher triceps skin-fold thickness and MAMC were associated with lower mortality when both variables were included in the same model and the HRs of each did not change appreciably (Table 2). Additional adjustment for height in the MAMC or triceps skin-fold thickness model did not significantly change the results (data not shown). When creatinine was removed from the models the HRs for MAMC and BMI were slightly stronger. For example, in adjusted continuous analysis the HRs per 1 s.d. increase were 0.83 (95% CI 0.75–0.91), 0.90 (95% CI 0.83–0.97), and 0.85 (95% CI 0.78–0.93) for triceps skin-fold thickness, MAMC, and BMI, respectively. Finally in adjusted continuous models that excluded patients who died in the first year (n = 204), the HRs per 1 s.d. increase were similar to those in the initial models: 0.87 (95% CI 0.78–0.97), 0.94 (95% CI 0.86–1.03), and 0.90 (95% CI 0.82–0.99) for triceps skin-fold thickness, MAMC, and BMI, respectively.
DISCUSSION
This study demonstrates that low muscle mass and low fat mass are each associated with higher all-cause mortality in HD patients. Although the relationship between triceps skin-fold thickness and mortality was linear, the risk associated with MAMC was particularly evident in the lowest quartile (Table 2). There was no significant interaction between triceps skin-fold thickness and muscle mass using either the quartiles or the continuous forms of the covariates. In addition, our results confirm the results of prior studies demonstrating that lower BMI is associated with higher all-cause mortality.
In our study, lower muscle mass is associated with worse survival, and confirm the results of prior studies, which have demonstrated the adverse prognostic significance of low muscle mass.10 Data from a French study of 1345 HD patients demonstrated that patients with the same BMI were not equally protected from death; only patients with BMI>25 kg/m2 and serum creatinine (as a proxy for muscle mass) >800 μmol/l (9.0 mg/dl) had lower mortality, whereas patients with serum creatinine <800 μmol/l and BMI>25 kg/m2 had higher annual mortality.11 Beddhu et al.12 demonstrated that the protective effect of high BMI was limited only to individuals with normal or high muscle mass using urine creatinine as a proxy of muscle mass in 70,028 Medicare HD patients. The drawback of these three studies is the indirect assessment of muscle mass from creatinine measurements, in contrast to its direct assessment in this report.
There are several reasons why low muscle mass may be associated with worse survival. First, lower muscle mass may reflect poor nutritional status.10 Second, low muscle mass may reflect a level of inflammation.13 Honda et al. demonstrated a significant increase of inflammation (CRP≥10 mg/l) in HD patients with low lean-body mass determined by DEXA. Third, muscle mass may be the compartment in which uremic toxins are distributed in HD patients. Gotch14 demonstrated that total body water and muscle mass are strongly correlated and muscle mass is the primary location of intracellular water. Therefore, patients with lower muscle mass may have a higher concentration of uremic toxins.
Our results also show that lower fat mass is associated with increased all-cause mortality, with the relationship being linear in adjusted analysis (Figure 2). These results are consistent with the finding of Kalantar-Zadeh et al.9 who evaluated body fat by near infrared and noted that low baseline body fat percentage and fat loss over time were independently associated with higher mortality in HD patients.
There are several reasons why lower fat mass may be associated with higher mortality in dialysis patients. First, lower fat mass may reflect the severity of underlying disease that we unable to adjust for despite multivariable analyses. Second, lower fat mass may reflect decreased energy stores for coping with catabolic stress of dialysis.9 Third, although body fat is associated with recruitment of macrophages and inflammation in the general population,15 this is not as clear in dialysis patients. In fact, no difference in inflammatory markers (interleukin-6, tumor necrosis factor-α, C-reactive protein) was noted in different body fat groups in several studies;9,13,16 however other studies have noted higher levels of inflammatory markers such as inflammatory high density lipoprotein cholesterol (HDL) in those with higher BMI.17,18 It remains to be determined whether the association between fat mass and inflammation is altered in the uremic state.
