Abstract
A prior study in New York City observed that airborne concentrations of three metals found in steel – iron, manganese, and chromium – are more than 100 times higher in the subway system than in aboveground air. To investigate the potential for health effects of exposure at these levels, we conducted a pilot study of subway workers comparing personal exposures to steel dust with biomarkers of metal exposure, oxidative stress, and DNA damage in blood and urine samples. Workers wore a personal air sampler operating at 4 L/m for one to three work shifts with blood and urine samples collected at the end of the final shift. We found that PM2.5 exposures varied among subway workers on the basis of job title and job activity. The subway workers’ mean time-weighted PM2.5 exposure was 52 µg/m3, with a median of 27 µg/m3, and a range of 6–469 µg/m3. The observed concentrations of PM2.5, iron, manganese, and chromium fell well below occupational standards. Biomarker concentrations among the 39 subway workers were compared with a group of 11 bus drivers, and a group of 25 suburban office workers. Concentrations of DNA–protein crosslinks and chromium in plasma were significantly higher in subway workers than in bus drivers, but no significant difference was observed for these biomarkers between subway workers and office workers. Urinary isoprostane concentrations were significantly correlated with the number of years working in the subway system, and were detected at higher, though not significantly higher, concentrations in subway workers than in bus drivers or office workers. At the group level, there was no consistent pattern of biomarker concentrations among subway workers significantly exceeding those of the bus drivers and office workers. At the individual level, steel dust exposure was not correlated with any of the biomarkers measured.
Keywords: Manganese, Chromium, PM2.5, Biomonitoring, Subway
1. Introduction
The subway is increasingly recognized as a unique and important microenvironment for air pollution research (Nieuwenhuijsen et al., 2007). Suspended particulate matter in subway systems differs from street-level particulate matter with respect to particulate morphology, size distribution, concentration, and chemical composition. Relative to street-level particles, subway particles are generally more angular in shape, larger in diameter, more abundant by mass, and contain higher levels of the metals found in steel (Seaton et al., 2005; Sitzmann et al., 1999). In New York City for example, the concentrations of iron (Fe), manganese (Mn), and chromium (Cr) in suspended particulate matter were found to be more than 100 times higher in the subway system than aboveground (Chillrud et al., 2004). Subway particles also have greater capacity than outdoor (street-level) particles to induce DNA damage and oxidative stress in cultured lung cells (Karlsson et al., 2005; Seaton et al., 2005). Given the elevated concentration of suspended particulate matter in the subway, the greater in vitro reactivity of subway particles, the large number of people exposed (millions per day), and the long-term nature of the exposure, it seems appropriate to assess whether exposure to suspended particulate matter in the subway could potentially pose a risk to human health. Because of the fundamental differences between subway particles and street-level particles, we cannot infer the potential health effects of exposure to suspended particulate matter in the subway based on the health effects known to be associated with exposure to ambient particulate matter (Seaton et al., 2005). In this pilot study, we focus our attention on the potential health effects of those metals that are most enriched in the New York City subway environment relative to street-level particulate matter: iron, manganese, and chromium (Chillrud et al., 2004).
Prior studies in several cities have shown that concentrations of suspended particulate matter (PM) in the subway are generally elevated above street-level concentrations (Table 1). Subway particulate matter concentrations vary widely as does the ratio of subway to street-level particulate matter concentrations. Factors thought to influence subway PM concentrations include station depth, date of construction, ventilation rate, proportion of frictional to regenerative braking, train frequency, wheel type (rubber versus steel), and the presence or absence of platformedge doors and/or air-conditioning in subway cars and stations. The New York City subway system currently uses steel wheels, ceramic composite brake shoes, and regenerative breaking (Cudahy, 2003). Subway cars have on-board air filtration/ conditioning and underground station depths for much of the system are shallow as compared with London or Washington, DC. In London, Toronto, and Stockholm, concentrations of particulate matter with an aerodynamic diameter ≤2.5 µm (PM2.5) are on the order of 10 times higher in the subway than aboveground, whereas in Guangzhou, subway PM2.5 concentrations are four times lower than the relatively high levels of outdoor PM2.5 (Chan et al., 2002; Crump, 2000; Johansson and Johansson, 2003; Seaton et al., 2005). While the difference between subway and street-level particulate matter concentrations will strongly influence how subway air quality is perceived by the public, it is only one of many parameters that determine the health risks associated with air pollution exposure in underground rail systems. Among other factors to consider are the number of particles per volume of air (number density), the particles’ chemical composition, toxicological properties, and size distribution.
Table 1.
Comparison of particulate matter (PM) concentrations in subway systems with PM concentrations in outdoor air samples.
| City | Year opened |
Subway PM2.5 (µg/m3) |
Subway sample n |
Outdoors PM2.5 (µg/m3) |
Outdoors sample n |
Source | Subway observation period |
|---|---|---|---|---|---|---|---|
| London (summer) |
1863 | 247 | 44 ridersa | 34.5 | 40 cyclists | Adams et al. (2001) | Duration of commute |
| London (winter) | 1863 | 157 | 12 ridersa | 23.5 | 56 cyclists | Adams et al. (2001) | Duration of commute |
| London | 1863 | 300; 310; 420 |
3 fixedb | ~30 | 2 fixed | Seaton et al. (2005) | 7 am–5 pm 3 days |
| New York | 1904 | 62 | 1 riderc2 samples | 13 ± 4 | 37 fixed | Chillrud et al. (2004) | 8 h samples |
| New York | 1904 | 56 ± 95 | 33 workersc | 13 ± 4 | 37 fixed | This study | 8–11 h work shifts 1–3 days |
| Stockholm | 1950 | 258 | 1 fixedb | 23 | 1 fixed | Johansson and Johansson (2003) | 7 am–7 pm 2 weeks |
| Toronto | 1954 | 159 | 6 fixedb | 15 | 185 personal | Crump (2000) | 8 h samples |
| Mexico City | 1969 | 61 | 18 ridersa | 71; 68 | 16 buses, 28 minibuses | Gomez-Perales et al. (2004) | Duration of commute |
| Helsinki | 1982 | 47; 60 | 2 fixedb | 10, 17 | 2 fixed | Aarnio et al. (2005) | Two weeks |
| Guangzhou | 1999 | 44 ± 11 | 14 ridersa | 106; 145 | 8 taxis, 15 buses | Chan et al. (2002) | Duration of commute |
| Rome | 1955 | PM10: 407 | 5 fixedb | PM10: 101 | 4 fixed | Ripanucci et al. (2006) | 8 am–6 pm 1 day |
| Prague | 1974 | PM10: 103 | 1 ridera 108 samples |
PM10: 74 | 1 person 108 samples |
Branis (2006) | Duration of commute |
Data are presented as arithmetic means ± arithmetic standard deviation when this information was available. Rail systems are shown according to the age of the subway system from oldest to newest.
Riders—these studies’ estimates of underground PM2.5 are representative of the time spent in subway stations and on subway trains by investigators or study subjects who wore personal air samplers. They are not representative of 24 h integrated exposure.
Fixed—these studies’ estimates of underground PM2.5 are based on stationary samples taken on or near the station platform
Excludes six workers who worked at an aboveground maintenance shop.
While choosing the subway as a mode of transport may have a relatively small effect on a commuter’s total daily PM2.5 exposure, the effect on their total daily particulate metal exposure will be more pronounced. Studies in Toronto and London designed to detect the effects of the introduction of the gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT) on automobile drivers’ manganese exposures inadvertently detected the effects of the manganese-enriched subway environment on subway commuters. The Toronto study found that the best predictor of manganese in personal air samples was time spent in the subway (Crump, 2000). The London study found that office workers, roughly half of whom commuted by subway, had blood manganese levels significantly higher than those of taxi drivers (Pfeifer et al., 1999). In New York, a study whose primary goal was to understand the personal exposure pathways of air toxics for high school students, found that subway steel dust was the dominant source of exposure to airborne iron, chromium, and manganese for the students enrolled in the study who used the subway (Chillrud et al., 2004).
The deleterious effects of particulate metal exposure at high concentrations have been documented in toxicological, animal, and epidemiological studies. When compared to aboveground PM, subway PM sampled in Stockholm was found to be eight times more likely to induce DNA damage and four times more likely to cause oxidative stress in cultured lung cells (Karlsson et al., 2005). Particles sampled from three London subway stations were found to have greater inflammatory potential and greater capacity to induce DNA damage in cultured human epithelial cells than aboveground particulate matter (Seaton et al., 2005). Karlsson et al. (2008) found that the genotoxicity of subway particles may be due to their ability to form intracellular reactive oxygen species (ROS). Karlsson et al. (2008) also found that subway dust was more genotoxic than Fe3O4, Fe2O3, CuO, or Cu/Zn particles, suggesting that the genotoxicity of subway dust could not be solely attributable to these constituent particles. Numerous epidemiological studies of welders have documented significant associations between exposure to welding fumes and disease endpoints (Antonini, 2003), such as pneumonia (Palmer et al., 2003), siderosis (Doherty et al., 2004), and neurological disorders such as Parkinsonism (Aschner et al., 1999). Welding fumes and subway particulates are both enriched in Fe, Mn, and Cr, though welding fumes have a finer size distribution and exposures typically occur at much higher concentrations than those that have been observed in subway environments.
