Abstract
Purpose
We examined the effects of genistein and/or Eukarion (EUK)-207 on radiation-induced lung damage and investigated whether treatment for 0–14 weeks (wks) post-irradiation (PI) would mitigate late lung injury.
Materials and Methods
The lungs of female Sprague-Dawley (SD) rats were irradiated with 10 Gy. EUK-207 was delivered by infusion and genistein was delivered as a dietary supplement starting immediately after irradiation (PI) and continuing until 14 wks PI. Rats were sacrificed at 0, 4, 8, 14 and 28 wks PI. Breathing rate was monitored and lung fibrosis assessed by lung hydroxyproline content at 28 wks. DNA damage was assessed by micronucleus (MN) assay and 8-hydroxy-2-deoxyguanosine (8-OHdG) levels. The expression of the cytokines Interleukin (IL)-1α, IL-1β, IL-6, Tumor necrotic factor (TNF)-α and Transforming growth factor (TGF)-β1, and macrophage activation were analysed by immunohistochemistry.
Results
Increases in breathing rate observed in the irradiated rats were significantly reduced by both drug treatments during the pneumonitis phase and the later fibrosis phase. The drug treatments decreased micronuclei (MN) formation from 4–14 wks but by 28 wks the MN levels had increased again. The 8-OHdG levels were lower in the drug treated animals at all time points. Hydroxyproline content and levels of activated macrophages were decreased at 28 wks in all drug treated rats. The treatments had limited effects on the expression of the cytokines.
Conclusion
Genistein, and EUK-207 can provide partial mitigation of radiation-induced lung damage out to at least 28 wks PI even after cessation of treatment at 14 wks PI.
Keywords: genistein, EUK-207, ROS, rat lung, radiation
Introduction
The lung is one of the most susceptible organs to potentially debilitating radiation toxicity. The mechanisms involved in the initiation and perpetuation of radiation-induced lung injury remain incompletely understood but the functional effects of irradiation on lung are normally separated into two phases. Radiation pneumonitis generally occurs between 2–4 months after irradiation and fibrosis tends to develop after 4–6 months and often follows acute pneumonitis (Marks et al. 2003). Patients with pneumonitis may present clinically with cough, dyspnea, fever, chest pain, or only with radiographic changes. Acute pneumonitis is treated with steroids, but can be life-threatening even with treatment. Lung fibrosis is regarded as permanent and can cause shortness of breath, coughing, diminished exercise tolerance and ultimately lead to severe respiratory distress, pulmonary hypertension, right heart failure, even death. Extensive data suggest that pneumonitis and fibrosis may arise as a result of the interaction of a cycle of chronic inflammation and oxidative damage initiated by radiation damage in the lung (Finkelstein et al. 1994, Fleckenstein et al. 2007, Gauter-Fleckenstein et al., Ghafoori et al. 2008, Johnston et al. 1996, Johnston et al. 2004, Rabbani et al. 2007, Robbins and Zhao 2004, Rube et al. 2008, Rube et al. 2004, Rube et al. 2004, Yakovlev et al., Zhao and Robbins 2009).
Ionizing radiation exerts immediate biological effects largely through the generation of reactive oxygen species (ROS) such as hydroxyl radicals (OH•) and to a lesser extent superoxide (O2−), and hydrogen peroxide (H2O2), produced directly by radiation. ROS are also induced by subsequent induction of inflammation and a cascade of cytokine activity (Rubin et al. 1995) and can oxidize cellular molecular components. ROS can further interact with nitric oxide to generate peroxynitrite, a reactive nitrogen species (RNOS) that can also chemically modify cellular biomolecules. Extensive studies implicate cellular DNA as the primary target for the biological and lethal effects of ionizing radiation (Iliakis et al. 1991, Olive et al. 1998, Rube et al. 2010). The main reaction products of DNA oxidation are base modifications, base loss, and strand breaks. ROS generated by ionizing radiation can react with the various DNA bases but reactions with guanine bases in DNA to form 8-hydroxy-2-deoxyguanosine (8-OHdG) has been reported as a key biomarker of oxidative damage (van Loon et al.2010).
Alveolar macrophages play an important role in the upregulation of the inflammatory process post irradiation. These inflammatory mediators are regarded as a source of proinflammatory cytokines and can help to drive the persistent cytokine cascade and chronic inflammatory state indicative of early and late radiation-induced pulmonary injury (Rubin et al. 1995). The production of ROS by exposure to radiation followed by the activation of inflammatory cells and the generation of persistent autocrine and paracrine messages from soluble mediators gives rise to changes in pulmonary cell populations and organization that are believed to be ultimately responsible for generating clinically evident early and late responses (Robbins and Zhao 2004, Rubin et al. 1992). The superoxide dismutases (SOD) are a family of metalloprotein enzymes that have a significant role in protection from oxidative damage. Since their discovery in 1969 (McCord and Fridovich 1969, 1969), there have been several reports that SOD can decrease radiation damage in a wide range of biological systems including lung (Abou-Seif et al. 2003, Epperly et al. 2000, Epperly et al. 2001, Kang et al. 2003, Khan et al. 2003, Petkau 1987, Quinlan et al. 1994, Rabbani et al. 2005, Tai et al. 2009, Tsan 1997). SOD functions by converting superoxide to H2O2 and O2. H2O2 is further reduced by catalase or glutathione peroxidase to water and oxygen (McCord and Fridovich 1969b).
We previously found that genistein, a soy isoflavone and Eukarion (EUK)-189, a superoxide dismutase-catalase mimetic showed some promising mitigating effects on radiation-induced lung damage in Sprague-Dawley (SD) rats (Calveley et al. 2010, Langan et al. 2006)). In the current study we addressed the questions of whether these drugs would give increased levels of mitigation when combined and whether discontinuation of treatment after the initial wave of pneumonitis (14 weeks PI) would allow for maintenance of the mitigation out to 28 weeks (wks) during the fibrosis phase. We used EUK-207 rather than EUK-189 in these studies because they have similar antioxidant activity but EUK-207 has improved pharmacokinetic properties, including greater stability and greater potency in certain cellular and in vivo models (Rosenthal et al. 2009, Vorotnikova et al. 2010).
Materials and Methods
Animal treatments
Female Sprague-Dawley (SD) rats (Charles River Laboratories International, Inc., Wilmington, MA, USA) aged seven to eight wks and weighing 180–200g were used in all experiments. The animals were housed in animal facilities accredited by the Canadian Council on Animal Care and treated in accordance with approved protocols. Each experimental time point was repeated with 3–4 rats and control (age-matched) animals were also studied at several time-points during the experiments. In the main experiment the rats were divided into six experimental groups: young controls sacrificed for analysis at the start of the experiment (N=4), control diet alone (N=12), irradiation only with control diet (N=16), irradiation with Genistein diet (N=16), irradiation with EUK-207 infusion (N=16) and irradiation with combined genistein diet and EUK-207 infusion (N=16). Rats were randomly selected (3 for control or 4 for treated groups) at the start of the experiment and sacrificed at each time point (4, 8, 14 and 28 wks PI) during the course of the experiment. No animals had to be prematurely sacrificed due to morbidity.
