Abstract
Background
The head and neck is the most common site of mucosal melanoma, a cancer with poor prognosis. In contrast to cutaneous melanoma, mucosal melanoma of the head and neck (MMHN) is uncommon, with limited data regarding outcomes and prognostic factors drawn from small, single-institution case series. In order to identify factors predictive of survival, we analyzed MMHN outcomes in a large US cohort.
Methods
MMHN cases (n = 815) diagnosed in the USA between 1973 and 2007 were analyzed in the Surveillance, Epidemiology, and End Results registry, and cause of death was individually determined in 778 (95.5%) cases. Kaplan–Meier survival analysis and Cox proportional hazards regression were used to analyze prognostic variables.
Results
Disease-specific survival status was determined in 778 (95.5%) cases. The 5- and 10-year rates of overall survival (OS) were 25.2 and 12.2%; disease-specific survival (DSS), 32.4 and 19.3%. On multivariable analysis, anatomic primary site was an independent predictor of OS and DSS, with tumors in the nasal cavity and oral cavity associated with survival superior to tumors in the nasopharynx and paranasal sinuses. Age > 70 years, tumor size, nodal status, and distant metastasis status were additional independent predictors of poorer survival.
Conclusions
In this large cohort of patients with MMHN, we have identified several novel factors robustly predictive of overall and melanoma-specific survival.
Mucosal melanoma is an uncommon subtype of malignant melanoma, representing 1% of all melanomas and 6% of head and neck melanomas in the USA.1,2 The head and neck is the most common anatomic site, accounting for 55% of mucosal cases.2,3 In contrast to the wealth of outcomes data regarding cutaneous melanoma, our understanding of this less prevalent entity has been drawn from an aggregate experience of approximately 1,000 cases, drawn from several single-institution case series. One large registry study, comprising 84,000 melanomas in the National Cancer Database treated between 1985 and 1994, included 1,074 cases of mucosal melanoma of the head and neck, but did not analyze outcomes or prognostic factors in detail.2
Reported survival rates for mucosal melanoma of the head and neck (MMHN) vary but are consistently poor, with 5-year overall survival ranging from 20 to 35% in the largest published retrospective studies.4,5 Several single-institution studies from tertiary-level referral centers, comprised of cohorts ranging from 28 to 61 patients, have identified a variety of candidate prognostic variables such as age, clinical stage, tumor thickness, and vascular invasion.5-7 Outcomes data are most generalizable when drawn from contemporary cohorts of large sample size, including a variety of treatment settings, and when multivariable analysis is used to identify factors with prognostic value that are subsequently validated in independent datasets. Therefore, in order to robustly identify factors that have the greatest prognostic significance in MMHN, we analyzed a large cohort of cases from a US cancer registry.
MATERIALS AND METHODS
A population-based cohort study was performed using the Surveillance, Epidemiology, and End Results (SEER) program (National Cancer Institute, Bethesda, MD), a national cancer registry that records and follows all patients with cancer diagnosed within defined geographic regions of the USA. Currently, the SEER program captures 26% of all cancers diagnosed in the USA.8 The SEER dataset has been utilized previously to analyze the outcomes and prognostic factors of melanoma and, in particular, rare malignancies in the head and neck.9,10
Data on 815 MMHN cases were extracted from the SEER 17 dataset for the period 1973–2007, using histology codes (International Classification of Diseases for Oncology, third edition) consistent with primary malignant melanoma originating from a mucosal head and neck subsite (oral cavity, oropharynx, larynx, hypopharynx, nasal cavity, paranasal sinuses, nasopharynx, middle ear, and trachea).11,12 Tumors originating from the external lip or esophagus were not included. Pertinent patient data including age, gender, year of diagnosis, primary site, mode of therapy, survival time, and cause of death were analyzed. Although American Joint Committee on Cancer (AJCC) staging information is not recorded in this dataset, details of tumor size, nodal status, and distant metastasis status at presentation are available.