The pro-inflammatory effects of soluble transition metals have been demonstrated in vitro (McNeilly et al., 2004), and animal models suggest that soluble transition metals, with iron the most prevalent species, may be the primary determinant of acute inflammatory response to ambient particulate matter (Costa and Dreher, 1997). In humans, instillation of metal-rich ambient PM2.5 in the lungs of healthy human volunteers was found to be associated with greater airway inflammation than instillation of ambient PM2.5 with lower concentrations of transition metals (Schaumann et al., 2004). If the transition metal content of particulate matter is a primary determinant of the severity of inflammatory response in vitro, and in controlled animal and human experiments, then the subway provides a suitable environment to determine whether an inflammatory response can also be induced by long-term exposure to particulate metals at lower concentrations than those used in the laboratory studies cited above.
While there are no known health effects of airborne particulate metal exposure at the levels observed in the study of New York City high school students (Chillrud et al., 2004), investigation of potential health effects from steel dust exposure among adult subway workers seems prudent, given that they spend a greater amount of time in the subway system and that their work-related activities should result in greater per-unit-time exposure levels than those of the public. If there are biological effects of exposure, they may be easier to detect among the subway worker population than among the subway-riding public.
While there have been numerous efforts to monitor air quality in subway systems (see Table 1) the present study is one of the first to couple personal air monitoring with biological monitoring of study participants. A similar study in Stockholm (Bigert, 2007) found that subway workers with greater exposure to subway particulate matter (platform workers), had significantly higher plasma concentrations of PAI-1, a biomarker of inflammatory response, than subway workers with lower exposures (ticket sellers).
In the present study, we characterize the particulate metal exposure of a cross-section of subway workers, and evaluate whether their exposure is associated with biological changes that can result in greater susceptibility to disease. We examine: (a) whether there is a dose–response relationship between exposure to subway particulate metals and levels of various biomarkers in the blood and urine of study participants, and (b) whether concentrations of biomarkers of metal exposure, oxidative stress, and DNA damage are significantly higher among subway workers than among office workers or bus drivers, and (c) whether associations between biomarker concentrations are indicative of potential biological mechanisms of damage.
2. Materials and methods
In this pilot study, the potential for biological effects due to steel dust exposure was assessed at the individual level by comparing the concentration of biomarkers in the subway workers’ blood and urine samples to the concentration of particulate metals in air sampled by their personal air monitors during one to three (8–11 h) work shifts. The potential for biological modification at the population level was evaluated by comparing biomarker concentrations in blood and urine samples from 39 subway workers with those from 11 bus drivers and 25 suburban office workers. No environmental monitoring data were collected for the control groups who were assumed to be minimally exposed to particulate iron, manganese, and chromium, based on questionnaire data, and prior ambient air sampling in the New York metropolitan area (Urban outdoor samples) and at the place of work of the office workers (Suburban outdoor samples). The biomarkers used in this study are listed in Table 2.
Table 2.
Biomarkers measured in urine, plasma and whole blood.
| Biomarker | Measured in: | As indicator of: |
|---|---|---|
| Mn | urine, plasma, whole blood | Mn exposure |
| Cr | urine, plasma, whole blood | Cr exposure |
| Pb | urine, plasma, whole blood | Pb exposure |
| BPDE | urine | exposure to PAHs |
| Creatinine | urine | hydration status |
| Isoprostanes | urine | lipid oxidation |
| 8-oxodG | urine | DNA oxidation |
| Plasma carbonyls | plasma | protein oxidation |
| DNA–protein crosslinks | lymphocytes | DNA damage |
Abbreviations: 8-oxodG, 8-oxodeoxyguanosine; BPDE, benzo(a)pyrene diol epoxide; Cr, chromium; DNA, deoxyribonucleic acid; isoprostanes, 15-F2t–isoprostane; Mn, manganese; PAHs, polycyclic aromatic hydrocarbons; Pb, lead.
Inclusion criteria for all subjects: we selected males, 18 years or older, who had worked continuously in the same job title for at least the past 2 years. Preference was given to non-smokers who did not wear personal protective equipment such as face masks or respirators. Women were excluded because their endogenous iron stores are generally lower than those in men, and since our small study size precluded us from adequately controlling for gender differences. Control subjects who rode the subway more than twice a month were excluded. The study protocol was approved by the Columbia University Medical Center Institutional Review Board.
2.1. Personal air monitoring and biological sample collection
We enrolled 39 New York City subway workers from a cross-section of job titles with a wide range of anticipated exposure levels. The typical duties of the various job titles are described below. The subway lines where the workers were monitored are given in parentheses. Workers recruited for the study included track construction crews who remove ballast, lay new track, and perform maintenance tasks (A,C,E,6,D,N,R). Track maintenance crews replace rails, change plates, put up conduit, and perform other tasks (4,6,S,N,7,2,3,F,J). Station cleaners hose down the subway platform, clean the tile wall opposite the platform while standing on the tracks, and flag oncoming trains (1,9,F,D,3,4,N,R,L). Each of the overhaul shop workers monitored worked aboveground and performed distinct tasks in distinct locations in this aboveground facility. Train operators drive the train from the lead car while train conductors operate the car doors from the middle of the train (1,9). Construction flaggers work in tunnels and on platforms signaling approaching trains to slow down and warning workers of oncoming trains (D,1,9,6). Refuse train workers spend approximately 3 h a day loading and unloading dumpsters at underground and aboveground stations (D,M,F,G,E). Most of their time is spent on the refuse train. The refuse train does not have an air-conditioning system and particulate matter levels on-board are higher than levels measured on trains with air conditioning and air filtration (Chillrud et al., 2004).
The personal air-monitoring campaign began in November 2004 and ended in February 2005. Station cleaners (n=6), overhaul shop workers (n=6), construction flaggers (n=6), track maintenance workers (n=6), and track construction workers (n=6) were monitored for three work shifts, while refuse train workers (n=3) were monitored for two shifts and train operators and conductors (n=6) for one shift. The monitoring period was shorter for those workers thought to have consistent exposures from one shift to the next. Track workers were monitored using a new filter for each work shift because their exposures were expected to be high and to vary appreciably depending on work location and activity. A composite multi-day filter was collected for construction flaggers, station cleaners, overhaul shop workers and refuse train workers, who were expected to have exposures that potentially could be too low to capture accurately in one work shift. All exposures were normalized for total volume of air sampled. At the end of each shift, workers returned the personal air-monitoring equipment and described the location and duration of their job activities.
Workers were outfitted with personal air monitors custom-built with a low profile and worn under the arm beneath work jackets to minimize the risk of injury from entanglement with passing trains. An air intake tube clipped to the outside of the worker’s lapel was attached to a size-selective cyclone with a 2.5 mm aerodynamic-diameter cut point (model KTL, BGI Inc.) when operated at 4 L/min ± 10%. Particulates were collected onto 37 mm Teflon membrane filters (Gelman Inc.) in plastic cassettes. The personal air monitor consisted of a Thomas rotary air pump (G12/04 EB) housed with a cooling fan in an acrylic tube held in the holster of a DeSantis Patriot shoulder harness. A rechargeable lithium ion battery array (Battery Specialties, 186502S-2P, 7.2V-4.0Ah), regulated to +12 v DC ± − 2% by an LM340 solid-state regulator, and a custom-designed circuit board fabricated to provide switched, fuse-protected power, were housed in the pouch under the opposite arm. The fixed voltage supply powered the timer and the air pump. Filters were weighed pre- and post-sampling on a microbalance after being conditioned in a temperature/humidity controlled environment for at least 24 h (by opening the petrislide filter container) and statically discharged via a polonium source. Filters were analyzed inside a class-100 flow bench for reflectance. Following reflectance measurements, filters were prepared for multi-element analysis by magnetic sector high-resolution inductively-coupled-plasma mass-spectrometry (HR-ICP-MS). Aliquots of Standard Reference Material (SRM) 1648 (Urban Particulate Matter) were weighed on a microbalance and digested several times during the course of the sample analyses. The SRM aliquots were digested using the same quantities of acids and microwave program. Recoveries for iron, manganese, and chromium were 93%, 86%, and 92%, respectively, of their reported values. Recoveries for all other measured analytes were within 25% of reported values and most were within 12% of reported values.
To compare particle size distribution in the subway to aboveground conditions, a light-scattering particle counter (Met-One 237b, Grants Pass, OR) was installed on a refuse collection train for 2 days in February, 2005. The air inlet tube of the particle counter was positioned outside the window of an empty conductor’s cabin. Particle counts were integrated every 30 s. Mean particle counts for the size fractions were compared during a period of approximately 1 h during which the subway train’s trajectory was either continuously underground or continuously aboveground.
At the conclusion of the final shift, each worker filled out a study questionnaire and provided a blood sample and a urine sample. The study questionnaire asked workers about their typical job activities, commuting habits, other potential exposures to particulate metals, diet, and use of vitamins and medications. To better understand potential determinants of exposure, questionnaire data for continuous variables (e.g. number of hours spent on track) were compared to average (filter-based) metal exposures. Binary variables (e.g. spends time scraping debris from track) were used to categorize subway workers into two groups that could then be compared for significant differences in exposure levels or biomarker concentrations (see section “Statistical methods” below for more details).
