Abstract
Purpose
On the basis of the known role of platelet-derived growth factor (PDGF)-BB/PDGF receptor (PDGFR) β in pericyte regulation, highly specific inhibitors of this target are needed. We tested the efficacy of a highly selective aptamer against PDGF-B with or without anti-VEGF therapy in ovarian cancer models.
Results
Bevacizumab inhibited tumor growth by 45% and 48% in the HeyA8 and SKOV3ip1 models, respectively. AX102 had minimal effect on the HeyA8 model, but increased tumor growth in the SKOV3ip1 model. However, bevacizumab plus AX102 was more effective than bevacizumab alone, and resulted in 76–88% inhibition of tumor growth in both models. A longitudinal study in the HeyA8 model using bioluminescence imaging showed that combination of bevacizumab, AX102 and paclitaxel caused tumor reduction by 65% (based on bioluminescence imaging). In the HeyA8 model, MVD and PCNA counts were significantly reduced in the bevacizumab treatment groups, and pericyte coverage was significantly decreased in the AX102 treatment groups. In the SKOV3ip1 model, MVD and PCNA was significantly reduced in the bevacizumab treatment group, and even lower in the bevacizumab and AX102 combination treatment group.
Experimental design
The therapeutic efficacy of targeting endothelial cells (bevacizumab) and/or pericytes (PDGF-aptamer, AX102) was examined using HeyA8 and SKOV3ip1 orthotopic models of ovarian cancer metastasis. Following therapy, tumors were examined for microvessel density (MVD), proliferating cell nuclear antigen (PCNA), and vascular maturation (pericyte coverage).
Conclusions
Dual targeting of endothelial cells and pericytes holds potential as an anti-vascular therapeutic approach in ovarian carcinoma.
Keywords: ovarian cancer, endothelial cell, pericyte, PDGF-B, AX102, aptamer, bevacizumab
Introduction
One of the major hurdles in the management of malignant tumors is metastasis, which is dependent on angiogenesis.1 Thus, angiogenesis has become an important target for cancer therapy. Among several anti-angiogenesis strategies, targeting endothelial cells using anti-VEGF approaches is considered to be the most efficacious.2 As the first anti-angiogenic agent approved and marketed in the United States, bevacizumab, a VEGF-A neutralizing monoclonal antibody, has shown promising results as a single agent in renal cancer.3 In combination with chemotherapy, bevacizumab has resulted in improved progression-free and overall survival in advanced colorectal4 and lung cancers.5 However, bevacizumab failed to show a survival benefit in a phase III clinical trial in metastatic breast cancer patients.6 In ovarian cancer, preclinical studies indicate that single agent bevacizumab can markedly reduce ascites,7 and is effective in reducing tumor burden in combination with paclitaxel.8 In phase II clinical trials, bevacizumab has resulted in encouraging anti-tumor activity,9 which has ushered in phase III investigation in both the primary and recurrent settings. Unfortunately, despite these exciting results, tumor progression eventually occurs. Therefore, novel therapeutic strategies and additional targets are urgently needed for further improvement of anti-angiogenesis therapy.
In a mature blood vessel, endothelium is surrounded by pericytes, which are mesenchymal in origin and are required for normal microvascular stability and function.10 Pericytes interact with endothelial cells by physically supporting and functionally regulating them. Pericytes also play a role in the regulation of blood flow and modulation of new blood vessel growth.11 Within the tumor vasculature, the role of pericytes is not clearly understood, but they appear to play a significant role in microvascular stability and function. Pericytes are abundant, but loosely attached to endothelial cells in tumor blood vessels, and paradoxically extend cytoplasmic processes away from the vessel wall. These alterations may cause weakening of the vessel wall, and increase the risk of hemorrhage.12,13 We and others have shown that the amount of pericyte coverage in different tumors ranges from extensive to little depending on tumor types as well as the markers used to identify pericytes.14–16 We have also demonstrated that pericytes provide survival cues to endothelial cells and may protect them from apoptotic stimuli. For example, pericytes may protect endothelial cells from anti-VEGF therapy. Therefore, pericytes are now being considered as a valid target for cancer therapy.