Our study confirms the previous finding of lower BMI being associated with mortality,3,4,6,19 but also suggest a ‘U’-shaped relationship (Figure 2). The protective effect of BMI seems to be maximal at BMI of 30 kg/m2, with increased risk particularly below, but also above, this threshold. These results are similar to those from the Dialysis Outcomes and Practice Patterns Study where a ‘J’-shaped curve was reported, with a BMI of 30–34.9 being most protective.6 These observations suggest that a BMI target of <25 kg/m2 (as defined in the general population) may not be optimal in dialysis patients whether they are on or off the transplant list.20
The strengths of our study include the following: First, our study is unique in that it used standardized anthropometric measurements to quantify fat and muscle mass. Studies have indicated that anthropometric measurements may be more closely correlated with the gold standard of DEXA than bioelectrical impedance in dialysis patients.21–23 We acknowledge, however, that all measures of body composition, particularly those related to fat free mass, may be affected by hydration status.24 Second, we had a large sample with detailed ascertainment of exposures, covariates, and outcomes. Third, our results are for the most part generalizable to maintenance HD patients since our mean (s.d.) of BMI (25.2 (5.1)) is comparable to that in the United States Renal Data System (USRDS), which has a mean (s.d.) BMI of 24.4 (5.3).25
Potential limitations of this study include first the fact that the skin-fold measurements in this study quantified peripheral rather than central fat tissue. Central fat, however, is thought to be a stronger risk factor for cardiovascular disease both in the general population26 and in patients during all stages of chronic kidney disease,27,28 and data from both human and animal studies suggest that there may be fat redistribution in kidney disease.29,30 Second, the formulae used for calculation of percent fat mass and muscle mass31,32 have not been validated for HD patients; therefore, we were unable to provide the percent of body fat mass and muscle mass in this study. Third, as in any observational study we cannot account for unmeasured or residual confounding. Fourth, the HEMO study excluded morbidly obese subjects with weight over 100 kg.
Conclusion
Both low muscle and low peripheral fat mass are associated with higher all-cause mortality in HD patients. These findings need to be reproduced in additional studies and may have important clinical implications regarding weight loss recommendations in dialysis patients on and off the transplant list.
MATERIALS AND METHODS
Study population
The HEMO study was a multi-center, randomized controlled, clinical trial with a 2 × 2 factorial design, comparing high dialysis dose (eKt/V 1.45) to standard dialysis dose (eKt/V 1.05), and membrane flux of low (mean β2-microglobulin clearance <10 ml/min) to high (mean β2-microglobulin clearance >20 ml/min and ultrafiltration coefficient >14 ml/h/mm Hg). The patients were aged 18 to 80 years old, undergoing in-center HD three times weekly, and had been on dialysis for at least 3 months. The exclusion criteria included unstable angina and New York Heart Association Class IV HF. The participants were evaluated during an 8- to 12-week baseline period between March 1995 and October 2000. They were excluded from randomization if they were unable to achieve a dialysis dose of eKt/V≥1.35 within 4.5 h, or if their residual kidney urea clearance was >1.5 ml/min/35 l of urea distribution volume. Patients with weight >100 kg, severe malnutrition indicated by a pre-dialysis albumin level of <2.8 g/dl, current malignances requiring radiation or chemotherapy, symptomatic acquired immunodeficiency syndrome, cirrhosis with hepatic encephalopathy, chronic pulmonary disease, and current hospitalization were also excluded. A total of 1846 patients were enrolled in this study. All patients received standard medical care for blood pressure control, calcium–phosphorus balance, anemia, and other parameter control by the Quality of Care Committee. Data collection ended in 31 December 2001. The range of follow-up period for individual patients was 0.9–6.6 years, depending on the date of randomization.33 Among the 1846 patients, 1709 had undergone measurements of triceps skin-fold thickness, MAMC, and BMI.
Outcome variable
All-cause mortality was ascertained through contact with each dialysis unit as well as through death certificates. Participants were censored at the time of death, transplantation, or withdrawal from the study or the study end date, 31 December 2001.
Body composition measurements
Certain anthropometric techniques assess body composition based on a model in which the body consists of two chemically distinct compartments: fat and fat-free mass. The fat-free mass consists of the muscle mass, soft lean tissues, and the skeleton. Triceps skin-fold thickness and MAMC are the most commonly used anthropometric measurements in assessing these body compartments.