A total of 28 mL of blood was collected into four 7 mL vacutainers by a physician or certified phlebotomist in a mobile blood donation vehicle parked near the worksite. Vacutainers were inverted 10 times to mix the anticoagulant (EDTA or sodium heparin) into the blood sample. Urine was collected into 50 mL acid-washed polypropylene tubes. During sample collection, urine and blood samples were stored in a cooler for no more than 3 h, then transferred to a laboratory refrigerator and prepared for analysis.
2.2. Biomarker selection
Iron, manganese, and chromium can have toxic biological effects by generating reactive oxygen species through Fenton or Fenton-like chemistry, inducing oxidative stress (Ali et al., 1995; Costa and Dreher, 1997; Shi et al., 1993). The increase in reactive oxygen species disrupts biochemical homeostasis, which can result in lipid peroxidation, DNA damage, and depletion of the antioxidants that mediate inflammatory response in epithelial cells (McNeilly et al., 2004; Stohs and Bagchi, 1995). We used 15-F2t-isoprostane (isoprostane), protein carbonyls, and 8-oxodeoxyguanosine (8-oxodG) as measures of lipid, protein, and DNA oxidation, respectively.
Blood, plasma, and urine manganese concentrations were measured as biomarkers of Mn exposure. However, Mn absorption and excretion are strongly regulated, maintaining stable tissue levels (Aschner et al., 2005). Since much of the variability in blood and urine Mn concentrations is unrelated to inhalation exposure, urinary and blood Mn may be useful as indicators of exposure on a group basis, but are not considered suitable for use as a biomarker of individual exposure (Apostoli et al., 2000).
DNA-protein crosslinks (DPC) in lymphocytes were quantified as a measure of DNA damage potentially resulting from inhalation exposure to chromium. Exposure to Cr(VI) has been shown to produce DPC in vitro and in vivo (Zhitkovich, 2002). At low and moderate exposures the dose-response curve has shown good sensitivity, and DPC are not affected by age, race, bodyweight, or gender (Zhitkovich, 2002). Intracellular reduction of Cr(VI) to Cr(III) results in the formation of Cr(III) adducts with DNA and proteins (Zhitkovich, 2002). These ternary Cr(III) DNA complexes have been found to be mutagenic in human cells (Voitkun et al., 1998). It is important to note that DPC can also be caused by exposure to nickel, arsenic, formaldehyde, radiation, and other factors (Barker et al., 2005) and that the assay used here is not specific to Cr-induced DNA lesions.
Urinary polycyclic aromatic hydrocarbon (PAH) metabolites, were measured as a marker of exposure to combustion generated aerosols, with the expectation that concentrations would be higher for bus drivers than for subway workers. In prior sampling, it was found that when compared to aboveground concentrations, the NYC subway did not have elevated airborne Pb concentrations on a per mass basis (e.g. ng Pb per mg PM2.5) (Chillrud et al., 2005). However, at the request of subway workers who collaborated in the planning of this study, we tested personal air samples and biological samples for lead content.
2.3. Analyses of biological samples
ICP-MS-DRC methods for measuring metals in whole blood, plasma and urine were developed according to published procedures (Pruszkowski et al., 1998; Stroh, 1993), with modifications for blood sample preparation as suggested by the Laboratory for ICP-MS Comparison Program, Institut National de Sante Publique du Quebec. Methods for plasma and urine sample preparation were developed in the Trace Metal Core Laboratory at the Mailman School of Public Health at Columbia University. DNA–protein crosslinks were measured in lymphocytes isolated from whole blood using a modified method described by Zhitkovich and Costa (1992), Kuykendall et al. (1996) and Quievryn and Zhitkovich (2000). Methods for the isoprostane and 8-oxodG assays are described in Rossner et al. (2006). Protocols for the measurement of PAH metabolites are described in Santella et al. (1994). The levels of protein carbonyl groups were assessed using a noncompetitive ELISA, as described in Buss et al. (1997), with some modifications following Marangon et al. (1999).
2.4. Statistical methods
Because of the small sample size and because many of the variables were not normally distributed, non-parametric statistical tests were used. These tests do not assume a normal distribution and are based on rank rather than the actual value of the quantity measured. All group-level comparisons discussed below are the results of Wilcoxon’s rank sum test for equality of medians (Mathworks Inc, 2002). Since multiple comparisons were made, we performed a Bonferroni adjustment of the critical level (p=0.05), dividing by the number of comparisons that were made (three) to obtain an adjusted critical level of p=0.016 (Feise, 2002). All individual level tests of association discussed below were performed using Spearman’s coefficient of rank correlation (rs).
2.5. Creatinine adjustment for urinary analytes
Urinary creatinine was measured as an indicator of hydration status and was used to normalize the concentrations of urinary biomarkers. Urinary analytes were adjusted for creatinine by dividing the analyte concentration (nmol/L or µg/L) by the creatinine concentration (mg/dL) to obtain a creatinine-adjusted analyte concentration (reported as nmol/mmol or ng/mg). However, urinary creatinine concentrations have also been found to be dependent on age, muscle mass, race, red meat intake, and other factors (Barr et al., 2005). At the individual level, partial correlation was used to control for the effects of creatinine and BMI on associations between urinary biomarkers and exposure metrics, as suggested by Barr et al. (2005). For group-level comparisons, results are shown with and without creatinine normalization.
3. Results and discussion
3.1. Personal air-monitoring results
Subway worker PM2.5 concentrations varied on the basis of job activity and job title (Table 3). The mean subway worker time-weighted PM2.5 exposure was 52 µg/m3, with a median of 27 µg/m3, and a range of 6–469 µg/m3. Generally, train operators, train conductors and station cleaners were exposed to the lowest PM2.5 and steel dust concentrations (in µg/m3). Construction flaggers, refuse train workers and overhaul shop workers were exposed to intermediate concentrations of PM2.5 and steel dust. Track construction and track maintenance workers were exposed to the highest concentrations of steel dust. Track maintainers were exposed to a mean PM2.5 concentration of 171 µg/m3. Exposures for the same individuals varied substantially from one night to the next, depending on job activity and job location. For example, scraping dry impacted material from the tracks was the dirtiest activity monitored, but when the impacted material was wet, exposures were reduced by a factor of eight.
Table 3.
Median concentrations of PM2.5, Fe, Cr, and Mn in subway worker personal samples compared to ambient and standard concentrations.
| n | PM2.5(µg/m3) |
Fe (µg/m3) |
Mn (µg/m3) |
Cr (µg/m3) |
Fe (as a % of PM2.5 by mass) Median |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | 5th–95th percentile |
Median | 5th–95th percentile |
Median | 5th–95th percentile |
Median | 5th–95th percentile |
|||
| OSHA 8h PELa | na | 5000b | na | 5000c | na | 20,000d | na | 5000e | na | na |
| All subway workers | 39 | 27 | 8–112 | 7 | 1–34 | 75 | 19–407 | 27 | 6–222 | 27 |
| Track maintenance | 6 | 61 | 38–439 | 16 | 10–156 | 153 | 97–1422 | 49 | 33–332 | 28 |
| Track construction | 6 | 53 | 40–80 | 16 | 9–20 | 121 | 83–160 | 57 | 35–86 | 25 |
| Refuse train | 3 | 27 | 27–40 | 11 | 8–13 | 103 | 76–118 | 37 | 25–40 | 34 |
| Construction flaggers | 6 | 21 | 17–70 | 7 | 5–22 | 66 | 52–231 | 21 | 13–79 | 32 |
| Operators and conductors | 6 | 15 | 7–18 | 6 | 3–7 | 64 | 36–72 | 17 | 10–19 | 42 |
| Overhaul shop | 6 | 20 | 8–75 | 3 | 0.4–22 | 62 | 22–283 | 25 | 6–174 | 12 |
| Station cleaners | 6 | 19 | 8–27 | 3 | 1–6 | 30 | 12–58 | 11 | 4–27 | 16 |
| Urban outdoor samplesf | 36 | 13 | 7–21 | 0.1 | 0.04–0.2 | 2 | 1–4 | 0.3 | 0.1–1 | 1 |
| Suburban outdoor samplesg |
20 | 9 | 5–16 | 0.07 | 0.03–0.12 | 1 | 1–4 | 0.3 | 0.1–1.1 | 1 |
na—not applicable.
OSHA 8 h permisssible exposure limits are for respirable particulate fraction ~PM4, and so are not directly comparable to personal samples which are all for the PM2.5 fraction.
For particulates not otherwise specified.
For Fe as oxide fumes.
OSHA TLV-TWA.
For Cr(VI), not total Cr.
Urban outdoor samples were collected over a period of 8 weeks outside the residences of high school students who participated in an earlier study (Chillrud et al., 2004). Each of the 36 samples integrates 48 h of monitoring. Participants lived in Manhattan, Brooklyn, Queens, and the Bronx. Results for urban outdoor air samples are given as an example of outdoor air quality in New York City.