Aptamers are a backbone-modified ssDNA or RNA oligonucleotides that bind tightly to a molecular target with high affinity and specificity.17 AX102 is a ssDNA aptamer that is highly selective for PDGF-B and is a modified version of the DNA aptamer NX1975/ARC126.18 It has been shown that AX102 effectively blocks PDGF-B actions, and reduces pericyte coverage of tumor vessels.19,20 Here, we investigated whether AX102 would enhance the anti-angiogenic activity of bevacizumab in orthotopic ovarian cancer models.
Results
Effect of dual targeting of endothelial cells and pericytes on ovarian cancer growth
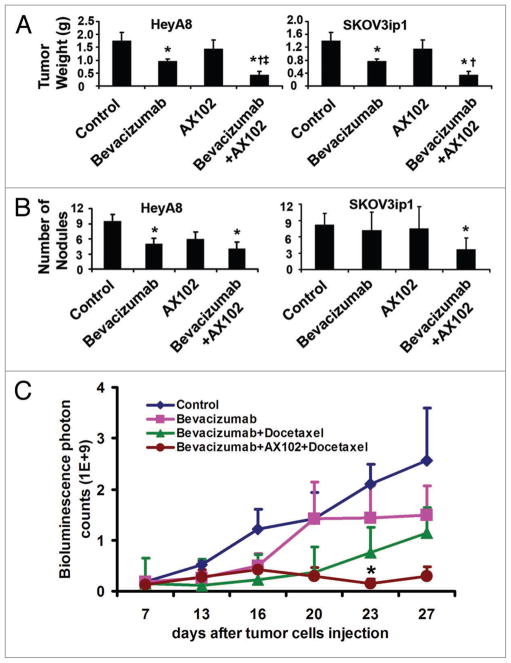
On the basis of our previous mechanistic observations demonstrating the protective effects of pericytes for endothelial cells,14,16 we tested the efficacy of dual pericyte (AX102) and endothelial (bevacizumab) targeting. HeyA8 or SKOV3ip1 cells were inoculated into the peritoneal cavity of nude mice, and 7 days later, mice were randomized into the following 4 groups: (1) vehicle control, (2) bevacizumab, (3) AX102 and (4) bevacizumab and AX102. In the HeyA8 model (Fig. 1A), bevacizumab treatment resulted in a 45% reduction in tumor weight compared to treatment with vehicle (p < 0.05), while AX102 alone had no effect on tumor weight. Bevacizumab in combination with AX102 resulted in 76% reduction in tumor weight compared to vehicle controls (p < 0.005) and 56% reduction in tumor weight in comparison to bevacizumab monotherapy (p < 0.05). Similarly, in the SKOV3ip1 model, bevacizumab reduced tumor weight by 45% in comparison to vehicle controls (p < 0.05). Combination therapy was even more effective (88% reduction in tumor weight; p < 0.02 compared to controls and p < 0.05 compared to bevacizumab monotherapy). However, treatment with AX102 alone significantly increased tumor weight compared to the vehicle control group (p < 0.05). To determine potential effects on metastases, we also counted the number of nodules, as shown in Figure 1B. In the HeyA8 model, both bevacizumab monotherapy and combination with AX102 significantly reduced tumor nodules by 47% and 58%, respectively in comparison to vehicle control (p < 0.05 for both). However, the difference between the bevacizumab and the combination groups was not statistically significant. In the SKOV3ip1 model, only the combination therapy of bevacizumab and AX102 significantly decreased tumor nodule number by 55% in comparison to vehicle control (p < 0.05). There were no obvious toxicities noted in any group.
Figure 1.