The AIN-76A diet (Harlan Teklad, Madison, WI, USA), a semi-purified casein-based diet containing no detectable phytoestrogens (limit of detection 5pmol/l) was selected as the control diet. The genistein diet was formulated from the control diet by supplementing with 750 mg/kg of genistein. The genistein was chemically synthesized (Toronto Research Chemicals Inc, Toronto, Ontario) and incorporated into the AIN-76A diet at Harlan Teklad. These diets were used in our previous study (Calveley et al. 2010). The diet has been reported to yield serum concentration levels in mice (1–2 umol/L) similar to those observed in humans consuming a diet containing modest amounts of soy products. Our measurements of dietary consumption in the rats indicated the food intake was 25–30g/day representing a genistein intake of 18.5–22.5 mg/rat/day. Assuming an oral bioavailability of approximately 10–15% the rats should have absorbed a dose of 10–13 mg/kg (Coldham et al. 2002, Soucy et al.2006, Wang et al. 2000). The Genistein diet was withdrawn at 14 wks after radiation and changed to control diet until 28 wks. Measurements in a separate group of 17 rats after 8, or 14 wks on the diet gave a mean plasma level of free genistein of 55.9 (SD 52.2) nmol/L with no significant difference between the time points (Calveley et al. 2010), although this likely represents only 3–5% of the total genistein in the plasma since most is conjugated (Michael McClain et al. 2006).
The salen-manganese(Mn) SOD-catalase mimetic, EUK-207 was custom-synthesized and characterized as described previously (Rosenthal et al. 2009). The animals received doses of 8mg/kg body weight/day administered by Alzet 2ML4 infusion pump (Alzet Osmotic Pumps, Durect Corporation, Cupertino, CA, USA) implanted subcutaneously (delivery rate 2.5 µl/hr) and changed every 4 wks until 14 wks after irradiation. For the drug combination (G+E) group, genistein was delivered in the diet and EUK-207 by Alzet pump. The genistein diet was initiated within 1 hr after irradiation and continued until 14 wks when the rats were switched to the AIN-76A control diet until 28 wks. EUK-207 was given by Alzet pumps inserted within 1 hr after irradiation and maintained until 14 wks after irradiation (pumps replaced every 4 wks). We selected EUK-207 for this study because it has similar antioxidant activity to other salen-Mn complexes, particularly EUK-189, the analog we have studied previously (Langan et al. 2006) but has greater stability (Rosenthal et al. 2009) and greater potency in cell culture systems (Rosenthal et al. 2009); Vorotnikova et al. 2010) than EUK-189. In addition, EUK-207 is more effective than EUK-189 at mitigating radiation-induced renal injury in a rat model (Rosenthal et al. 2009), while the two compounds have comparable effectiveness in a mouse model for age-associated cognitive impairment (Clausen et al. 2010, Liu et al. 2003).
Irradiation
The rats were anesthetized by isoflurane inhalation and placed in custom-designed Lucite holding containers. Two circular surface collimators, made of lead, were inserted on both sides of the jig to confine the radiation only to the lung volume. All irradiated animals received a single dose of 10 Gy delivered by a double-headed 100 kVp X-ray unit, with a half-value layer (HVL) of 4mm Al, operating at 10 mA with a dose-rate of 10.7 Gy per minute (Bristow et al. 1990). Initial studies with a digital x-ray unit (Faxitron, Lincolnshire, IL, USA) had indicated that lead shielding around the lungs using a circular field of 4 cm diameter for irradiation would allow whole lung irradiation. However, subsequent studies revealed that only approximately 90% of the lung was actually in the field due to the geometry of the system and a small volume of the lower lung (~ 10%) was out of the radiation field.
Lung Extraction and Immunohistochemistry
For the lung tissue removal from the euthanized animals, alpha MEM (Minimum Essential Medium) media supplemented with antibiotics was perfused through the right ventricle of the hearts of deeply anaesthetized (ketamine/xylazine) animals to remove as much blood in the lungs as possible. The lung lobes were then separated and the upper and lower lobes of right lung were used for hydroxyproline assay and micronuclear assay respectively. The whole left lobe of the lung was used for immunohistochemical analysis. In the majority of the animals we injected a volume (0.5–1.0 ml) of 10% formalin into the left lobe of the lung to expand the alveoli and then placed the lobes in 10% formalin for at least 48 hours for fixation. The whole left lobe of lungs was embedded in paraffin and sections 5µm thick were cut and placed on slides in preparation for immunohistochemical staining as described in our previous paper (Calveley et al. 2005). Slides were incubated with primary antibodies for the activated macrophage marker ED-1 (MCA341, 1:100, AbD Serotec, Oxford, UK), for cytokines interleukin (IL)-1α (sc-1254, 1:100, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), IL-1β (AAR15G, 1: 1000, AbD Serotec), IL-6 (sc-1265, 1:200, Santa Cruz Biotechnology Inc), Tumour necrosis factor (TNF)-α (sc-1357, 1:200, Santa Cruz Biotechnology Inc), Tumour growth factor (TGF)-beta (MCA797, 1:50, AbD Serotec,) and for 8-hydroxy-2-deoxyguanosine (8-OHdG) (MOG-110P, 1:1000, JaICA, Shizuoka, Japan). Secondary antibodies included biotinylated anti-mouse immunoglobulin G (IgG) (BA-1000, 1:200, Vector Laboratories, Burlingame, CA, USA), biotinylated anti-rabbit IgG (BA-1000, 1: 200, Vector Laboratories) and biotinylated anti-goat IgG (BA-5000, 1: 300, Vector Laboratories,) for 30 minutes at room temperature.
Image Analysis
Following staining, the slides were scanned using the ScanScope XT (Aperio Technologies, Vista, CA, USA). This is a brightfield scanner that digitizes the whole microscope slide at 20× and 40× magnification and provides high resolution images. The images can then be viewed with ImageScope (Aperio Technologies) for quantitative analysis. Using the Positive Pixel Algorithm, the whole slide was analyzed and the number of positive pixels/number of positive and negative pixels × 100 was recorded (% positivity). The positive pixel count algorithm is used to quantify the amount of a specific stain present in a scanned slide image. We specified a color (range of hues and saturation) and three intensity ranges (weak, positive, and strong). For pixels which satisfied the color specification, the algorithm counted the number and intensity-sum in each intensity range, along with three dimensional quantities: average intensity, ratio of strong/total number, and average intensity of weak+positive pixels. The algorithm has a set of default input parameters when first selected. These inputs were pre-configured for brown color quantification in the three intensity ranges (220-175, 175-100, and 100-0). Pixels which are stained, but do not fall into the positive-color specification, are considered negative and are counted as well, so that the fraction of positive to total stained pixels is determined. We analyzed 1 section from each rat for each time point.
Assessment of fibrosis
To assess fibrosis, part of the upper right lung tissue was analyzed for hydroxyproline content using a colorimetric assay to quantify the amount of hydroxyproline (OH-Pro) in papain (P3125, Sigma-Aldrich Canada, Oakville, ON, Canada) digested rat lung tissue. Lung tissue (100 mg) was digested at 60°C for 48 hours and then subjected to acid hydrolysis for 18 hours at 110°C. Free hydroxyproline was released from protein and peptides into the solution that was then neutralized. The hydroxyproline was oxidized into a pyrrole with chloramine T (857319, Sigma-Aldrich). This intermediate turns pink in color with the addition of Ehrlich`s Reagent (4-dimethylaminobenzaldehyde) (156477, Sigma-Aldrich). The samples were loaded into a 96-well microplate and the absorbance was measured at 560 nm using a plate reader. The concentrations of the samples were determined from a standard curve using cis-4-hydroxy-L-proline (H1637, Sigma-Aldrich).