Suburban outdoor samples were collected over a period of 8 weeks as part of an earlier study (Chillrud et al., 2004). The samples were collected outside the workplace (in Palisades, NY) of the office workers used as a control group in this study. Each of the 20 samples integrates 48 h of monitoring.
As a point of reference, the 24 h EPA National Ambient Air Quality Standard for PM2.5 is 35 µg/m3 (EPA, 2008), while the 24 h WHO Air Quality Guideline for PM2.5 is 25 µg/m3 (WHO, 2006). However, these air quality standards are not ideal benchmarks for evaluating health risks in this case because they are intended to be compared with stationary samples from outdoor environments averaged over 24 h, rather than personal air samples from enclosed spaces averaged over 8 h work shifts. Differences between subway and aboveground particle morphology, composition and size distribution further limit the value of comparisons to outdoor PM2.5 guidelines. Additionally, results from personal and stationary air samples are not always well correlated; stationary air-sampling methods can underestimate personal air pollution exposures (Violante et al., 2006).
As expected, Fe, Mn, and Cr in subway worker personal PM2.5 samples fell well below OSHA guidelines for respirable PM (~PM4, see Table 3). The median subway worker manganese concentration was 75 ng/m3, with a range of 11–1582 ng/m3. The median Pb particulate concentration for subway workers was 16 ng/m3 (5th–95th percentile: 5–83 ng/m3). This exceeded median Pb concentrations of urban outdoor samples (6 ng/m3, 5th-95th percentile: 3–13 ng/m3) collected outside residences in an earlier study (Chillrud et al., 2004), but was well below the EPA NAAQS (150 ng/m3, rolling 3-month average) and OSHA PEL (50,000 ng/m3). Furthermore, the difference was largely due to higher particulate matter concentrations in the subway. On a per mass basis (pg of Pb per µg of PM2.5), the median Pb concentration for subway workers (573 ppm, 5th–95th percentile: 367– 1478 ppm) approximated the median urban outdoor Pb concentration (538 ppm, 5th–95th percentile: 317–937 ppm).
Since a quantitative characterization of exposure levels for the various job titles was lacking at the outset of this study, it was necessary to collect this information by enrolling subway workers from a cross-section of job titles with a wide range of exposure levels. This allowed us to explore dose–response relationships at the individual level. However, the wide range of exposure levels also weakened our ability to detect biological differences between the exposed and control groups at the group level. The exposure levels for various job titles determined in this study may provide a useful basis for developing enrollment criteria in future studies of subway workers.
3.2. Determinants of exposure
Self-reported information about work location and work activity provided some details regarding potential determinants of exposure. Workers who spent time on the subway tracks, spent time scraping, or were exposed to second-hand smoke on the job were exposed to PM2.5, Fe, Mn, and Cr (ng/m3) concentrations 2–6 times higher than those who did not. The six workers who spent any time scraping had fewer years of experience, on average, working in the subway system (4.7 years) than those who did not (11.3 years). This observation raises the possibility that individual exposures may decrease over time as workers self-select out of job activities with higher exposures.
The percentage of iron in the particulate matter to which subway workers were exposed varied widely, from 14% for overhaul shop workers to 43% for train operators and conductors. Track workers who often actively disturb sedimented debris and were exposed to mean PM2.5 concentrations of 113 µg/m3, consisting of 27% iron by mass. In contrast, train operators and conductors are exposed to particulate matter that is already suspended or that has been re-suspended by the train. On average the train operator’s and conductor’s exposure to PM2.5 was 13 µg/ m3, consisting of 43% iron by mass. This relatively high percentage of iron is consistent with the high levels of particulate metals detected in particulate matter vacuumed from 13 air-conditioning filters, which act as bulk samplers of suspended particulates in the subway system. Filters from eight different subway lines contained an average of 43 ± 3.7% iron, 0.35 ± 0.04% manganese, and 0.16 ± 0.04% chromium (mean ± one standard deviation).
3.3. Chemical composition and size distribution of subway particulate matter
Fe/Mn ratios for subway worker personal air samples were consistent with personal samples of subway-riding high school students collected in an earlier study (Chillrud et al., 2004). The subway worker personal samples had Fe/Mn ratios of 103±10 and Cr/Mn ratios of 0.36±0.16 (7one standard deviation). The subway-riding student personal samples had Fe/Mn ratios of 104 and Cr/Mn ratios of 0.33 (Chillrud et al., 2004). These Fe/Mn and Cr/Mn ratios suggest that Fe and Mn found in the subway is not derived from soil blown in from aboveground. Typical crustal ratios are 55 for Fe/Mn and 0.11 for Cr/Mn (Turekian and Wedepohl, 1961).
Observations from a light-scattering particle counter drawing air from outside an operating refuse train indicated that particles collected when the train was underground were greater in number in every size fraction, and had a coarser size distribution than particles collected when the train was aboveground. In the 0.3–0.5 mm, 0.5– 0.7 µm, 0.7–1.0 µm, 1.0–2.5 µm, 2.5–5.0 µm, and >5.0 µm size fractions, the ratios of subway particle counts to aboveground particle counts were 2.4, 6.1, 7.4, 7.7, 7.3, and 10.7, respectively.
Particle count observations were also made on the 42nd Street Shuttle platform in the 42nd Street/Times Square station over a period of 3 weeks in May 2005. These data were compared to aboveground observations collected over 6 weeks during the summer of 1999 outside a residential building in Harlem. The ratios of subway particle counts to aboveground particle counts in the same size fractions listed above were 0.7, 1.6, 2.5, 5.2, 11.1, and 22.5, respectively. These observations support the claim that larger diameter steel dust particles derived from frictional abrasion are an important component of subway PM while smaller-diameter combustion-derived particles represent a more important component of aboveground PM (Karlsson et al., 2005; Sitzmann et al., 1999). Fig. 1 shows a scanning electron micrograph (SEM) of particulate matter collected from a high-volume sampler deployed at the 42nd Street/ Times Square station.
Fig. 1.
Scanning electron micrograph (SEM) of particulate matter collected by a high-volume stationary sampler at the 42nd Street/Times Square station in New York City. The image demonstrates the wide range of particle sizes present (submicron to super-micron). Most particles are angular in shape, consistent with abrasion of metal surfaces. The personal samples collected from subway workers excluded PM larger than 2.5 µm in mean aerodynamic diameter. Energy-dispersive X-ray microanalysis of dozens of particles provided their elemental composition; the vast majority were rich in iron oxides and presumably subway-derived. Spectra and micrographs by Dee Breger, Director of Microscopy, Drexel University.
3.4. Demographic characteristics of study subjects
A summary of the demographic characteristics of the control and exposed groups is given in Table 4. Results are reported here for both bus drivers and office workers, however, neither of these groups is drawn from an ideal control population. The bus driver group is small (n=11) and is subject to stresses and air pollution exposures that are distinct from those experienced by subway workers. The office workers recruited lived mostly outside of New York City (88% compared to 28% of subway workers) and had a significantly lower body mass index on average than subway workers. While information about participant’s race was not collected as part of the study, we estimated that 26 of the 39 subway workers were African-American, while 21 of the 25 office workers were non-hispanic whites. Given these substantive differences between control and exposed populations, observed differences in biomarker concentrations cannot be attributed with certainty to differences in particulate metal exposure.
Table 4.
Demographic data for subway workers, bus drivers and office workers.
| Subway (n=39) |
Bus drivers (n=11) |
Office workers (n=25) |
||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Age (years) | 48 | 38–53 | 45 | 41–48 | 44 | 37–51 |
| Years in current job title | 10 | 4–14 | 9 | 4–20 | 6 | 2–14 |
| Height (inches) | 70 | 68–71 | 68 | 67–69 | 70 | 68–73 |
| Weight (lbs) | 200 | 180–224 | 205 | 187–240 | 190 | 165–210 |
| BMI | 29 | 27–33 | 31 | 28–34 | 26** | 24–28 |
| % who live in NYC | 72 | na | 91 | na | 12*** | na |
| % former smokers | 36 | na | 10 | na | 17 | na |
| % current smokers | 3 | na | 0 | na | 0 | na |
| % live with smoker | 13 | na | 10 | na | 0 | na |
IQR—inter-quartile range, 25th–75th percentile.
na—percentiles were not calculated for binary survey results.
Significant difference between subway worker and this group using Wilcoxon’s rank sum test for equality of medians.
p < 0.01
p < 0.001
3.5. Biological monitoring: overview
Strong evidence for steel dust-related biological effects would consist of a significant difference between the biomarker concentrations of the control and exposed groups for a biomarker that also shows a significant association at the individual level with steel dust exposure measured in personal air monitoring samples. No such evidence was found. Urinary isoprostanes, Cr in plasma, and DPC were detected at higher levels in subway workers than in the bus drivers control group (Table 5), but these biomarkers were not found to be associated with steel dust exposures. At the individual level, none of the biomarkers measured were significantly associated with the airborne Fe, Mn, or Cr concentrations (in ng/m3) measured on PM2.5 filters; in other words, no dose–response relationship was apparent (Table 6). It is possible that the biomarkers of oxidative stress selected for this study (protein carbonyls, urine 8oxo-dG, and isoprostanes) were too specific, and therefore did not adequately capture general oxidative response.