Effect of dual targeting of endothelial cells and pericyte on human ovarian cancer growth. (A) Mean tumor weights with standard errors are shown. (B) Mean number of tumor nodules with standard errors is shown. Mice were injected with HeyA8, or SKOV3ip1 human ovarian cancer cells. Seven days later, mice were randomized to receive treatment: (1) vehicle control; (2) bevacizumab alone (6.25 mg/kg, i.p. twice per week); (3) AX102 (50 mg/kg, i.p., daily) alone; (4) bevacizumab (6.25 mg/kg, i.p. twice per week) plus AX102 (50 mg/kg, i.p., daily). *p < 0.05 compared to vehicle controls; †p < 0.05 compared to bevacizumab alone. (C) Longitudinal assessment of tumor growth. Mice were injected i.p. with HeyA8-Luc cells, and seven days later were randomized to receive treatment: (1) Vehicle control; (2) bevacizumab (6.25 mg/kg, i.p. twice per week) alone; (3) bevacizumab (6.25 mg/kg, i.p. twice per week) plus docetaxel (2 mg/kg, i.p. weekly); (4) bevacizumab (6.25 mg/kg, i.p. twice per week) plus AX102 (50 mg/kg, i.p., daily) plus docetaxel (2 mg/kg, i.p. weekly). The in vivo bioluminescence imaging was conducted longitudinally at different time points. *p < 0.05 compared to other groups.
We next examined the effect of dual endothelial and pericyte targeting in combination with chemotherapy on longitudinal tumor growth using in vivo bioluminescence imaging.14 The HeyA8-Luciferase cells were injected into the peritoneal cavity of mice, and baseline imaging was performed on day 7. Mice were then randomized into 4 groups: (1) control, (2) bevacizumab, (3) bevacizumab plus docetaxel, (4) bevacizumab and AX102 plus docetaxel. Therapy was started on day 7. The in vivo bioluminescence imaging was performed at 3–4 day intervals and the experiment was stopped when control mice became moribund. Bevacizumab alone inhibited tumor growth, with greater inhibition noted in combination with docetaxel chemotherapy. Furthermore, the combination therapy of bevacizumab, AX102 and docetaxel resulted in reduction of established tumors by 65% based on photon counts after 16 days of treatment (Fig. 1C). To assess the ability of AX102 to block PDGF-BB, we measured the expression of phosphorylated PDGFR-β in tumor tissues by immunofluorescence staining. We observed that AX102 treatment resulted in significantly reduced PDGFR-β phosphorylation (Suppl. Fig. 1).
Effect of dual endothelial and pericyte targeting on tumor vasculature and tumor cell proliferation
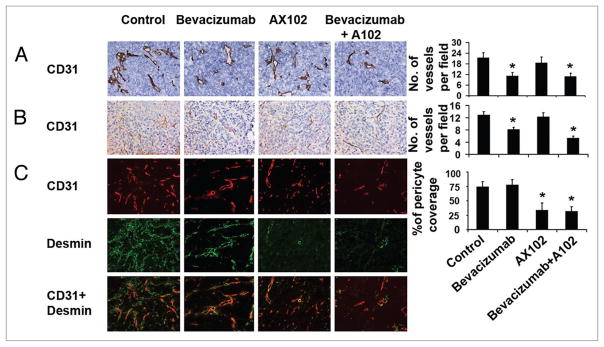
To examine potential effects of therapy on the tumor vasculature, we first examined vessel density. IHC staining for CD31 was performed on tumor tissues from mice following in vivo treatment with bevacizumab, AX102 or the combination of these two agents. In the HeyA8 model (Fig. 2A), MVD was reduced by 49% in the bevacizumab treatment groups compared to control (p < 0.05). AX102 monotherapy resulted in slightly decreased MVD compared to control, but the difference was not statistically significant (p > 0.05). Addition of AX102 to bevacizumab treatment resulted in a 48% reduction in tumor MVD in comparison to the control group, this was essentially equivalent to monotherapy with bevacizumab. In the SKOV3ip1 model, MVD was reduced by 37% in the bevacizumab treatment groups compared to control (p < 0.05, Fig. 2B). Addition of AX102 to bevacizumab treatment produced a greater decrease in tumor MVD of 59% in comparison to control treatment (p < 0.05). AX102 monotherapy had no effect on MVD (p > 0.05).
Figure 2.