Breathing Rate Measurement
We measured the breathing frequency of rats using a respiration rate monitor (Columbus Instruments, Columbus, Ohio, USA) as a measure of functional damage. The rats were acclimatized to the method several times in the 2 wks preceding the start of the experiment. Breathing frequencies were measured once a week at 2, 4 and 6 wks and then weekly from 7 to 28 wks post irradiation. The breathing rate of each animal was measured for two minutes, after an initial 45 second acclimatization period. Breathing rate was determined by taking the mean of a maximum of five 6 second intervals of calm breathing within the two minutes measurement period.
Micronuclei Assay
An assay of micronuclei in lung cells was used to assess DNA damage in the rat lungs at different times following irradiation as described previously (Calveley et al. 2005, Khan et al. 1998, Khan et al. 2003). Briefly, upon sacrifice one lobe (lower right) from each rat was used for the micronuclei assay. The tissue was minced and digested with 0.25% trypsin (T3924, Sigma-Aldrich), for 80 minutes at 37°C. The digest was mixed with an equal volume of culture medium, filtered, centrifuged and suspended in culture medium. The cell suspension was plated on single-chambered sterile Permanox slides for 24 hours in alpha MEM plus 10% fetal calf serum (FCS). The culture medium was then replaced with medium containing 3 µg/ml Cytochalasin B (C6762, Sigma-Aldrich) to halt cytoplasmic division to produce binucleated cells. The cells were fixed after 72 hours with 95% methanol following incubation with a hypotonic solution for 10 minutes. For scoring, the slides were stained with Acridine Orange (Difco, Burlington, ON, Canada) and 400–500 binucleate cells were scored for micronuclei under a fluorescent microscope. The results were recorded as number of micronuclei per 1000 binucleated cells.
Statistical analysis
Multiple linear regressions and Tukey’s method for the adjustment of least square means in multiple comparisons were used for analysis of the data sets. A p value less than 0.05 was considered as significantly different. Mixed modeling was also used to examine time trends in the data. SAS (enterprise guide-4) software was used for the analysis. In all figures the error bars represent standard errors.
Results
Breathing rate
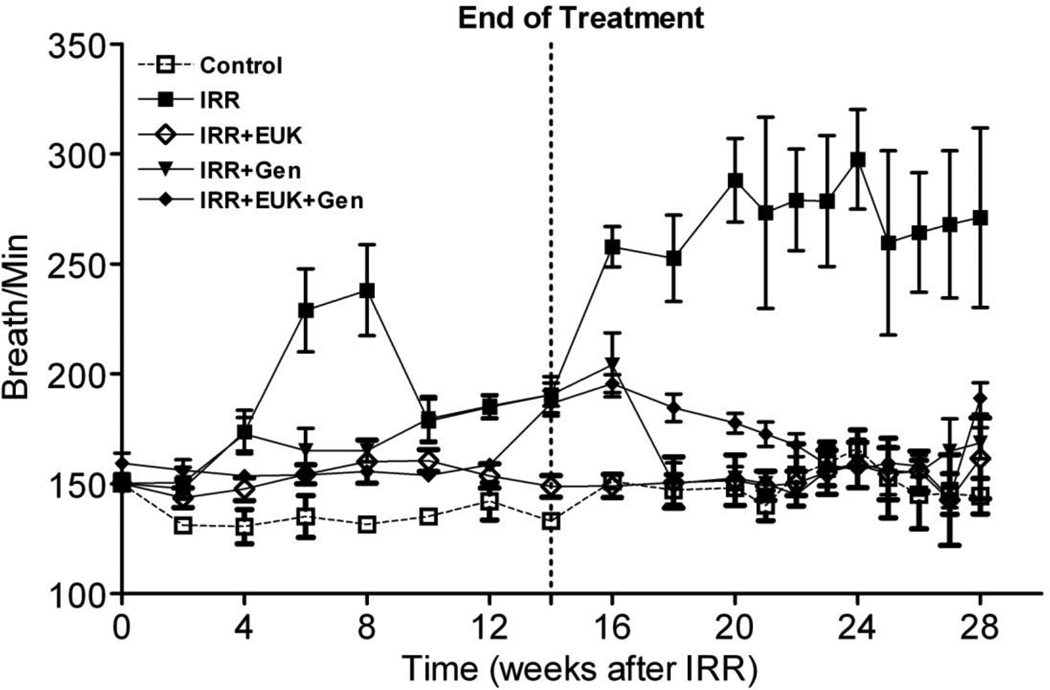
Figure 1 shows the average breathing rate for the rats over 28 wks following irradiation. For rats on the control diet with no radiation their breathing rate stayed at approximately 140–150 breaths per minutes, over the 28 wks. At 28 wks, the body weight of the normal control rats was higher than the irradiated rats. The body weight increased more gradually in the radiation-only group of rats than in other treated groups during the pneumonitis time window (4–10 wks). However, by 28 wks there was no difference in body weight among the treated rats (data not shown). The rats given 10 Gy and put on the control diet demonstrated two waves of increase in breathing rate over the period. The first increase over 4–10 wks peaked at approximately 240 bpm at 6 wks PI. A second increase in breathing rate to similar high levels occurred starting about 14 wks PI and continued to increase till 20 wks and then plateaued until sacrifice at 28 wks. The rats on the genistein diet, or EUK-207 or their combination (G+E) all showed a significant reduction (p<0.05) in breathing rate relative to the radiation-only group over the whole time period. For the genistein diet and the combined group there was a significant increase (p<0.05) relative to the control from 8–28 wks but the EUK-207 treatment group showed no significant increase relative to the control over the entire time period. It is noteworthy that the treatments significantly mitigated the second rise in breathing rate at greater than 14 wks even though the treatment stopped at 14 wks.
Figure 1.
Breathing rate (mean breaths per minute) as a function of time after irradiation (10 Gy) for rats receiving no radiation or radiation only on the control diet, rats irradiated with 10 Gy lung irradiation with genistein, EUK-207 or combination treatment (G+E). All drug treatments started immediately after irradiation and finished at 14 wks. Each point represents the mean (±Standard Error of the Mean -SEM) for all rats available for analysis at the different times.