To study survival characteristics of this cohort, the cause of death was able to be determined in 778 (95.5%) cases. Death was considered disease specific if the cause of death was melanoma, or was attributed to the same primary site as the index melanoma. Cases attributed to other causes, or to other anatomic sites, were not considered disease specific. Cases coded as “miscellaneous,” “other cause of death” or “in situ, benign or unknown behavior neoplasm” (5.5% of cohort) were considered indeterminate and censored at the time of death. Any death ascribed to the eye, orbit or brain in which the patient had a single primary located in the ethmoid sinus or nasal cavity was considered a melanoma-specific death.
Kaplan–Meier analysis and Cox proportional hazards regression models were used to analyze associations between available variables (age, gender, race, head and neck subsite, tumor size, nodal metastasis status at presentation, distant metastasis status at presentation, and treatment modality) and overall (OS) and disease-specific survival (DSS). Variables with P < 0.10 on univariate analysis were incorporated into a multivariable model. Data were extracted in SEER*Stat (build 6.6.2, March 2010; NCI, Bethesda, MD), and statistical analyses were performed with SPSS (version 16.0; SPSS Inc., Chicago, IL).
RESULTS
Patient and tumor characteristics for 815 patients are summarized in Table 1. Of included patients, 46.1% were men and 53.9% women. Median age at diagnosis was 72 years (range 17–100 years, mean 68.7 years). The majority of the patients were White (87.9%), followed by Asian or Pacific Islander (7.7%), Black (4.3%), and American Indian/Alaska Native (0.2%). The nasal cavity (49.1%) was the most common primary site of MMHN, followed by the paranasal sinuses (23.1%), oral cavity (18.8%), and nasopharynx (5.5%). At presentation, 13% of patients had nodal metastases. By subsite, the incidence of nodal metastases was 6.3% for nasopharynx tumors, 9.0% for oral cavity, 13.4% for nasal cavity, 14.3% for paranasal sinus, and 20.0% for oropharynx tumors.
TABLE 1.
Patient and tumor characteristics (n = 815)
| Age (years) | |
| <50 | 98 (12.0 %) |
| 50–69 | 275 (33.7%) |
| ≥70 | 442 (54.2%) |
| Period of diagnosis | |
| 1970 s | 40 (4.9%) |
| 1980 s | 105 (12.9%) |
| 1990 s | 209 (25.6%) |
| 2000 s | 461 (56.6%) |
| Sex | |
| Male | 382 (46.9%) |
| Female | 433 (53.1%) |
| Race | |
| White | 711 (87.5%) |
| Black | 38 (4.7%) |
| Asian or Pacific Islander | 62 (7.6%) |
| American Indian or Alaska Native | 2 (0.3%) |
| Tumor size (cm) | |
| ≤2 | 115 (37%) |
| >2 and ≤4 | 125 (40.2%) |
| >4 | 71 (22.8%) |
| Lymph node status | |
| Negative | 481 (87%) |
| Positive | 72 (13%) |
| Stage | |
| Local | 273 (39.3%) |
| Regional | 289 (41.6%) |
| Distant | 132 (19.0%) |
| Primary site | |
| Nasal cavity | 400 (49.1%) |
| Paranasal sinus | 188 (23.1%) |
| Nasopharynx | 45 (5.5%) |
| Oral cavity | 153 (18.8%) |
| Oropharynx | 26 (3.2%) |
| Other (middle ear, trachea) | 3 (0.4%) |
The three-, five-, and ten-year overall survival (OS) probabilities were 37.2, 25.2, and 12.2%. Three-, five-, and ten-year disease-specific survival (DSS) probabilities were 44.4, 34.4, and 19.3% (Fig. 1). The estimated mean OS time was 58.3 months, and mean DSS time was 87.9 months. Univariate and multivariable analyses of survival were performed for both OS and DSS (Tables 2 and 3). On univariate analysis, age, primary subsite, tumor size, lymph node status, distant metastasis status, and treatment modality were significantly associated with OS (Fig. 2) and DSS (Fig. 3).
FIG. 1.