Effect of dual targeting of endothelial and pericyte on tumor vasculature. (A) Frozen tumor sections were stained for CD31 to reveal microvessel density using immunohistochemical staining in HeyA8 model. (B) In SKOV3 ip1 model, paraffin embedded sections were used for CD31 staining. Microvessels were counted and graphed. (C) Dual immunofluorescence staining for CD31 (endothelial marker, red) and desmin (pericyte marker, green) was performed to determine the extent of pericyte coverage in HeyA8 model. The percentage of vessels with at least 50% coverage of associated desmin-positive cells were recorded. *p < 0.05 compared to vehicle controls. †p < 0.05 compared to bevacizumab alone. Images were taken at original magnification x200.
Next, we examined pericyte coverage in the HeyA8 model using double immunofluorescence staining for CD31 (endothelial cell marker) and desmin (pericyte marker; Fig. 2C). Treatment with bevacizumab had no effect on pericyte coverage while treatment with AX102 significantly decreased pericyte coverage by 66% compared to controls (p < 0.01). Bevacizumab in combination with AX102 produced a 68% decrease in pericyte coverage compared to the control group, and no significant additional decrease in pericyte coverage was noted with combination therapy in comprison to AX102 alone.
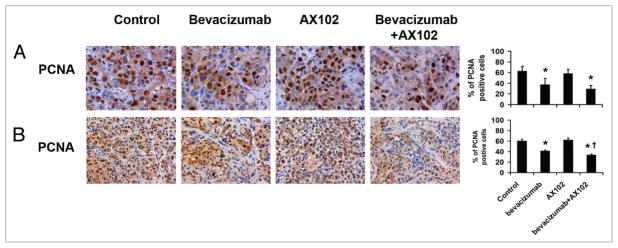
Since anti-angiogenic therapies can indirectly affect tumor cell proliferation, we next examined PCNA counts following treatment. In the HeyA8 model, bevacizumab alone reduced tumor cell proliferation by 41% in comparison to controls (p < 0.05, Fig. 3A). AX102 treatment alone had no significant effect on tumor cell proliferation in this model, and its addition to bevacizumab produced a 53% decrease in PCNA staining (p < 0.05), which was not significantly different from bevacizumab therapy alone. In the SKOV3ip1 model, the PCNA counts were reduced by 32% in the bevacizumab treatment groups compared to control (p < 0.05), as shown in Figure 3B. Addition of AX102 to bevacizumab treatment produced a greater decrease in tumor PCNA of 45% in comparison to control turmors (p < 0.05 in comparison to the control group and the bevacizumab group). There was a non-significant increase in proliferation in response to AX102 monotherapy. We also examined the effect on downstream markers such as MAPK (p42/44) phosphorylation using immunohistochemical staining. As expected, AX102-based therapy decreased MAPK (p42/44) phosphorylaton (Suppl. Fig. 1).
Figure 3.
Effect of dual targeting of endothelial cells and pericytes on tumor proliferation. Tumor sections were stained for PCNA to reveal tumor cell proliferation in (A) HeyA8 model and (B) SKOV3 ip1 model. PCNA positive cells were counted and graphed. *p < 0.05 compared to vehicle controls. †p < 0.05 compared to bevacizumab alone. Images were taken at original magnification x400 for (A) and x200 for (B).
Discussion
The key findings from our study are that pharmacological targeting of both endothelial cells and pericytes is highly efficacious in ovarian carcinoma and resulted in reduced tumor growth in orthotopic ovarian cancer mouse models. This effect may be due, in part, to enhanced anti-angiogenesis effects following reduction of pericyte coverage in the tumor vasculature.