DNA damage
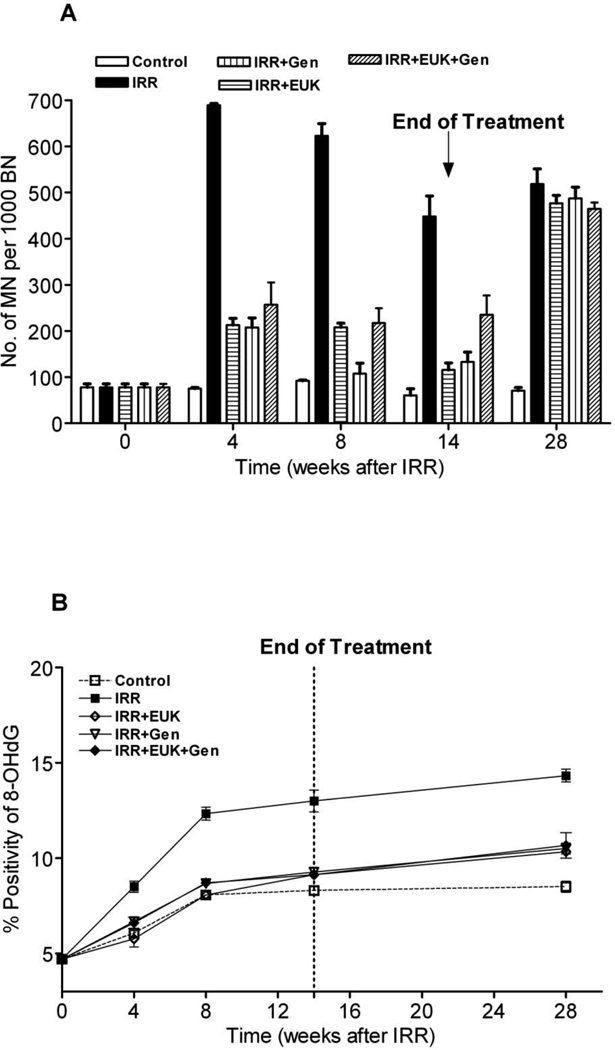
Figure 2A shows the micronuclei (MN) counts at 4, 8, 14 and 28 wks after irradiation in comparison to the control values at time zero for all the treatment groups. MN numbers were significantly increased at 4 wks after radiation to greater than 7-fold higher than the untreated group (p<0.01). All the drug-treated groups showed a significant reduction (about 3-fold) in MN levels compared to the radiation-only group at this time (p<0.01). The MN levels in the radiation-only group remained significantly higher than the control and drug-treated groups at 8 and 14 wks (p<0.01). However, after stopping the drug treatment at 14 wks after irradiation, the MN formation in all the drug-treated groups increased again to the high level seen in the radiation-only group at 28 wks.
Figure 2.
A) Micronucleus yield (MN/1000BN) in fibroblasts from rat lungs and B) percent positivity of 8-OHdG staining following lung irradiation (10 Gy) with Genistein, EUK-207 and combination (G+E) treatment. All drug treatments started immediately after irradiation and finished at 14 wks. The assays were performed at 0, 4, 8, 14 and 28 wks following irradiation. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar (A) or each point (B) represents the mean (±SEM).
To further examine the possible role of ROS in causing DNA damage we assessed 8-hydroxy-2-deoxyguanosine (8-OHdG) staining in the lung tissue. Figure 2B shows quantitation of the staining in the various groups. Following irradiation the positivity of staining of 8-OHdG increased rapidly in the first 8 wks and then more slowly to 28 wks. The drug-treated groups also showed an increase but this was significantly less at 8 wks than that observed for radiation alone. From 8–28 wks the drug-treated groups increased with a similar slope to that of the radiation-only group and over this time range there was a significant difference between the drug-treated groups and the radiation-only group (p<0.01). Thus even though the drug treatment ended at 14 wks, the 8-OHdG levels in the drug treated rats did not increase to the levels observed in the radiation-only rats in contrast to the MN levels. Interestingly the control rats also showed an increase in 8-OHdG levels in the first 8 wks suggesting that this increase is partly due to aging. The levels of 8-OHdG in the drug-treated groups were all similar and not significantly different from the control group except for the combined treatment group at 28 wks (p<0.05).
Fibrosis
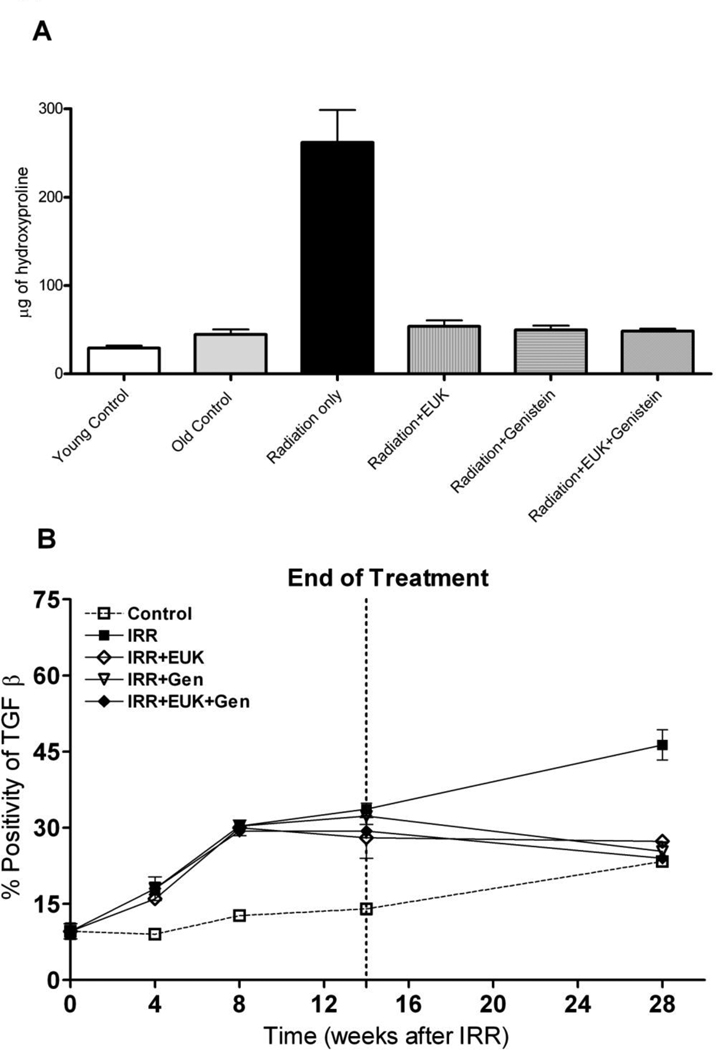
Figure 3A shows the hydroxyproline content of the lung tissue at 28 wks after PI. We found that the rats given radiation only showed a significant increase in hydroxyproline content relative to the young control and normal control rats (p<0.01). The treatment with Genistein and EUK-207 alone and their combination showed a significant decrease in hydroxyproline content to the level of the normal control rats (p< 0.01). The cytokine TGF-β has been widely associated with radiation-induced fibrosis in lung (Anscher et al. 2008, Finkelstein et al. 1994, Ritzenthaler et al. 1993, Roberts et al. 1986, Rube et al. 2000, Sime et al. 1997). Figure 3B shows TGF-β staining in the lungs of the various treatment groups. Following irradiation the positivity of TGF-β staining increased steadily from 4 wks to 28 wks compared to control rat group. There was a similar increased trend of positivity of TGF-β staining in the drug-treated groups following irradiation until 14 wks. However, at 28 wks, there was a significant decrease (p<0.01) in positive staining for these groups relative to the radiation-only group to levels similar to that seen in control rats.
Figure 3.
A) Hydroxyproline content (µg of hydroxyproline/100 mg of wet lung tissue) at 28 wks following lung irradiation (10 Gy) with Genistein, EUK-207 and combination (G+E) treatment. All drug treatments started immediately after irradiation and finished at 14 wks. Each bar represents the mean (±SEM). B) Protein expression of TGF-β at 0, 4, 8, 14 and 28 wks following lung irradiation (10 Gy) with Genistein, EUK-207 and combination (G+E) treatment. All drug treatments started immediately after irradiation and finished at 14 wks. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each point represents the mean (±SEM).