Table 5.
Comparison of median concentrations of biomarkers in urine, plasma and whole blood.
| Subway workers |
Bus drivers |
Office workers |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | p valuea | Median | IQR | p valuea | |
| Urinary, raw | ||||||||
| Isoprostanes (nmol/L) | 19 | 14–35 | 12 | 9–18 | 0.09 | 10 | 5–24 | 0.03 |
| 8-oxodG (nmol/L) | 298 | 184–383 | 340 | 254–426 | 0.34 | 279 | 159–366 | 0.77 |
| BPDE (pmol/mL) | 41 | 28–69 | 58 | 40–87 | 0.08 | 29 | 21–45 | 0.08 |
| Mn in urine (µg/L) | 1.7 | 1.5–2.2 | 1.8 | 1.6–2.1 | 0.61 | 1.7 | 1.2–2.4 | 0.96 |
| Cr in urine (µg/L) | 0.14 | 0.06–0.32 | 0.20 | 0.12–0.23 | 0.69 | 0.08 | 0.03–0.30 | 0.35 |
| Pb in urine (µg/L) | 1.3 | 0.8–1.9 | 0.9 | 0.8–1.5 | 0.57 | 1.5 | 0.7–2.3 | 0.95 |
| Creatinine (mg/dL) | 214 | 123–271 | 187 | 124–225 | 0.26 | 128 | 73–184 | <0.01 |
| Urinary, creatinine-normalized | ||||||||
| Isoprostanes/creatinine (nmol/mmol) | 1.1 | 0.7–1.6 | 0.8 | 0.7–1.0 | 0.09 | 1.0 | 0.7–1.3 | 0.33 |
| 8-oxodG/creatinine (nmol/mmol) | 17 | 13–20 | 22 | 15–40 | 0.16 | 25 | 19–31 | <0.01 |
| BPDE/creatinine (nmol/mmol) | 2.6 | 1.7–4.8 | 5.0 | 2.9–6.7 | 0.06 | 3.3 | 1.7–4.8 | 0.54 |
| Urinary Mn/creatinine (ng/mg) | 0.80 | 0.69–1.12 | 0.97 | 0.85–1.36 | 0.13 | 1.3 | 1.0–1.7 | <0.01 |
| Urinary Cr/creatinine (ng/mg) | 0.08 | 0.04–0.16 | 0.11 | 0.07–0.17 | 0.48 | 0.11 | 0.06–0.21 | 0.54 |
| Urinary Pb/creatinine (ng/mg) | 0.68 | 0.48–0.98 | 0.66 | 0.54–1.06 | 1.00 | 0.92 | 0.68–1.23 | 0.04 |
| Plasma | ||||||||
| Protein Carbonyls (nmol carbonyl/ml plasma) | 17 | 16–19 | 18 | 16–20 | 0.49 | 15 | 14–17 | 0.01 |
| Mn in plasma (µg/L) | 3.7 | 3.1–4.3 | 3.2 | 3.0–3.4 | 0.05 | 3.8 | 3.5–4.1 | 0.61 |
| Cr in plasma (µg/L) | 0.27 | 0.23–0.32 | 0.20 | 0.18–0.24 | <0.01 | 0.28 | 0.24–0.31 | 0.95 |
| Pb in plasma (µg/L) | 0.23 | 0.15–0.36 | 0.22 | 0.14–0.34 | 0.80 | 0.26 | 0.20–0.39 | 0.31 |
| Whole blood | ||||||||
| DNA–protein crosslinks (%) | 5.3 | 3.9–7.9 | 2.5 | 2.0–3.2 | <0.01 | 4.4 | 2.5–6.9 | 0.29 |
| Mn in blood (µg/L) | 12 | 11–14 | 13 | 12–15 | 0.20 | 12 | 11–14 | 0.90 |
| Cr in blood (µg/L) | Not reportedb | Not reportedb | Not reportedb | |||||
| Pb in blood (µg/L) | 17 | 13–23 | 18 | 16–21 | 0.65 | 18 | 16–23 | 0.38 |
Abbreviations: 8-oxodG, 8-oxodeoxyguanosine; BPDE, benzo(a)pyrene diol epoxide; Cr, chromium; DNA, deoxyribonucleic acid; Isoprostanes, 15-F2t–isoprostane; Mn, Manganese; Pb, lead.
p values—significance levels are based on comparisons between the control group (either bus drivers or office workers) and the exposed group (subway workers). Significance levels are calculated based on Wilcoxon’s rank sum test for equality of means and are indicated as follows. Results for biomarkers with a significant difference between subway workers and the bus driver group or subway workers and the office worker group, are shown in bold.
Not reported—chromium in blood results are not reported because concentrations were found to be inversely proportional to sample volume indicating that the sodium heparin anticoagulant may have contaminated the samples with detectable amounts of chromium.
Table 6.
Spearman’s coefficient of rank correlation for individual level association between biomarker concentrations and exposure metrics.
| PM2.5 (µg/m3) | Fe (ng/m3) | Mn (ng/m3) | Cr (ng/m3) | Fe (as a % of PM2.5 by mass) | |
|---|---|---|---|---|---|
| Urinary, raw | |||||
| Isoprostanes (nmol/L) | −0.17 | −0.17 | −0.18 | −0.27 | −0.01 |
| 8-oxodG (nmol/L) | −0.06 | −0.13 | −0.20 | −0.23 | −0.19 |
| BPDE (pmol/mL) | −0.19 | −0.10 | −0.14 | −0.20 | 0.30 |
| Mn in urine (µg/L) | −0.18 | −0.18 | −0.21 | −0.25 | −0.01 |
| Cr in urine (µg/L) | −0.02 | −0.10 | −0.12 | −0.12 | −0.23 |
| Pb in urine (µg/L) | −0.18 | −0.24 | −0.24 | −0.32* | −0.18 |
| Creatinine (mg/dL) | −0.19 | −0.24 | −0.29 | −0.35* | −0.08 |
| Urinary, creatinine-normalized | |||||
| Isoprostanes/creatinine (nmol/mmol) | −0.12 | −0.10 | −0.09 | −0.13 | −0.03 |
| 8-oxodG/creatinine (nmol/mmol) | 0.19 | 0.17 | 0.13 | 0.21 | −0.14 |
| BPDE/creatinine (nmol/mmol) | −0.14 | −0.01 | 0.05 | 0.07 | 0.36* |
| Urinary Mn/creatinine (ng/mg) | −0.10 | −0.05 | 0.01 | 0.06 | −0.03 |
| Urinary Cr/creatinine (ng/mg) | 0.12 | 0.06 | 0.10 | 0.10 | −0.15 |
| Urinary Pb/creatinine (ng/mg) | 0.12 | 0.10 | 0.17 | 0.16 | −0.16 |
| Plasma | |||||
| Protein carbonyls (nmol carbonyl/mL plasma) | −0.08 | −0.01 | 0.06 | 0.00 | 0.16 |
| Mn in plasma (µg/L) | −0.14 | −0.23 | −0.22 | −0.24 | −0.37* |
| Cr in plasma (µg/L) | 0.13 | 0.13 | 0.08 | 0.07 | −0.19 |
| Pb in plasma (µg/L) | 0.06 | − 0.04 | 0.03 | 0.04 | −0.48** |
| Whole blood | |||||
| DNA–protein crosslinks (%) | 0.18 | 0.19 | 0.16 | 0.16 | 0.05 |
| Mn in blood (µg/L) | 0.13 | 0.18 | 0.21 | 0.13 | 0.22 |
| Cr in blood (µg/L) | n/a | n/a | n/a | n/a | n/a |
| Pb in blood (µg/L) | 0.16 | 0.11 | 0.17 | 0.12 | − 0.12 |
n/a—chromium in blood results are not reported because concentrations were found to be inversely proportional to sample volume indicating that the sodium heparin anticoagulant may have contaminated the samples with detectable amounts of chromium.
Abbreviations: 8-oxodG, 8-oxodeoxyguanosine; BPDE, benzo(a)pyrene diol epoxide; Cr, chromium; DNA, deoxyribonucleic acid, isoprostanes, 15-F2t–isoprostane;Mn, manganese; Pb, lead.
Significant correlations between biomarkers and exposure metrics are indicated as follows.
p < 0.05
p < 0.01
3.6. Urinary creatinine, manganese, and 8-oxodG
Among all study subjects, creatinine was significantly correlated with BMI (rs=0.30, p=0.008, n=74), but not with age or red meat consumption. There were differences in creatinine status between the relatively sedentary office workers, the bus drivers, and the more active subway workers. Creatinine-normalized urinary manganese and 8-oxodG concentrations were significantly higher in the office worker control group samples than in subway worker samples, but the differences was driven entirely by differences in creatinine, i.e. there was no significant difference in the raw urinary manganese and 8-oxodG concentrations.