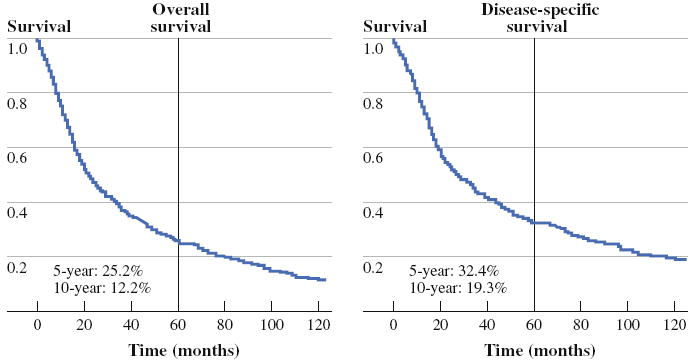
Overall survival and disease-specific survival for entire cohort
TABLE 2.
Factors predictive of overall survival
| Variable | Univariate analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-Value | Hazard ratio | 95% CI | P-Value | |
| Age (years) | <0.001 | <0.001 | ||||
| <50 | 1.00 | 1.00 | ||||
| 50–69 | 1.33 | 1.12–1.61 | 1.52 | 1.03–2.22 | ||
| ≥70 | 1.99 | 1.89–2.13 | 2.65 | 2.37–2.98 | ||
| Sex | 0.08 | 0.69 | ||||
| Male | 1.00 | 1.00 | ||||
| Female | 0.87 | 0.74–1.02 | 0.96 | 0.80–1.16 | ||
| Decade of diagnosis | 0.66 | |||||
| 1970 s | 0.93 | 0.72–1.19 | ||||
| 1980 s | 1.11 | 0.93–1.32 | ||||
| 1990 s | 0.96 | 0.96–1.11 | ||||
| 2000 s | 1.00 | |||||
| Race | 0.13 | |||||
| White | 1.00 | |||||
| Black | 1.26 | 0.87–1.82 | ||||
| Asian/Pacific Islander | 0.73 | 0.53–1.01 | ||||
| American Indian or Alaska Native | 1.41 | 0.35–5.66 | ||||
| Primary site | <0.001 | 0.03 | ||||
| Oral cavity | 0.70 | 0.58–0.85 | 0.88 | 0.60–1.29 | ||
| Oropharynx | 1.19 | 0.82–1.70 | 0.66 | 0.27–1.64 | ||
| Nasal cavity | 0.80 | 0.69–0.93 | 0.73 | 0.50–1.07 | ||
| Nasopharynx | 1.09 | 0.84–1.43 | 1.63 | 0.91–2.93 | ||
| Paranasal sinus | 1.00 | 1.00 | ||||
| Size (cm) | <0.001 | 0.002 | ||||
| ≤2 | 1.00 | 1.00 | ||||
| 2–4 | 1.60 | 1.33–1.94 | 1.43 | 1.13–1.82 | ||
| >4 | 1.59 | 1.30–1.96 | 1.49 | 1.14–1.75 | ||
| Lymph node status | <0.001 | 0.001 | ||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 1.37 | 1.18–1.61 | 1.59 | 1.22–2.08 | ||
| Distant metastases | <0.001 | <0.001 | ||||
| None | 1.00 | 1.00 | ||||
| Present | 1.64 | 1.47–1.82 | 1.75 | 1.33–2.27 | ||
| Treatment | <0.001 | 0.67 | ||||
| Surgery | 1.00 | 1.00 | ||||
| Surgery + radiation | 1.04 | 0.91–1.19 | 0.84 | 0.83–0.86 | ||
| Radiation | 1.47 | 1.30–1.69 | 0.93 | 0.67–1.32 | ||
| None | 2.27 | 1.84–2.76 | 1.20 | 0.93–1.53 | ||
TABLE 3.