Radiation-induced inflammation
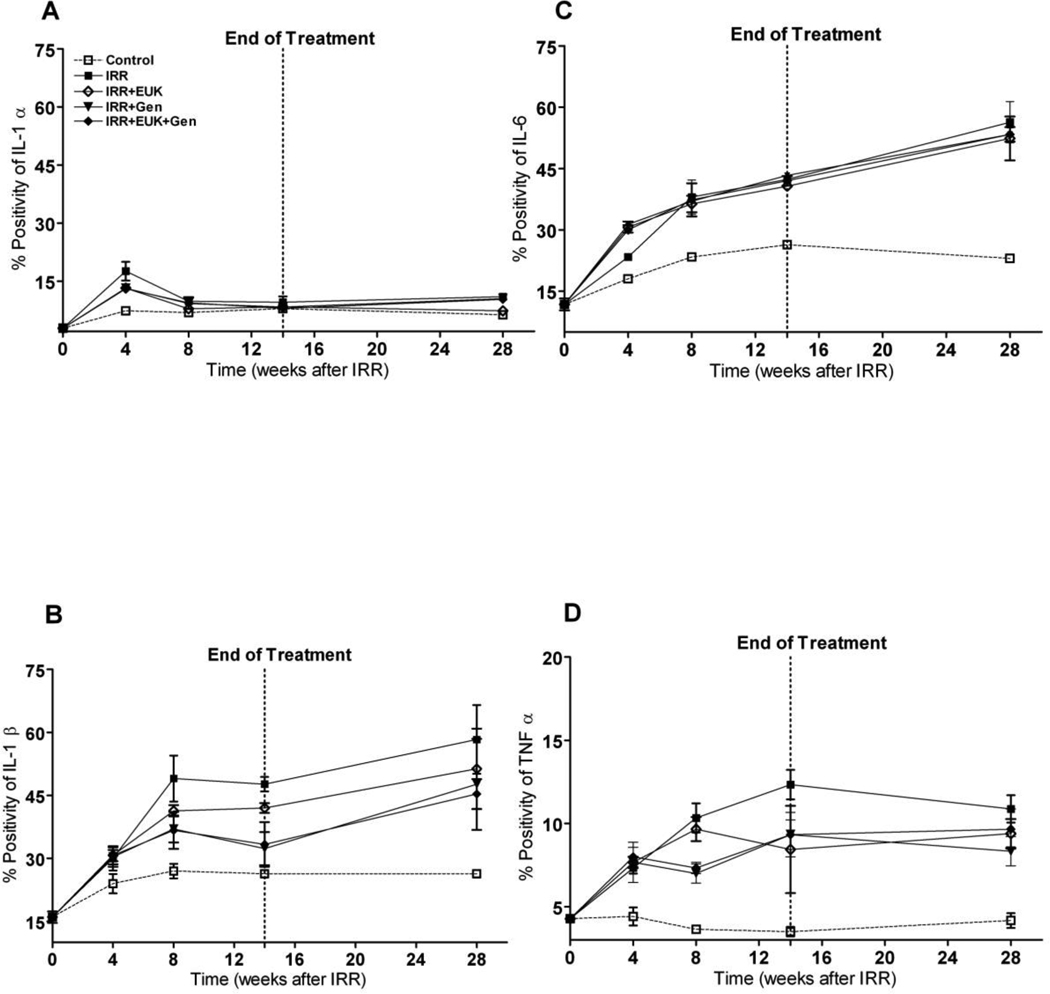
The expression of the inflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α, as analysed by immunohistochemical staining, in lung tissue from rats sacrificed at the different time points is shown in Figure 4. Irradiation caused an increase in all four cytokines over the course of the experiment although the pattern of the increases was somewhat different for the four cytokines. In general the staining was diffusely spread throughout the lung and did not appear to be specifically limited to one cell type. For IL-1α, IL-1β and IL-6 the control rats also showed a small increase with age but this was not seen for TNF–α. For IL-1α (Figure 4A) there was a large increase at 4 wks PI (significantly different from the control p<0.01) followed by decreased levels at 8 wks and no change out to 28 wks. The drug treatment groups showed a similar pattern with no significant differences to the radiation-only group of rats over the whole time course of the study. Expression of IL-1β (Figure 4B) was significantly increased after irradiation from 8 wks until 28 wks compared to the normal control rats (p<0.01). The drug treatment groups showed a similar trend of increased expressions of IL-1β until 28 wks but were intermediate between the control and radiation-only groups. Statistical significance varied but generally these groups were significantly different from both the control and the radiation-only group (p<0.05) For IL-6 expressions (Figure 4C) all the treated groups showed a steady and significant increase from 4–28 wks compared to normal control rats (p<0.01) but the drug treatments showed no difference from the radiation-only group. A significant increase in expression of TNF-α (Figure 4D) was observed in all irradiated rats from 4 to 28 wks after radiation compared to normal control rats (p<0.01). The drug treatments involving the genistein diet caused a significant reduction from the radiation-only group when analyzed over the period 8–28 wks (p<0.05) but spot analysis specifically at the 28 wks time point showed similar levels of expression to the radiation-only group. The EUK-207 treatment group was not significantly different from the radiation-only group at any time point.
Figure 4.
Protein expression of cytokines IL-1α (A), IL-1β (B), IL-6 (C), TNF-α (D) staining at 0, 4, 8, 14 and 28 wks following lung irradiation (10 Gy) with Genistein, EUK-207 and combination (G+E) treatment. All drug treatments started immediately after irradiation and finished at 14 wks. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar represents the mean (±SEM).
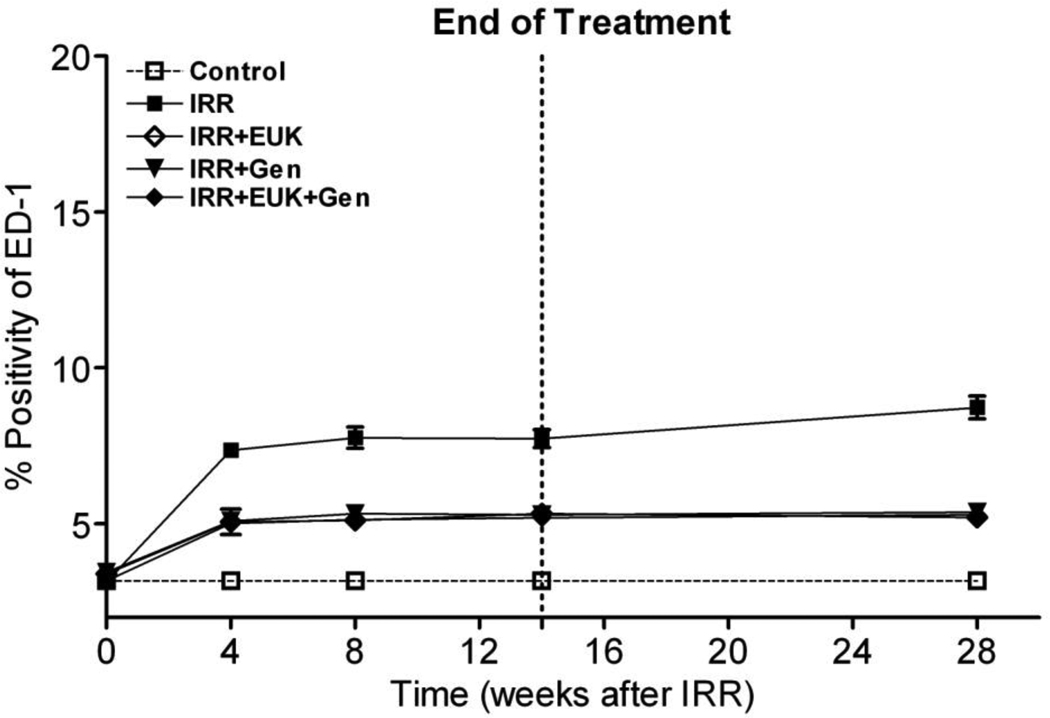
Further confirmation of the induction of a prolonged inflammatory response was seen in the analysis of macrophage activation as demonstrated by ED-1 staining in Figure 5. The positivity of ED-1 staining increased sharply at 4 wks and then increased more gradually out to 28 wks in the radiation-only group. These changes were significant relative to normal control rats (p<0.01). All the drug treatment groups showed a significant increase in ED-1 staining relative to the control group (p<0.01) but this was significantly less than that seen in the radiation-only group over the whole period from 4 wks to 28 wks (p<0.01).