Anthropometric measurements34 were obtained by researchers trained on standard techniques35 and were performed immediately after a dialysis session during the baseline period. Triceps skin-fold thickness and MAMC were obtained from the non-access side of the body using 2–4 repeat measurements. Triceps skin-fold thickness was used to assess fat mass. MAMC was used to assess muscle mass. MAMC was calculated using the standard formula MAMC (cm) = Mid-upper arm circumference −π ★ Triceps skin-fold thickness (cm).36 Height and post-dialysis (dry) weight were used to calculate the BMI (weight in kg/(height in m)2).
Covariates
Age, sex, race, smoking history, and dialysis duration were obtained through history. Race was classified as Black or non-Black. Diabetes was defined based on current or past use of oral hypoglycemics. Cerebral vascular disease, peripheral vascular disease, ischemic heart disease, other heart disease, and HF were defined using the index of Co-Existing Disease (ICED), a coding system that classifies the presence and severity of different diseases.37–39 Pre-dialysis serum albumin was determined monthly at the HEMO central laboratory. Serum creatinine, calcium, and phosphorous levels were obtained from local laboratory measurements.
Statistical analysis
Means and s.d.s were calculated for continuous variables, and proportions were calculated for categorical variables. χ2-Test or analysis of variance was used to compare the proportion or means among the different quartiles of triceps skin-fold thickness. The Pearson correlations between triceps skin-fold thickness, MAMC, and BMI were calculated.
The adjusted functional forms of triceps skin-fold thickness, MAMC, and BMI, and their associations with all-cause mortality were examined using restricted cubic splines and graphically presented using the Design Library of the Statistical Software R.40,41
We used stratified Cox regression models allowing different baseline hazard functions for the 15 Clinical Centers to evaluate the risk factors for all-cause mortality. Univariate analysis was performed to examine the relationship between each of the body composition variables and all-cause mortality. Body composition variables were initially evaluated as continuous variables per 1 s.d. increase so as to enable comparisons between them, and subsequently in quartiles.
In the multivariable models age, sex, race, and randomization status were included in all models and other variables were added if P<0.05 in stepwise selection. The interaction of baseline albumin with follow-up time was included in the models to account for a decline in the association of baseline albumin with all-cause mortality over time. Other variables did not violate the proportional hazards assumption.
Finally, interactions were performed between MAMC and triceps skin-fold thickness, as well as each of the body composition measures with sex, race, diabetes, ischemic heart disease, HF, other heart disease, and smoking.
Sensitivity analyses
First, we included triceps skin-fold thickness and MAMC in the same model so as to evaluate whether they confounded the importance of each other. Second, we adjusted for height because BMI adjusts for height. Third, we excluded serum creatinine from the multivariable models because of potential colinearity between triceps skin-fold thickness, MAMC, and serum creatinine. Fourth, we excluded patients who died within the first year so as to evaluate whether the relationships are consistent over time.