VEGF-targeted therapies have improved patient survival in many cancer types.9,21–23 However, despite the initial response rates, most patients develop progressive disease. Among the anti-VEGF resistance mechanisms, pericytes are thought to play an important role. We have observed that in normal tissues, pericytes are associated tightly with endothelial cells and extensively cover normal vasculature. In ovarian cancer, however, pericytes are loosely associated with endothelial cells and oriented irregularly relative to the stroma.13 We and others have shown that pericytes play important roles in the maintenance of ovarian cancer vasculature.16 A key regulator for pericyte development and function is the PDGF ligand/receptor system. PDGF is composed of A, B, C & D polypeptide chains that form heterodimer PDGF-AB and homodimers PDGF-AA, BB, CC and DD. Its biological activities are mediated through two tyrosine kinase receptors, PDGFR-α and -β. PDGF-AA, BB, AB and CC bind to PDGFR-α, whereas PDGF-BB and DD interacts with PDGFR-β.24,25 PDGF-BB and PDGF-DD have been shown to recruit pericytes and may play a role in tumor vessel stabilization. PDGF-AA and PDGF-CC promote recruitment of PDGFR-α positive fibroblasts, which produce VEGF, FGF2, and enhance tumor angiogenesis.26,27 We have previously demonstrated that PDGF-AA and BB ligands are expressed in most ovarian cancer samples, and 40% of tumor cells were positive for PDGFR-β expression.28 In the tumor microenvironment, the tumor and endothelial cells produce PDGF-BB, which recruits pericytes and promotes VEGF production by pericytes. This provides a survival advantage to endothelial cells by preventing apoptosis.16 Tumor vessels lacking pericytes appear to be more dependent on VEGF for their survival, and in turn more vulnerable to VEGF blockade. Thus, targeting pericytes may enhance the anti-angiogenic effects of VEGF blockade.
PDGF-B produced by tumor and endothelial cells plays a critical role in pericyte recruitment,29 therefore, specific PDGF-B blockade may target angiogenesis by preventing the recruitment of pericytes and disturbing the interaction of endothelial cells and pericytes. In the present study, we demonstrated that AX102, a highly selective PDGF-B aptamer significantly decreased pericyte coverage in the HeyA8 tumor tissue. Similar observations have been documented with xenografts of Lewis lung carcinomas (LLC) and pancreatic cancers by Sennino et al.19 However, the latter group noted vessel regression after pericyte loss, while we observed no significant effect on MVD after pericyte loss through AX102 treatment. These disparate observations may be due to different levels of PDGF/PDGFR in the tumor microenvironment and the relationship of pericytes and endothelium in different tumor models. We also demonstrated that dual targeting of pericytes and endothelial cells is more efficacious than targeting endothelium alone. While AX102 monotherapy was not effective (both in tumor weight and tumor nodules number), combination with bevacizumab was highly efficacious. These results are consistent with our previous studies using AEE-788 (a dual tyrosine kinase inhibitor of EGFR and VEGFR) and imatinib, and VEGF-trap and PDGF-trap.14–16 However, AX102 specifically targets PDGF-B, while imatinib targets PDGF-β and other molecules such as c-kit. Thus, AX102 is potentially more attractive in terms of targeting pericytes.
Our finding that AX102 monotherapy does not reduce tumor growth is not surprising and is supported by previous studies. 14–16,19,30 In the present study, AX102 monotherapy had no significant effect on MVD and tumor cell proliferation. In the SKOV3ip1 model, AX102 enhanced the effect of bevacizumab on MVD and PCNA reduction. Mechanistically, this finding is supported by the findings that disruption of pericytes enhances vascular leakiness and thus increases tumor edema. Since pericytes regulate endothelial cell function by inhibiting their proliferation and provide protection against apoptosis, the reduction of pericytes may weaken their effect on inhibiting endothelial cell proliferation. However, some studies have demonstrated that PDGF inhibition significantly reduced tumor bone metastasis of human breast and prostate cancer xenografts.31 Studies from Guo and colleagues showed that overexpression of PDGF-BB in glioblastoma cells led to increased pericyte coverage and enhanced tumor growth in the brain microenvironment.29 These different results may be due to different levels of PDGF/PDGFR expression and differences in the tumor microenvironment. In addition to PDGF-B/PDGFR-β, pericyte functions may also be regulated by other pathways, such as Ang1/Tie2, S1P/Edg-1, TGFβ1/Alk5 and matrix metalloproteinases (MMPs).13 These factors could offer additional opportunities for therapeutic pericyte targeting.
In summary, our data demonstrate that strategies targeting both endothelial cells and pericytes are more effective than targeting either cell type alone. Inhibition of both VEGF and PDGF-B signaling appears to be a promising antiangiogenic strategy that leads to tumor vessel destabilization and tumor regression. This study provides the preclinical rationale for the development of more effective therapies for human ovarian cancer.