Figure 5.
ED-1 staining for activated macrophages in the rat lung at 0, 4, 8, 14 and 28 wks following lung irradiation (10 Gy) with genistein, EUK-207 and combination (G+E) treatment. All drug treatments started immediately after irradiation and finished at 14 wks. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar represents the mean (±SEM).
Discussion
As previously, we observed that SD rats irradiated to the lungs demonstrated two waves of increase in breathing rate over the period of our study (Calveley et al. 2010). The first increase occurred over 4–10 wks PI, a timing consistent with pneumonitis. The second increase started after about 14 wks PI and continued out to sacrifice at 28 wks. This would be consistent with the development of fibrosis in these rats. The rats on the genistein diet, the EUK-207 infusion and their combination (G+E) showed significantly reduced breathing rate increases during both periods of breathing rate increase.
In our previous study (Calveley et al. 2010) we also observed that genistein treatment similar to that used in the current study could reduce the early breathing rate increase induced by 18 Gy (Co-60 γ-rays) to the whole SD rat lung but we did not observe a reduction in the second wave of breathing rate increase in the rats that survived to 28 wks. This difference may be related to the fact that many of the animals had to be sacrificed during the course of the previous experiment because lung problems caused severe morbidity. This was not apparent in the current study suggesting that the treatment was less severe. A possible explanation may be that in the current study approximately 10% of the lower lung was not in the direct radiation field. Also different irradiation facilities were used in the two studies (due to the decommissioning of the Co-60 treatment facility) with different dose rates and different expected relative biological effectiveness. The difference in the doses delivered was designed to address these latter two issues based on our previous experience (Newcomb et al. 1993, Haston et al. 1994) but the accommodation may not have been exact. Regardless, the difference in the results from these two experiments could be very important, since it suggests the possibility that the mitigation of radiation-induced pneumonitis and functional lung damage may be more effective at lower levels of overall lung damage.
We have previously reported, using rodent models, that radiation induces significant expression of DNA damage in lung in the form of micronuclei (MN) in pulmonary fibroblasts using a well established cytokinesis-block MN assay (Calveley et al. 2005, Calveley et al. 2010, Khan et al. 1998, Khan et al. 2003, Langan et al. 2006, Para et al. 2009)). In the current studies we demonstrated a significant (P<0.01) reduction in MN formation in fibroblasts from rats treated with either EUK-207 or genistein at 4, 8 and 14 wks post irradiation while the animals were being treated with the drugs. Interestingly, however, we observed a rebound in the level of MN in these groups to levels similar to that in the radiation only group at 28 wks when the animals had been removed from the drug treatment. Since the drug treatments were initiated after the radiation treatment, the early reduction in DNA damage cannot be due to the scavenging of the initial radiation-produced radicals. Thus these findings suggest that the DNA damage leading to the MN observed probably occurs as a result of secondary radicals induced at later times, possibly due to leakage of Reactive Oxygen Species (ROS) from damaged mitochondria or produced by the radiation-induced inflammatory response. The rebound in the number of micronuclei observed after the cessation of the drug treatment is consistent with the idea that DNA damaging ROS are being continually produced in the lung tissue.
Measurement of oxidative DNA damage as assessed by levels of 8-OHdG, did not show as much of a rebound effect. The reasons for the discrepancy between these two measures of DNA damage are currently unclear but may relate to differential increases in levels of antioxidant or DNA repair enzymes induced by the treatment and the possibility that different radicals are responsible for the two types of damage. It is also noteworthy that MN formation reflects strand breakage which may be a more difficult lesion to repair than induction of 8-OHdG. Regardless of the mechanism, comparison of the MN data with the breathing rate and fibrosis data suggest that the presence of DNA damage in the fibroblasts is not critical for this functional lung damage, since we observed significant mitigation in the breathing rate and in fibrosis at 28 wks by all the drug treatments. These observations on breathing rate and fibrosis are more consistent with the results from the 8-OHdG staining but these differences raise the question of the importance of oxidative stress in the induction of this functional damage.
Activation of alveolar macrophages is generally thought to play an important role in the upregulation of the inflammatory process PI and to be largely responsible for the persistent cytokine cascade and chronic inflammatory state indicative of early and late radiation-induced lung injury (Johnston et al. 2004) Consistent with this concept we saw an early significant increase in ED-1 staining which was maintained throughout the experiment (4–28 wks) in the radiation-only group of rats. The irradiated and drug-treated groups of rats also showed a significant increase in ED-1 staining relative to the control but this was significantly damped relative to the radiation only group and was not increased after 14 wks when the drug treatment ceased. The activation of macrophages was consistent with the rapid increases in the expression of the inflammatory cytokines IL-1α, IL-1β, IL-6, TNF-α observed but the effects of the drug treatments on the ED-1 levels was not very consistent with the limited effects of the drug treatment on the levels of cytokines present in the tissue. Only IL-1β and TNF-α levels were reduced in the drug treated groups and, in the case of TNF-α, this occurred only in the animals consuming genistein as we have observed previously (Calveley et al. 2010). These results may suggest that other inflammatory cell populations (e.g. lymphocytes, neutrophils) or lung parenchymal cells can also be stimulated to produce these cytokines as time progresses PI, consistent with our observation of diffuse staining for these cytokines in the lung tissue and with the work of others (Rube et al 2000, 2005).
The late phase of fibrosis is characterized by increased collagen deposition and depends on the species and strain of animal, suggesting a genetic variability (Down and Yanch 2010, Franko et al. 1991, Franko et al. 1996, Haston et al. 2002, Haston et al. 2007, van Eerde et al. 2001). We analyzed hydroxyproline content in pieces of lung tissue at 28 wks PI. The results showed a significant increase in hydroxyproline content in the irradiation-only group of rats compared to young control and normal control rats. The treatment with genistein, EUK-207 or their combinations resulted in significantly lower hydroxyproline content to levels which were not significantly different from the levels in the control rats, indicating that the level of fibrosis 28 wks after radiation is significantly attenuated by both drug treatments
Other groups have demonstrated the partial efficacy of SOD in preventing radiation damage using different modes of drug delivery. Epperly at al. (Epperly et al. 1998, 2000, 2001) demonstrated that intratracheal injection of Mn-SOD plasmid-liposome gene therapy prior to irradiation may attenuate lung damage and improve survival in mice. However, the SOD plasma-liposome gene therapy must be administered early, in order to enable time for the antioxidant enzyme to be expressed. Whereas, the direct administration of SOD by intratracheal or other means is of limited effectiveness because, as a protein, SOD would have limited access to target cells and tissues. Therefore, the development and testing of small molecule SOD-mimetics is a potentially valuable approach. The combined SOD and catalase activities of low molecular weight Mn complexes like EUK-207 provides a further advantage, since SOD does not scavenge hydrogen peroxide but, instead, generates it. Similar to our findings, Rabbani et al. (Rabbani et al. 2007a, Rabbani et al. 2007b) reported that the chronic administration of the Mn porphyrin catalytic antioxidant Aeolus (AEOL)10150 via osmotic pump in female Fisher rats (1 week and 10 wks after irradiation) showed protective effects against radiation-induced lung injury. They also found that administering the drug before and for a short time after irradiation had no significant benefit. They strongly suggested that long term administration of the drug can significantly reduce lung damage, inflammation, oxidative stress, tissue hypoxia, etc. This finding validated their previous work in which constitutive overexpression of extracellular SOD had been shown to be effective in a mouse model of lung injury (Kang et al. 2003, Rabbani et al. 2005), though, like EUK-207, AEOL 10150 also acts intracellularly. Interestingly, these studies used 28 Gy single-dose irradiation to the right hemithorax, which if given to the whole lung would have been lethal. Their results may thus be consistent with our findings which suggested that less severe overall damage may be more amenable to protection/mitigation.