3.7. PAH metabolites
As expected, concentrations of PAH metabolites were higher for bus drivers than for subway workers or office workers. While personal inhalation exposure to PAH was not measured, the difference in PAH metabolite concentrations suggests that occupational air pollution exposures and the risk conveyed by those exposures are different for subway workers and bus drivers as a result of their distinct workplace environments. Consumption of meat cooked at high-temperatures can also be an important source of PAH exposure (Sinha et al., 2005). The self-reported weekly rate of consumption of red meat for bus drivers (mean: 1.7 times per week) was not greater than that of subway workers (mean: 2.4 times per week) or office workers (mean: 2.2 times per week). No information regarding cooking method was collected.
3.8. Protein carbonyls
Subway workers had significantly higher concentrations of protein carbonyls than office workers (p=0.01, see Table 5). The bus drivers control group also had elevated levels of protein carbonyls relative to the office workers (p=0.03). This suggests that the difference between subway worker and office worker protein carbonyl concentrations may not have been due to differences steel dust exposures. Protein carbonyls were significantly correlated with blood Mn for subway workers (rs=0.38, p=0.024, n=36), but not for the office workers (rs= 0.18, p=0.40, n=25) or bus drivers (rs=0.23, p=0.50, n=11).
3.9. Lead
Median subway worker Pb levels in blood, plasma, and urine approximated or were lower than the median control group Pb levels. Mean Pb blood levels among all subway workers (1.92 µg/dL), bus drivers (1.88 µg/dL), and office workers (2.23 µg/dL) approximated the mean Pb blood levels for adult males (n=762) living in New York City reported in the NYC Health and Nutrition Examination Survey (NYCHANES) (2.1 µg/dL) (McKelvey et al., 2007). One subway worker, no office workers, and no bus drivers exceeded the NYCHANES 95th percentile (5.87 µg/dL) for adult males (McKelvey et al., 2007). The subway worker Pb blood level that exceeded the NYCHANES 95th percentile of 5.87 µg/deciliter (dL) did not exceed the CDC’s blood lead action level for children (10 µg/dL).
3.10. Urinary isoprostanes
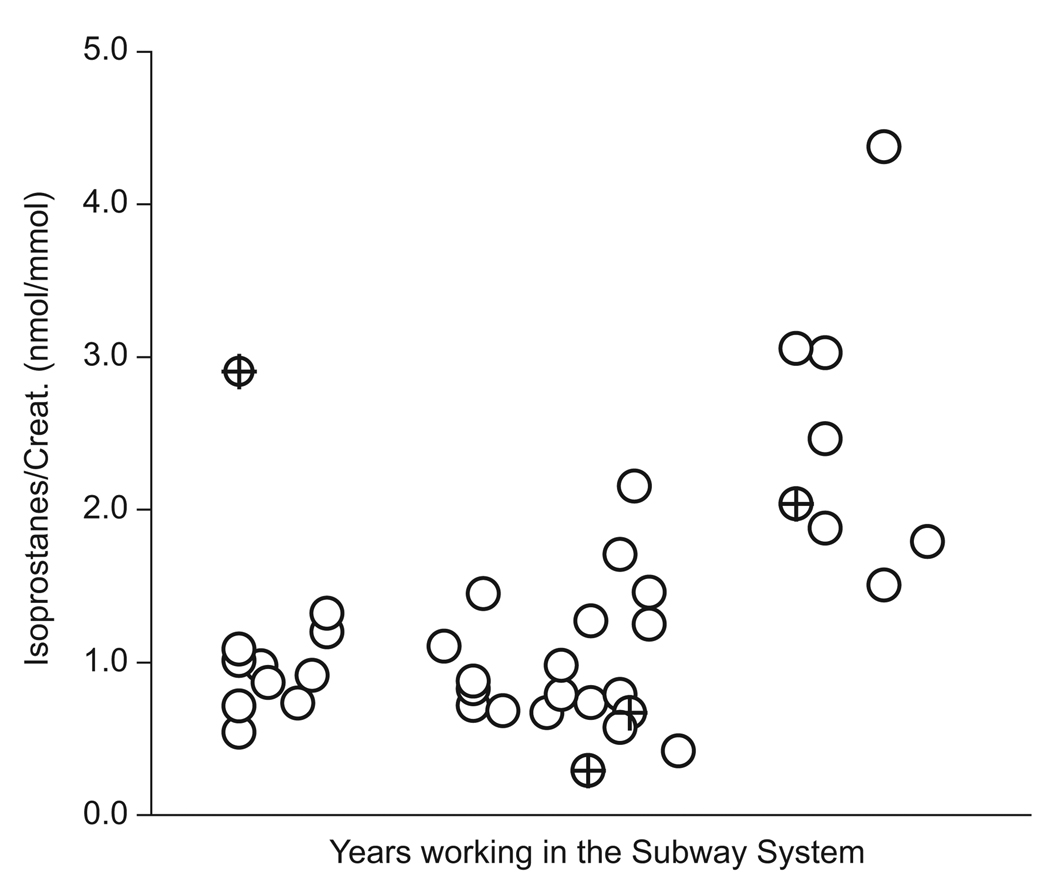
Urinary isoprostane concentrations were higher in subway workers than in office workers (p=0.026) or in bus drivers (p=0.092). When isoprostane concentrations were normalized for creatinine, concentrations remained higher among subway workers than office workers or bus drivers, though these differences were not statistically significant, see Table 5. Total years of subway work was significantly correlated with isoprostane concentrations both before (rs=0.42, p=0.008) and after controlling for the effect of creatinine concentrations (rs=0.53, p=0.0005, see Fig. 2). This relationship remains significant when BMI (rs=0.52, p=0.012) and age (rs=0.43, p=0.024) are added to the regression model. Of the 39 subway workers, four held jobs before they began working for the Transit Authority that had potential for high exposures to particulate metals. When these four workers are excluded, the strength of the adjusted isoprostane—years of subway work relationship remains approximately the same (rs=0.54, p=0.001).
Fig. 2.
Total years of subway work was significantly correlated with isoprostane concentrations (rs=0.42, p=0.008). The relationship remains significant after controlling for the effects of creatinine, BMI, and age on isoprostanes. Of the 39 subway workers, four held jobs before they began working for the Transit Authority that had the potential for high exposures to particulate metals. A crosshatch is used to indicated the sample points corresponding to these four workers. When these four workers were excluded, the isoprostane—years of subway work relationship strengthens to rs=0.54, p=0.001. No values are shown on the x-axis in order to protect the privacy of study participants.
We are not aware of any prior study that has found a relationship between isoprostane concentrations and cumulative exposure to transition metals. The possibility that isoprostane levels respond to steel dust exposure is consistent with earlier studies that found that asymptomatic shipyard welders in South Korea had serum isoprostane concentrations 2.4 times higher than an unexposed office worker control group (Han et al., 2005), and that MnCl2 exposure induces changes in isoprostane concentrations in the nematode C. Elegans (Aschner, 2006).
3.11. DNA–protein crosslinks and chromium in plasma
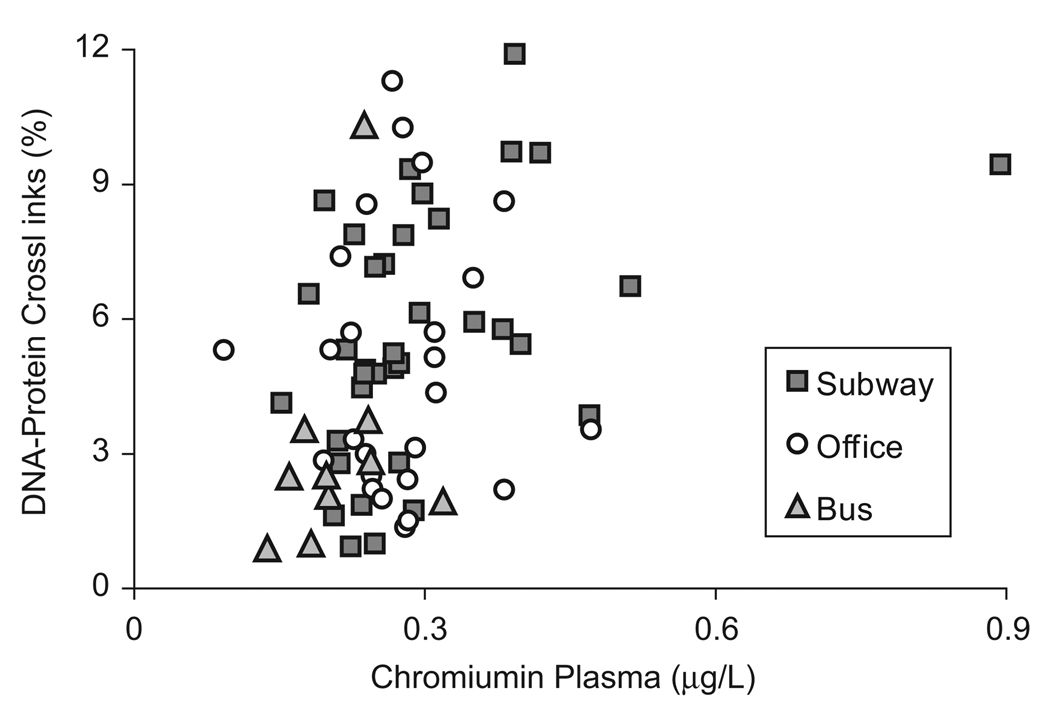
Subway workers (n=36) had significantly higher concentrations of chromium in plasma (p=0.005) and DNA–protein crosslinks (DPC) in lymphocytes (p=0.005) than the bus driver control group (n=11). There was no significant difference between subway workers’ and office workers’ plasma Cr and DPC concentrations. Among all study subjects, the percent of DPC was significantly correlated with the concentrations of chromium in plasma (rs=0.34, p=0.003, n=71, Fig. 3). Among the subway workers, the strength of the association was the same (rs=0.48, p=0.003, n=36), while for the office workers (rs=0.02, p=0.94, n=25) and bus drivers (rs=0.36, p=0.31, n=10), the relationship between DPC and Cr in plasma was not significant. It is also worth noting that plasma Cr concentrations were significantly elevated (p=0.017) for the 12 subway workers who reported working in areas where metal was being cut (0.37 µg/L) versus the 24 workers who did not (0.27 µg/L). Several studies have shown that DPC are responsive to chromium exposure. For example, Medeiros et al. (2003) found that both Cr(VI) and Cr(III) exposed workers had elevated levels of plasma Cr and DPC as compared to controls, and an in vitro study by Zhitkovich et al. (1996) showed that erythrocyte chromium levels were correlated with lymphocyte DPC.