Materials and Methods
Cell lines and culture conditions
Highly metastatic human ovarian cancer cell lines HeyA8 and SKOV3ip1, as described previously, were used in the present study.32 Cells were cultured in RPMI1640 medium supplemented with 15% fetal bovine serum and 0.5% gentamicin, maintained on plastic and incubated at 37°C in a mixture of 5% CO2 and 95% air. The tumor cells were free of pathogenic murine viruses and Mycoplasma (assayed by M.A. Bioproducts, Walkersville, MD). The cells were maintained for less than 10 weeks after recovery from frozen stock.
Animals
Female athymic nude mice (NCr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were kept under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in agreement with current regulations and standards of the NIH, the United States Department of Health and Human Services, and the United States Department of Agriculture. According to institutional guidelines, mice used in these experiments were 8–12 weeks old.
Drugs and reagents
Bevacizumab and docetaxel were purchased from the M.D. Anderson Cancer Center pharmacy (Houston, TX). AX102 was generously provided by Archemix Corp. (Cambridge, MA). AX102 is a 34-nucleotide DNA aptamer that specifically binds to and inhibits the activity of PDGF-B.
Orthotopic implantation of tumor cells and tumor collection procedures
Sub-confluent cultures of HeyA8 and SKOV3ip1 cells were lifted with either 0.25% trypsin-EDTA (Life Technologies, for SKOV3 ip1) or 0.1% EDTA (for HeyA8), neutralized with RPMI 1640 medium containing 10% fetal bovine serum, centrifuged at 1,000 rpm for 5 minutes, washed in serum-free medium and resuspended in HBSS (Invitrogen). Single-cell suspensions with >95% viability, as determined by trypan blue exclusion, were used for the in vivo injections. Cells were then injected intraperitoneally (i.p) into mice at a concentration of 2.5 × 105 cells/0.2 ml for HeyA8 cells and 1 × 106 cells/0.2 ml for SKOV3ip1 cells. Mice were sacrificed at 28–42 days after tumor cell implantation, and body weight, tumor weight and location, number of nodules, and the amount of ascites were recorded. Tumor tissues were collected for further studies. For immunohistochemical (IHC) and H&E-staining procedures, tumors were fixed in formalin and embedded in paraffin. For immunofluorescence staining, tumors were embedded in OCT compound (Miles, Inc., Elkhart, IN), frozen rapidly in liquid nitrogen, and stored at −80°C. For western blot analysis, tumors were snap frozen rapidly in liquid nitrogen and stored at −80°C.
Treatment and data collection
For therapy experiments, mice were injected i.p. with HeyA8 and SKOV3ip1 cells as detailed above and randomized into 4 groups (n = 10/group). Seven days after tumor cells injection, the following treatments were initiated: (1) vehicle control; (2) bevacizumab (6.25 mg/kg, i.p. twice per week) alone; (3) AX102 (50 mg/kg, i.p., daily) alone; (4) bevacizumab (6.25 mg/kg, i.p. twice per week) plus AX102 (50 mg/kg, i.p., daily). Following 3 weeks of therapy for the HeyA8 model and 5 weeks of therapy for the SKOV3ip1 model, mice were sacrificed when mice in the control group became moribund.
For longitudinal assessment, mice were injected i.p. with the HeyA8-Luc cells at 2.5 × 105 cells/mouse. Seven days later, mice were randomized into one of the following four groups (n = 10/group): (1) vehicle control; (2) bevacizumab (6.25 mg/kg, i.p. twice per week) alone; (3) bevacizumab (6.25 mg/kg, i.p. twice per week) plus docetaxel (2 mg/kg, i.p. weekly); (4) bevacizumab (6.25 mg/kg, i.p. twice per week) plus AX102 (50 mg/kg, i.p., daily) plus docetaxel (2 mg/kg, i.p. weekly). In vivo bioluminescence imaging was conducted twice a week on a cryogenically cooled IVIS 100 imaging system (Xenogen Corp., Alameda, CA), as described previously,14 and the data were analyzed with Living Image software coupled to the IVIS system. The experiment was concluded when mice in any group became moribund.