Overall our study clearly demonstrated that genistein, EUK-207 and their combination provided substantial mitigation against the functional effects of lung irradiation particularly breathing rate changes consistent with pneumonitis. Furthermore, even after stopping the drugs at 14 wks following radiation this mitigation persisted out to at least 28 wks, when the experiment was terminated. The extent of the inflammatory response and of radiation-induced fibrosis was also reduced by the drug treatment suggesting the possibility that reductions in the inflammatory process during the pneumonitis stage may lead to a partial short-circuiting of the chronic cyclic inflammatory response induced by lung irradiation and thus to more long term benefit in functional outcome. Whether this effect would be prolonged beyond 28 wks remains to be determined. DNA damage was also mitigated in the rat lungs by the drug treatment but for the MN endpoint this mitigation did not seem to be prolonged after the end of the drug treatment. The combination of the two drugs did not demonstrate effects greater than either drug alone. This finding is consistent with our understanding that both act via an antioxidant mechanism. Genistein is known to inhibit NFkB activation, one of the major activators of the inflammatory response, and it has antioxidant activity. EUK-207 and other salen-manganese complexes have SOD and catalase activities, and suppress the activation of transcription factors associated with oxidative stress, such as Nuclear Factor Kappa B (NFkB) and Activator Protein-1 (AP-1), in vivo (Peng et al. 2005, Rong et al. 1999, Zhang et al. 2004). Further studies are needed to elucidate in more detail whether different doses or scheduling of the drugs would further improve their mitigating effects. In addition, it would be of interest to examine whether combinations of genistein or EUK-207 with other radiation mitigators such as angiotensin converting enzyme (ACE) inhibitors (Ghosh et al. 2009), potentially acting via distinct mechanisms, would be more effective than either drug alone.
Acknowledgements
This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and by funds from the Canadian Institutes of Health Research (#144089). Partial support was also provided by the Ontario Ministry of Health and Long Term Care (OMHLTC). The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
Disclosure Statement:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abou-Seif MA, El-Naggar MM, El-Far M, Ramadan M, Salah N. Amelioration of radiation-induced oxidative stress and biochemical alteration by SOD model compounds in pre-treated gamma-irradiated rats. Clinica Chimica Acta. 2003;337:23–33. doi: 10.1016/s0009-8981(03)00192-x. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. The Journal of Biological Chemistry. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Zgonjanin L, Rabbani ZN, Corbley MJ, Fu K, Sun L, Lee WC, Ling LE, Vujaskovic Z. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. International Journal of Radiation Oncology Biology Physics. 2008;71:829–837. doi: 10.1016/j.ijrobp.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Baxa DM, Yoshimura FK. Genistein reduces NF-kappa B in T lymphoma cells via a caspase-mediated cleavage of I kappa B alpha. Biochemical Pharmacology. 2003;66:1009–1018. doi: 10.1016/s0006-2952(03)00415-5. [DOI] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Comparison between in vitro radiosensitivity and in vivo radioresponse of murine tumor cell lines. II: International Journal of Radiation Oncology Biology Physics. 1990;18:331–345. doi: 10.1016/0360-3016(90)90098-5. [DOI] [PubMed] [Google Scholar]
- Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, Hill RP. Genistein can mitigate the effect of radiation on rat lung tissue. Radiation Research. 2010;173:602–611. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. International Journal of Radiation Biology. 2005;81:887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- Choi C, Cho H, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFkappaB activation in RAW 264.7 macrophages. Bioscience Biotechnology and Biochemistry. 2003;67:1916–1922. doi: 10.1271/bbb.67.1916. [DOI] [PubMed] [Google Scholar]
- Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiology of Aging. 2010;31:425–433. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldham NG, Zhang AQ, Key P, Sauer MJ. Absolute bioavailability of (14C) genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. European Journal of drug metabolism and pharmacokinetics. 2002;27:249–258. doi: 10.1007/BF03192335. [DOI] [PubMed] [Google Scholar]
- Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Archives of Biochemistry and Biophysics. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- Down JD, Yanch JC. Identifying the high radiosensitivity of the lungs of C57L mice in a model of total-body irradiation and bone marrow transplantation. Radiation Research. 2010;174:258–263. doi: 10.1667/RR2149.1. [DOI] [PubMed] [Google Scholar]
- Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, Greenberger J. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Therapy. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Therapy. 2000;7:1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated radiation. International Journal of Cancer. 2001;96:221–231. doi: 10.1002/ijc.1023. [DOI] [PubMed] [Google Scholar]
- Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. International Journal of Radiation Oncology Biology Physics. 1994;28:621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Seminars in Radiation Oncology. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Franko AJ, Sharplin J, Ward WF, Hinz JM. The genetic basis of strain-dependent differences in the early phase of radiation injury in mouse lung. Radiation Research. 1991;126:349–356. [PubMed] [Google Scholar]
- Franko AJ, Sharplin J, Ward WF, Taylor JM. Evidence for two patterns of inheritance of sensitivity to induction of lung fibrosis in mice by radiation, one of which involves two genes. Radiation Research. 1996;146:68–74. [PubMed] [Google Scholar]
- Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radical Biology and Medicine. 2010;48:1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafoori P, Marks LB, Vujaskovic Z, Kelsey CR. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22:37–47. discussion 52-33. [PubMed] [Google Scholar]
- Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. International Journal of Radiation Oncology Biology Physics. 2009;75:1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haston CK, Begin M, Dorion G, Cory SM. Distinct loci influence radiation-induced alveolitis from fibrosing alveolitis in the mouse. Cancer Research. 2007;67:10796–10803. doi: 10.1158/0008-5472.CAN-07-2733. [DOI] [PubMed] [Google Scholar]
- Haston CK, Hill RP, Newcomb CH, Van Dyk J. Radiation-induced lung damage in rats: the influence of fraction spacing on effect per fraction. International Journal of Radiation Oncology Biology Physics. 1994;28:633–640. doi: 10.1016/0360-3016(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Haston CK, Zhou X, Gumbiner-Russo L, Irani R, Dejournett R, Gu X, Weil M, Amos CI, Travis EL. Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Research. 2002;62:3782–3788. [PubMed] [Google Scholar]
- Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991 Dec;13:641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiation Research. 1996;145:762–767. [PubMed] [Google Scholar]
- Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Experimental Lung Research. 2004;30:369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- Kang JL, Lee HW, Lee HS, Pack IS, Chong Y, Castranova V, Koh Y. Genistein prevents nuclear factor-kappa B activation and acute lung injury induced by lipopolysaccharide. American Journal of Respiratory and Critical Care Medicine. 2001;164:2206–2212. doi: 10.1164/ajrccm.164.12.2104017. [DOI] [PubMed] [Google Scholar]
- Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. International Journal of Radiation Oncology Biology Physics. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. International Journal of Radiation Oncology Biology Physics. 1998;40:467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- Khan MA, Van Dyk J, Yeung IW, Hill RP. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiotherapy and Oncology. 2003;66:95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiotherapy and Oncology. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proceedings of the National Academy of Sciences USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Seminars in Radiation Oncology. 2003;13:333–345. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) The Journal of Biological Chemistry. 1969;244:6049–6055. [PubMed] [Google Scholar]
- McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. The Journal of Biological Chemistry. 1969;244:6056–6063. [PubMed] [Google Scholar]
- Michael McClain R, Wolz E, Davidovich A, Bausch J. Genetic toxicity studies with gensitein. Food Chemistry and Toxicity. 2006;44:42–55. doi: 10.1016/j.fct.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Newcomb CH, Van Dyk J, Hill RP. Evaluation of isoeffect formulae for predicting radiation-induced lung damage. Radiotherapy and Oncology. 1993;26:51–63. doi: 10.1016/0167-8140(93)90026-5. [DOI] [PubMed] [Google Scholar]
- Olive PL. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiation Research. 1998 Nov;150(5 Supplement):S42–S51. [PubMed] [Google Scholar]
- Para AE, Bezjak A, Yeung IW, Van Dyk J, Hill RP. Effects of genistein following fractionated lung irradiation in mice. Radiotherapy and Oncology. 2009;92:500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. The Journal of Biological Chemistry. 2005;280:29194–29198. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Petkau A. Role of superoxide dismutase in modification of radiation injury. British Journal of Cancer. 1987 Suppl 8:87–95. [PMC free article] [PubMed] [Google Scholar]
- Quinlan T, Spivack S, Mossman BT. Regulation of antioxidant enzymes in lung after oxidant injury. Environmental Health Perspectives. 1994;102 Suppl 2:79–87. doi: 10.1289/ehp.9410279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BioMedCentral Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. International Journal of Radiation Oncology Biology Physics. 2007a;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radical Research. 2007b;41:1273–1282. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler JD, Goldstein RH, Fine A, Smith BD. Regulation of the alpha 1(I) collagen promoter via a transforming growth factor-beta activation element. The Journal of Biological Chemistry. 1993;268:13625–13631. [PubMed] [Google Scholar]
- Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. International Journal of Radiation Biology. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proceedings of the National Academy of Sciences USA. 1999;96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RA, Huffman KD, Fisette LW, Damphousse CA, Callaway WB, Malfroy B, Doctrow SR. Orally available Mn porphyrins with superoxide dismutase and catalase activities. The Journal of Biological Inorganic Chemistry. 2009;14:979–991. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rube CE, Fricke A, Wendorf J, Stutzel A, Kuhne M, Ong MF, Lipp P, Rube C. Accumulation of DNA double-strand breaks in normal tissues after fractionated irradiation. 2010. International Journal of Radiation Oncology Biology Physics. 2010;76:1206–1213. doi: 10.1016/j.ijrobp.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Rube CE, Palm J, Erren M, Fleckenstein J, Konig J, Remberger K, Rube C. Cytokine plasma levels: reliable predictors for radiation pneumonitis? PLoS One. 2008;3:e2898. doi: 10.1371/journal.pone.0002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. International Journal of Radiation Oncology Biology Physics. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- Rube C, Uthe D, Wilfert F, Ludwig D, Yang K, Konig J, Palm J, Schuck A, Remberger K, Rube C. The bronchiolar epithelium as a prominent source of proinflammatory cytokines after lung irradiation. International Journal of Radiation Oncology Biology Physics. 2005;61:1482–1492. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- Rube CE, Wilfert F, Palm J, Konig J, Burdak-Rothkamm S, Liu L, Schuck A, Willich N, Rube C. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlentherapie und Onkologie. 2004;180:442–448. doi: 10.1007/s00066-004-1265-7. [DOI] [PubMed] [Google Scholar]
- Rube CE, Wilfert F, Uthe D, Konig J, Liu L, Schuck A, Willich N, Remberger K, Rube C. Increased expression of pro-inflammatory cytokines as a cause of lung toxicity after combined treatment with gemcitabine and thoracic irradiation. Radiotherapy and Oncology. 2004;72:231–241. doi: 10.1016/j.radonc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. 2010 International Journal of Radiation Oncology Biology Physics. 1992;24:93–101. doi: 10.1016/0360-3016(92)91027-k. [DOI] [PubMed] [Google Scholar]
- Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. 2010 International Journal of Radiation Oncology Biology Physics. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. Journal of Clinical Investigation. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy NV, Parkinson HD, Sochaski MA, Borghoff SJ. Kinetics of genistein and its conjugated metabolites in pregnant sprague-dawley rats following single and repeated genistein administration. Toxicological Sciences. 2006;90:230–240. doi: 10.1093/toxsci/kfj077. [DOI] [PubMed] [Google Scholar]
- Tai Y, Inoue H, Sakurai T, Yamada H, Morito M, Ide F, Mishima K, Saito I. Protective effect of lecithinized SOD on reactive oxygen species-induced xerostomia. Radiation Research. 2009;172:331–338. doi: 10.1667/RR1557.1. [DOI] [PubMed] [Google Scholar]
- Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity. Proceedings of the Society for Experimental Biology and Medicine. 1997;214:107–113. doi: 10.3181/00379727-214-44076. [DOI] [PubMed] [Google Scholar]
- Van Eerde MR, Kampinga HH, Szabo BG, Vujaskovic Z. Comparison of three rat strains for development of radiation-induced lung injury after hemithoracic irradiation. Radiotherapy and Oncology. 2001;58:313–316. doi: 10.1016/s0167-8140(00)00301-7. [DOI] [PubMed] [Google Scholar]
- Van Loon B, Markkanen E, Hubscher U. Oxygen as a friend and enemy: How to combat the mutational potential of 8-oxo-guanine. DNA Repair. 2010;9:604–616. doi: 10.1016/j.dnarep.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiation Research. 2010;173:748–759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Lapcik O, Hampl R, Uehara M, Al-Maharik N, Stumpf K, Mikola H, Wahala K, Adlercreutz H. Time-resolved fluoroimmunoassay of plasma daidzein and genistein. Steriod. 2000;65:339–348. doi: 10.1016/s0039-128x(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Yakovlev VA, Rabender CS, Sankala H, Gauter-Fleckenstein B, Fleckenstein K, Batinic-Haberle I, Jackson I, Vujaskovic Z, Anscher MS, Mikkelsen RB, Graves PR. Proteomic Analysis of Radiation-Induced Changes in Rat Lung: Modulation by the Superoxide Dismutase Mimetic MnTE-2-PyP(5+) International Journal of Radiation Oncology Biology Physics. 2010;78:547–554. doi: 10.1016/j.ijrobp.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. The Journal of the Federation of American Societies for Experimental Biology. 2004;18:1547–1549. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Current Medicinal Chemistry. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]