Fig. 3.
The correlation between DNA–protein crosslinks and chromium in plasma is significant for all study subjects (rs=0.34, p=0.003). Since Spearman’s coefficient of rank correlation, rs, is not based on observed values but on rank, the subway worker outlier does not have a disproportionate effect on the significance of the correlation. The DPC–plasma Cr relationship remains significant when the point is removed (rs=0.32, p=0.007).
3.12. Intra-individual versus inter-individual biomarker variability
While screening studies have found that urinary isoprostanes, 8-oxodG, and plasma protein carbonyl content demonstrate suggestive or consistent responses to iron exposure (Schumann et al., 2005), their suitability as indicators of a biological response to inhalation transition metal exposure is also dependent on intra- and inter-individual variability. Measurement of urinary isoprostane concentrations has proved to be a reliable means to assess oxidative stress in vivo (Montuschi et al., 2004). While most studies have found no significant intra-individual daily variability and limited day-to-day variability in urinary isoprostane concentrations (Montuschi et al., 2004), at least one study (Helmersson and Basu, 2001) found significant diurnal variation in healthy adults. Between subject variability of blood 8-oxodG has also been found to be consistently greater than within subject variability suggesting that it is appropriate to use single measurements of this biomarker as an indicator of oxidative DNA damage in healthy individuals (Kato et al., 2006). However, Kanabrocki et al. (2002) found significant diurnal variation in 8-oxodG (potentially as a result of diurnal variation in oxidative stress), which would suggest that the results of spot samples such as those used in this study should be interpreted with caution.
3.13. Evaluating the potential for health risks
The concentrations of particulate metals at which health risks have been established far exceed the concentrations observed in this study. For example, cases of manganism or manganese-induced Parkinsonism are typically associated with chronic exposure to airborne manganese concentrations in excess of 1000–5000 µg/m3 (Aschner et al., 2005). Symptoms of neurofunc-tional changes and elevated manganese levels in blood and urine have been reported with exposure to levels as low as 70 µg/m3 (Lucchini et al., 1999). By contrast, the highest time-weighted concentrations of manganese found in this study were in the range of 1–2 µg/m3, with a median value of 0.075 µg/m3. We found no dose–response relationship between particulate metal concentrations measured from PM2.5 filters and the biological markers measured in blood and urine samples.
In no instance were subway worker biomarker concentrations significantly elevated above both the office worker and the bus driver control group concentrations. Given these results, and because the levels of steel dust observed in this study fell well below levels with known health risks, we conclude that the potential for health impacts in the worker population studied, at the levels observed, appears to be low. It is not clear whether the lack of a consistent pattern of positive associations between pollutant dose and biomarker response is due to poor biomarker selection, limited statistical power, or levels of particulate metals that are so low that they genuinely do not present a health risk. Therefore further investigation may be warranted, especially for those groups with the highest subway dust exposures (track maintenance and track construction workers). Given the small size of this study, and potential differences between the workers studied and the general public, the results of this study should not be interpreted to apply to the general subway ridership, which includes susceptible subgroups such as children, the elderly, and people with pre-existing respiratory conditions.
Acknowledgments
We thank the members of Local 100 of the Transit Workers Union and the MTA Office of System Safety. We are grateful for technical expertise provided by Dee Breger, Manuel Gonzalez, Dr. Marc Grodman, Robert Ortiz, Slavenka Sedlar, and Galina Zimberg. The EPA has not officially endorsed this publication and the views expressed herein may not reflect the views of the EPA. This is LDEO contribution no. 7264.
Footnotes
Funding sources: Funds for this research were provided by the NIEHS Center for Environmental Health in Northern Manhattan (P30ES009089). The US EPA Science to Achieve Results (STAR) Program (Grant no. 91615901-0).
Human subjects study information: This study protocol was approved by the Columbia University Medical Center Institutional Review Board (Protocol no.: IRB-AAAA0655).
References
- Aarnio P, Yli-Tuomi T, Kousa A, Makela T, Hirsikko A, Hameri K, Raisanen M, Hillamo R, Koskentalo T, Jantunen M. The concentrations and composition of and exposure to fine particles (PM2.5) in the Helsinki subway system. Atmospheric Environment. 2005;39:5059–5066. [Google Scholar]
- Adams HS, Nieuwenhuijsen MJ, Colvile RN, McMullen MAS, Khandelwal P. Fine particle (PM2.5) personal exposure levels in transport microenvironments, London, UK. Science of the Total Environment. 2001;279:29–44. doi: 10.1016/s0048-9697(01)00723-9. [DOI] [PubMed] [Google Scholar]
- Ali SF, Duhart HM, Newport GD, Lipe GW, Slikker W. Manganeseinduced reactive oxygen secies—comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4:329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Antonini JM. Health effects of welding. Critical Reviews in Toxicology. 2003;33:61–103. doi: 10.1080/713611032. [DOI] [PubMed] [Google Scholar]
- Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? American Journal of Industrial Medicine. 2000;37:283–290. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aschner M. Personal Communication. Nashville, TN: Department of Pediatrics Vanderbilt University School of Medicine; 2006. [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Critical Reviews in Toxicology. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–180. [PubMed] [Google Scholar]
- Barker S, Weinfeld M, Murray D. DNA-protein crosslinks: their induction, repair, and biological consequences. Mutation Research—Reviews in Mutation Research. 2005;589:111–135. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environmental Health Perspectives. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigert C. Cardiovascular Disease among Professional Drivers and Subway Staff in Stockholm. Stockholm: Department of Public Health Sciences, Karolinska Institutet; 2007. p. 44. Ph.D. Thesis. [Google Scholar]
- Branis M. The contribution of ambient sources to particulate pollution in spaces and trains of the Prague underground transport system. Atmospheric Environment. 2006;40:348–356. [Google Scholar]
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radical Biology and Medicine. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Chan LY, Lau WL, Zou SC, Cao ZX, Lai SC. Exposure level of carbon monoxide and respirable suspended particulate in public transportation modes while commuting in urban area of Guangzhou, China. Atmospheric Environment. 2002;36:5831–5840. [Google Scholar]
- Chillrud SN, Epstein D, Ross JM, Sax SN, Pederson D, Spengler JD, Kinney PL. Elevated airborne exposures of teenagers to manganese, chromium, and iron from steel dust and New York City’s subway system. Environmental Science & Technology. 2004;38:732–737. doi: 10.1021/es034734y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillrud SN, Grass D, Ross JM, Coulibaly D, Slavkovich V, Epstein D, Sax SN, Pederson D, Johnson D, Spengler JD, Kinney PL, Simpson HJ, Brandt-Rauf P. Steel dust in the New York City subway system as a source of manganese, chromium, and iron exposures for transit workers. Journal of Urban Health—Bulletin of the New York Academy of Medicine. 2005;82:33–42. doi: 10.1093/jurban/jti006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environmental Health Perspectives. 1997;105:1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump KS. Manganese exposures in Toronto during use of the gasoline additive, methylcyclopentadienyl manganese tricarbonyl. Journal of Exposure Analysis and Environmental Epidemiology. 2000;10:227–239. doi: 10.1038/sj.jea.7500085. [DOI] [PubMed] [Google Scholar]
- Cudahy B. A Century of Subways: Celebration 100 Years of New York’s Underground Railways. New York: Fordham University Press; 2003. [Google Scholar]
- Doherty MJ, Healy M, Richardson SG, Fisher NC. Total body iron overload in welder’s siderosis. Occupational and Environmental Medicine. 2004;61:82–85. [PMC free article] [PubMed] [Google Scholar]