Immunofluorescence staining
Sections were fixed in cold acetone for 30 minutes, and blocked with protein blocker (4% of fish gel) for 1 hour at room temperature. For dual immunofluorescence staining for CD31 and desmin, the sections were probed with CD31 antibody (1:500, BD Pharmingen, San Diego, CA) at 4°C overnight, after washing with phosphate-bufferred saline (PBS), the sections were then incubated with Alexa 594-conjugated anti-rat antibody (1:1,000, Invitrogen, Eugene, OR) for 1 hour at room temperature. After extensive washing with PBS, samples were next probed with anti-desmin antibody (1:400, DakoCytomation, Denmark) for 2 hours, followed by washing with PBS and incubation with Alexa 488-conjugated anti-rabbit antibody (1:1,000, Invitrogen) for 1 hour at room temperature.
Immunohistochemical (IHC) staining for proliferating cell nuclear antigen (PCNA) and CD31
For using formalin-fixed, paraffin-embedded tissue, sections were deparaffinized in xylene, rehydrated in graded alcohol, and transferred to PBS. After antigen retrieval with citrate buffer (pH 6.0) for PCNA, and proteinase K for CD31, the endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 15 minutes, and the nonspecific epitopes were blocked with fragment block (1:10, Jackson ImmunoResearch Laboratories) overnight at 4°C. Sections were next incubated with protein blocker (4% of fish gel) for 1 hour at room temperature, followed by incubation with the anti-PCNA PC10 antibody (1:50; DAKO) or anti CD31 (1:200, PharMingen) overnight at 4°C. After washing with PBS, for PCNA staining, sections were incubated with horseradish peroxidase (HRP)-conjugated rat anti-mouse IgG2a (1:100, Serotec, Harlan Bioproducts for Science, Inc.,) for 1 hour, for CD31 staining, sections were incubated with rat probe (BioCare) for 20 minutes, followed by incubation with rat HRP polymer (BioCare) for 20 minutes at room temperature. Finally, Visualization was attained with 3,3′-diaminobenzidine (Research Genetics) and counter-staining with Gil’s hematoxylin (BioGenex Laboratories). For using frozen sections for IHC staining, sections were fixed in cold acetone for 30 minutes, washed with PBS, blocked with protein blocker (4% of fish gel), and then were incubated with rat monoclonal anti-mouse CD31 (1:800, PharMingen) overnight at 4°C, washed with PBS and then incubated with HRP-conjugated goat anti-rat IgG (1:200, Jackson ImmunoResearch Laboratories) for 1 hour. Visualization was attained with 3,3′-diaminobenzidine (Research Genetics) and counterstaining with Gil’s hematoxylin (BioGenex Laboratories).
Quantification of pericyte coverage, microvessel density (MVD) and PCNA
For quantification, 5 samples from each group were examined. To quantify pericyte coverage, the percentage of vessels with at least 50% coverage of associated desminpositive cells was determined in 5 random 0.159-mm2 fields at x200 magnification for each sample. MVD was quantified by the number of lumen-like structures adjacent to CD31-positive endothelial cells within 5 randomly selected 0.159 mm2 fields at x200 magnification. PCNA was determined by the percentage of PCNA-positive cells in 5 randomly selected 0.159 mm2 fields at x200 magnification.
Statistical analysis
All measurements were represented as the average ± S.E. of the mean. Continuous variables were analyzed by two-tailed Student’s t test or analysis of variance (ANOVA) (for all groups) if normally distributed, and the nonparametric Mann-Whitney rank sum test (for all groups) if the distribution is unkonwn. A Bonferroni adjustment to α (default value 0.05) was made based on the number of pairwise comparisons within a treatment experiment using the formula: a (α) = 0.05/k, where K = number of comparisons against control. A p-value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Cancer Institute (T32 Training Grant CA101642), the Ovarian Cancer Research Fund Program Project Development Grant, the University of Texas, M.D. Anderson Cancer Center Ovarian Cancer Specialized Program of Research Excellence (P50 CA083639), the EIF Foundation, the National Institute of Health (CA110793, CA109298), the Gynecologic Cancer Foundation, the Zarrow Foundation, the Betty Ann Asche Murray Distinguished Professorship, and the Marcus Foundation. M.M.S. is supported by the GCF-Molly Cade ovarian cancer research grant and the NIH/NICHD Baylor WRHR scholarship grant (HD050128).