Acknowledgments
The participation by patients and the staff in the HEMO study is greatly appreciated. An abstract representing this work was published at the National Kidney Foundation Annual Meeting in Nashville, TN, in 2009. This work was supported by grants T32 DK07777 and K24 DK078204 from the National Institutes of Health.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of US adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 3.Johansen KL, Young B, Kaysen GA, et al. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 5.Kopple JD, Zhu X, Lew NL, et al. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Leavey SF, McCullough K, Hecking E, et al. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16:2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 8.Axelsson J, Rashid Qureshi A, Suliman ME, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr. 2004;80:1222–1229. doi: 10.1093/ajcn/80.5.1222. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Kuwae N, Wu DY, et al. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–210. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 10.Ikizler TA, Wingard RL, Harvell J, et al. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int. 1999;55:1945–1951. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Moreau-Gaudry X, Jean G, Berrerd-Genet L, et al. Are all body mass indexes (BMI) equally protective in maintenance haemodialysis. J Am Soc Nephrol. 2007;18:725A. [Google Scholar]
- 12.Beddhu S, Pappas LM, Ramkumar N, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 13.Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86:633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 14.Gotch FA. Kt/V is the best dialysis dose parameter. Blood Purif. 2000;18:276–285. doi: 10.1159/000014449. [DOI] [PubMed] [Google Scholar]
- 15.Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxford) 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 16.Kakiya R, Shoji T, Tsujimoto Y, et al. Body fat mass and lean mass as predictors of survival in hemodialysis patients. Kidney Int. 2006;70:549–556. doi: 10.1038/sj.ki.5000331. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Kopple JD, Kamranpour N, et al. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 2007;72:1149–1156. doi: 10.1038/sj.ki.5002491. [DOI] [PubMed] [Google Scholar]
- 19.Beddhu S, Cheung AK, Larive B, et al. Inflammation and inverse associations of body mass index and serum creatinine with mortality in hemodialysis patients. J Ren Nutr. 2007;17:372–380. doi: 10.1053/j.jrn.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivas TR, Meier-Kriesche HU. Obesity and kidney transplantation. Contrib Nephrol. 2006;151:19–41. doi: 10.1159/000095317. [DOI] [PubMed] [Google Scholar]
- 21.Avesani CM, Draibe SA, Kamimura MA, et al. Assessment of body composition by dual energy X-ray absorptiometry, skinfold thickness and creatinine kinetics in chronic kidney disease patients. Nephrol Dial Transplant. 2004;19:2289–2295. doi: 10.1093/ndt/gfh381. [DOI] [PubMed] [Google Scholar]
- 22.Kamimura MA, Avesani CM, Cendoroglo M, et al. Comparison of skinfold thicknesses and bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body fat in patients on long-term haemodialysis therapy. Nephrol Dial Transplant. 2003;18:101–105. doi: 10.1093/ndt/18.1.101. [DOI] [PubMed] [Google Scholar]
- 23.Kamimura MA, Jose Dos Santos NS, Avesani CM, et al. Comparison of three methods for the determination of body fat in patients on long-term hemodialysis therapy. J Am Diet Assoc. 2003;103:195–199. doi: 10.1053/jada.2003.50024. [DOI] [PubMed] [Google Scholar]
- 24.Stenver DI, Gotfredsen A, Hilsted J, et al. Body composition in hemodialysis patients measured by dual-energy X-ray absorptiometry. Am J Nephrol. 1995;15:105–110. doi: 10.1159/000168812. [DOI] [PubMed] [Google Scholar]
- 25.Leavey SF, Strawderman RL, Jones CA, et al. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31:997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 26.Bays H, Blonde L, Rosenson R. Adiposopathy: how do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther. 2006;4:871–895. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 27.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postorino M, Marino C, Tripepi G, et al. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Liu Z, Li S, et al. The relationship between body fat distribution and renal damage in Chinese with obesity. Exp Clin Endocrinol Diabetes. 2008;116:99–103. doi: 10.1055/s-2007-992117. [DOI] [PubMed] [Google Scholar]
- 30.Pinto-Sietsma SJ, Navis G, Janssen WM, et al. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41:733–741. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 31.Siri W. Sciences NAO. Techniques for Measuring Body Composition. National Research Council; Washington, DC: 1961. Body composition from fluid spaces and density: analysis of methods; pp. 223–244. [Google Scholar]
- 32.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 33.Greene T, Beck GJ, Gassman JJ, et al. Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials. 2000;21:502–525.0. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 34.Lohman T, Martorell R, Roche AF. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL: 1988. [Google Scholar]
- 35.US Department of Health and Human Services INI. Anthropometric procedure video. 1996. [Google Scholar]
- 36.Gibson RS. A Laboratory Manual. Oxford University Press; New York: 1993. Nutritional Assessment. [Google Scholar]
- 37.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 39.Greenfield S, Apolone G, McNeil BJ, et al. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31:141–154. doi: 10.1097/00005650-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Harrell FE. Design: S functions for biostatistical/epidemiologic modeling, testing, estimation, validation, graphics, and prediction. 2003. [Google Scholar]
- 41.Team. RDC: R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]