- EPA, U.S. Integrated Science Assessment for Particulate Matter (External Review Draft) Washington, DC: US Environmental Protection Agency; 2008. [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology. 2002;2:8–11. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Perales JE, Colvile RN, Nieuwenhuijsen MJ, Fernandez-Bremauntz A, Gutierrez-Avedoy VJ, Paramo-Figueroa VH, Blanco-Jimenez S, Bueno-Lopez E, Mandujano F, Bernabe-Cabanillas R, Ortiz-Segovia E. Commuters’ exposure to PM2.5, CO, and benzene in public transport in the metropolitan area of Mexico City. Atmospheric Environment. 2004;38:1219–1229. [Google Scholar]
- Han SG, Kim Y, Kashon ML, Pack DL, Castranova V, Vallyathan V. Correlates of oxidative stress and free-radical activity in serum from asymptomatic shipyard welders. American Journal of Respiratory and Critical Care Medicine. 2005;172:1541–1548. doi: 10.1164/rccm.200409-1222OC. [DOI] [PubMed] [Google Scholar]
- Helmersson J, Basu S. F-2-isoprostane and prostaglandin F-2 alpha metabolite excretion rate and day to day variation in healthy humans. Prostaglandins Leukotrienes and Essential Fatty Acids. 2001;65:99–102. doi: 10.1054/plef.2001.0295. [DOI] [PubMed] [Google Scholar]
- Johansson C, Johansson PA. Particulate matter in the underground of Stockholm. Atmospheric Environment. 2003;37:3–9. [Google Scholar]
- Kanabrocki EL, Murray D, Hermida RC, Scott GS, Bremner WF, Ryan MD, Ayala DE, Third J, Shirazi P, Nemchausky BA, Hooper DC. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiology International. 2002;19:423–439. doi: 10.1081/cbi-120002914. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Holgersson A, Moller L. Mechanisms related to the genotoxicity of particles in the subway and from other sources. Chemical Research in Toxicology. 2008;21:726–731. doi: 10.1021/tx7003568. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Nilsson L, Moller L. Subway particles are more genotoxic than street particles and induce oxidative stress in cultured human lung cells. Chemical Research in Toxicology. 2005;18:19–23. doi: 10.1021/tx049723c. [DOI] [PubMed] [Google Scholar]
- Kato I, Ren J, Heilbrun LK, Djuric Z. Intra- and inter-individual variability in measurements of biomarkers for oxidative damage in vivo: nutrition and Breast Health Study. Biomarkers. 2006;11:143–152. doi: 10.1080/13547500600565693. [DOI] [PubMed] [Google Scholar]
- Kuykendall JR, Kerger BD, Jarvi EJ, Corbett GE, Paustenbach DJ. Measurement of DNA-protein cross-links in human leukocytes following acute ingestion of chromium in drinking water. Carcinogenesis. 1996;17:1971–1977. doi: 10.1093/carcin/17.9.1971. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, Mergler D, Sassine MP, Palmi S, Alessio L. Long term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–297. [PubMed] [Google Scholar]
- Marangon K, Devaraj S, Jialal I. Measurement of protein carbonyls in plasma of smokers and in oxidized LDL by an ELISA. Clinical Chemistry. 1999;45:577–578. [PubMed] [Google Scholar]
- Mathworks Inc. Statistical Toolbox User’s Guide: Version 4. Natick, MA: Mathworks Inc; 2002. [Google Scholar]
- McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, Palmer CD, Parsons PJ. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environmental Health Perspectives. 2007;115:1435–1441. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly JD, Heal MR, Beverland IJ, Howe A, Gibson MD, Hibbs LR, MacNee W, Donaldson K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicology and Applied Pharmacology. 2004;196:95–107. doi: 10.1016/j.taap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Medeiros MG, Rodrigues AS, Batoreu MC, Laires A, Rueff J, Zhitkovich A. Elevated levels of DNA-protein crosslinks and micronuclei in peripheral lymphocytes of tannery workers exposed to trivalent chromium. Mutagenesis. 2003;18:19–24. doi: 10.1093/mutage/18.1.19. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. Faseb Journal. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, Gomez-Perales JE, Colvile RN. Levels of particulate air pollution, its elemental composition, determinants and health effects in metro systems. Atmospheric Environment. 2007;41:7995–8006. [Google Scholar]
- Palmer KT, Poole J, Ayres JG, Mann J, Burge PS, Coggon D. Exposure to metal fume and infectious pneumonia. American Journal of Epidemiology. 2003;157:227–233. doi: 10.1093/aje/kwf188. [DOI] [PubMed] [Google Scholar]
- Pfeifer GD, Harrison RM, Lynam DR. Personal exposures to airborne metals in London taxi drivers and office workers in 1995 and 1996. Science of the Total Environment. 1999;235:253–260. doi: 10.1016/s0048-9697(99)00201-6. [DOI] [PubMed] [Google Scholar]
- Pruszkowski E, Neubauer K, Thomas R. An overview of clinical applications by inductively coupled plasma mass spectrometry. Atomic Spectroscopy. 1998;19:111–115. [Google Scholar]
- Quievryn G, Zhitkovich A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis. 2000;21:1573–1580. [PubMed] [Google Scholar]
- Ripanucci G, Grana M, Vicentini L, Magrini A, Bergamaschi A. Dust in the underground railway tunnels of an Italian town. Journal of Occupational and Environmental Hygiene. 2006;3:16–25. doi: 10.1080/15459620500444004. [DOI] [PubMed] [Google Scholar]
- Rossner P, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL, Eng SM, Gaudet MM, Neugut AI, Santella RM. Relationship between urinary 15-F-2t isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2006;15:639–644. doi: 10.1158/1055-9965.EPI-05-0554. [DOI] [PubMed] [Google Scholar]
- Santella RM, Nunes MG, Blaskovic R, Perera FP, Tang DL, Beachman A, Lin JH, Deleo VA. Quantitation of polycyclic aromatic-hydrocarbons, 1-hydroxypyrene, and mutagenicity in urine of coal-tar-treated psoriasis patients and untreated volunteers. Cancer Epidemiology Biomarkers & Prevention. 1994;3:137–140. [PubMed] [Google Scholar]
- Schaumann F, Borm PJA, Herbrich A, Knoch J, Pitz M, Schins RPF, Luettig B, Hohlfeld JM, Heinrich J, Krug N. Metal-rich ambient particles (particulate matter(2.5)) cause airway inflammation in healthy subjects. American Journal of Respiratory and Critical Care Medicine. 2004;170:898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Schumann K, Kroll S, Weiss G, Frank J, Biesalski HK, Daniel H, Friel J, Solomons NW. Monitoring of hematological, inflammatory and oxidative reactions to acute oral iron exposure in human volunteers: preliminary screening for selection of potentially-responsive biomarkers. Toxicology. 2005;212:10–23. doi: 10.1016/j.tox.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Seaton A, Cherrie J, Dennekamp M, Donaldson K, Hurley JF, Tran CL. The London underground: dust and hazards to health. Occupational and Environmental Medicine. 2005;62:355–362. doi: 10.1136/oem.2004.014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XL, Dalal NS, Kasprzak KS. Generation of free-radicals from hydrogen-peroxide and lipid hydroperoxides in the presence of Cr(III) Archives of Biochemistry and Biophysics. 1993;302:294–299. doi: 10.1006/abbi.1993.1213. [DOI] [PubMed] [Google Scholar]
- Sinha R, Peters U, Cross AJ, Kulldorff M, Weissfeld JL, Pinsky PF, Rothman N, Hayes RB. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Research. 2005;65:8034–8041. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- Sitzmann B, Kendall M, Watt J, Williams I. Characterisation of airborne particles in London by computer-controlled scanning electron microscopy. Science of the Total Environment. 1999;241:63–73. [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal-ions. Free Radical Biology and Medicine. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Stroh A. Determination of Pb and Cd in whole-blood using isotope-dilution ICP-MS. Atomic Spectroscopy. 1993;14:141–143. [Google Scholar]
- Turekian KK, Wedepohl KH. Distribution of the elements in some major units of the Earth’s crust. Geological Society of America Bulletin. 1961;72:175–191. [Google Scholar]
- Violante FS, Barbieri A, Curti S, Sanguinetti G, Graziosi F, Mattioli S. Urban atmospheric pollution: personal exposure versus fixed monitoring station measurements. Chemosphere. 2006;64:1722–1729. doi: 10.1016/j.chemosphere.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Voitkun V, Zhitkovich A, Costa M. Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Research. 1998;26:2024–2030. doi: 10.1093/nar/26.8.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Air Quality Guidelines: Global Update 2005. Germany: World Health Organization; 2006. [Google Scholar]
- Zhitkovich A. Chromium: exposure, toxicity, and biomonitoring approaches. In: Wilson SH, Suk WA, editors. Biomarkers of Environmentally Associated Disease: Technologies, Concepts, and Perspectives. Boca Raton: Lewis Publishers; 2002. p. 582. ([8] of plates ill. (some col.) 24 cm) [Google Scholar]
- Zhitkovich A, Costa M. A simple, sensitive assay to detect DNA-protein cross-links in intact-cells and in vivo. Carcinogenesis. 1992;13:1485–1489. doi: 10.1093/carcin/13.8.1485. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A, Lukanova A, Popov T, Taioli E, Cohen H, Costa M, Toniolo P. DNA-protein crosslinks in peripheral lymphocytes of individuals exposed to hexavalent chromium compounds. Biomarkers. 1996;1:86–93. doi: 10.3109/13547509609088675. [DOI] [PubMed] [Google Scholar]