Footnotes
References
- 1.Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 2.Kuo CJ, Farnebo F, Yu EY, Christofferson R, Swearingen RA, Carter R, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci USA. 2001;98:4605–10. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–5. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 6.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 7.Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998;153:1249–56. doi: 10.1016/S0002-9440(10)65669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L, Hofmann J, Zaloudek C, Ferrara N, Hamilton T, Jaffe RB. Vascular endothelial growth factor immunoneutralization plus Paclitaxel markedly reduces tumor burden and ascites in athymic mouse model of ovarian cancer. Am J Pathol. 2002;161:1917–24. doi: 10.1016/S0002-9440(10)64467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 10.Sims DE. The pericyte—a review. Tissue Cell. 1986;18:153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 11.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 12.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Chunhua Lu, Sood Anil. Role of pericytes in angiogenesis. In: Teicher BA, Ellis LM, editors. Angiogenesis agents in cancer therapy. 2. Humana Press, Inc; Totowa (NJ): pp. 117–32. [Google Scholar]
- 14.Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–17. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 15.Lu C, Landen C, Kim T, Kamat A, Han L, Li Y, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for human ovarian cancer. Proc Am Assoc Cancer Res. 2006 doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 16.Lu C, Thaker PH, Lin YG, Spannuth W, Landen CN, Merritt WM, et al. Impact of vessel maturation on antiangiogenic therapy in ovarian cancer. Am J Obstet Gynecol. 2008;198:477. doi: 10.1016/j.ajog.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aptamers WJ. Encyclopedia of analytical chemistry. 2000:4848–71. [Google Scholar]
- 18.Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–84. [PubMed] [Google Scholar]
- 19.Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, et al. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–67. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floege J, Ostendorf T, Janssen U, Burg M, Radeke HH, Vargeese C, et al. Novel approach to specific growth factor inhibition in vivo: antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am J Pathol. 1999;154:169–79. doi: 10.1016/S0002-9440(10)65263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 22.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 23.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JD, Haldeman BA, Grant FJ, Murray MJ, Seifert RA, Bowen-Pope DF, et al. Platelet-derived growth factor (PDGF) stimulates PDGF receptor subunit dimerization and intersubunit transphosphorylation. J Biol Chem. 1991;266:8987–92. [PubMed] [Google Scholar]
- 25.Betsholtz C, Karlsson L, Lindahl P. Developmental roles of platelet-derived growth factors. Bioessays. 2001;23:494–507. doi: 10.1002/bies.1069. [DOI] [PubMed] [Google Scholar]
- 26.Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–10. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tejada ML, Yu L, Dong J, Jung K, Meng G, Peale FV, et al. Tumor-driven paracrine platelet-derived growth factor receptor alpha signaling is a key determinant of stromal cell recruitment in a model of human lung carcinoma. Clin Cancer Res. 2006;12:2676–88. doi: 10.1158/1078-0432.CCR-05-1770. [DOI] [PubMed] [Google Scholar]
- 28.Apte SM, Bucana CD, Killion JJ, Gershenson DM, Fidler IJ. Expression of platelet-derived growth factor and activated receptor in clinical specimens of epithelial ovarian cancer and ovarian carcinoma cell lines. Gynecol Oncol. 2004;93:78–86. doi: 10.1016/j.ygyno.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 29.Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–93. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, et al. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117:2114–22. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lev DC, Kim SJ, Onn A, Stone V, Nam DH, Yazici S, et al. Inhibition of platelet-derived growth factor receptor signaling restricts the growth of human breast cancer in the bone of nude mice. Clin Cancer Res. 2005;11:306–14. [PubMed] [Google Scholar]
- 32.Apte SM, Fan D, Killion JJ, Fidler IJ. Targeting the platelet-derived growth factor receptor in antivascular therapy for human ovarian carcinoma. Clin Cancer Res. 2004;10:897–908. doi: 10.1158/1078-0432.ccr-1151-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.