Factors predictive of disease-specific survival
| Variable | Univariate analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-Value | Hazard ratio | 95% CI | P-Value | |
| Age (years) | 0.005 | 0.003 | ||||
| <50 | 1.00 | 1.00 | ||||
| 50–69 | 1.16 | 0.96–1.41 | 1.19 | 0.81–1.79 | ||
| ≥70 | 1.44 | 1.37–1.54 | 1.57 | 1.44–1.75 | ||
| Sex | 0.09 | 0.61 | ||||
| Male | 1.00 | 1.00 | ||||
| Female | 0.92 | 0.84–1.01 | 1.06 | 0.85–1.92 | ||
| Decade of diagnosis | 0.91 | |||||
| 1970 s | 0.96 | 0.72–1.27 | ||||
| 1980 s | 1.08 | 0.88–1.31 | ||||
| 1990 s | 1.00 | 0.84–1.17 | ||||
| 2000 s | 1.00 | |||||
| Race | 0.50 | |||||
| White | 1.00 | |||||
| Black | 1.01 | 0.64–1.60 | ||||
| Asian/Pacific Islander | 0.79 | 0.55–1.13 | ||||
| American Indian or Alaska Native | 1.76 | 0.44–7.06 | ||||
| Primary site | <0.001 | 0.001 | ||||
| Oral cavity | 0.65 | 0.52–0.81 | 0.71 | 0.45–1.11 | ||
| Oropharynx | 1.29 | 0.87–1.91 | 0.46 | 0.16–1.33 | ||
| Nasal cavity | 0.74 | 0.62–0.88 | 0.75 | 0.48–1.17 | ||
| Nasopharynx | 1.13 | 0.84–1.52 | 2.29 | 1.19–4.42 | ||
| Paranasal sinus | 1.00 | 1.00 | ||||
| Size (cm) | <0.001 | 0.004 | ||||
| ≤2 | 1.00 | 1.00 | ||||
| 2–4 | 1.83 | 1.79–2.13 | 1.56 | 1.48–1.61 | ||
| >4 | 1.69 | 1.33–2.17 | 1.59 | 1.15–2.17 | ||
| Lymph node status | <0.001 | 0.004 | ||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 1.41 | 1.19–1.64 | 1.59 | 1.16–2.13 | ||
| Distant metastases | <0.001 | <0.001 | ||||
| None | 1.00 | 1.00 | ||||
| Present | 1.75 | 1.56–1.96 | 1.92 | 1.43–2.63 | ||
| Treatment modality | <0.001 | 0.41 | ||||
| Surgery | 1.00 | 1.00 | ||||
| Surgery + radiation | 1.16 | 1.09–1.16 | 0.84 | 0.82–1.28 | ||
| Radiation | 1.56 | 1.35–1.72 | 0.78 | 0.77–1.18 | ||
| None | 2.38 | 2.09–2.59 | 0.93 | 0.69–1.85 | ||
FIG. 2.
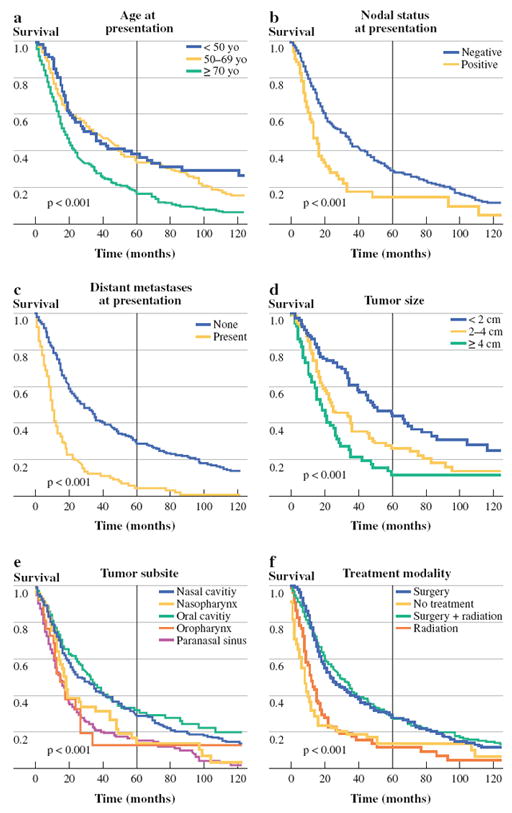
Factors associated with overall survival, univariate analysis: a age at presentation, b nodal status at presentation, c distant metastases at presentation, d tumor size, e tumor subsite, and f treatment modality
FIG. 3.
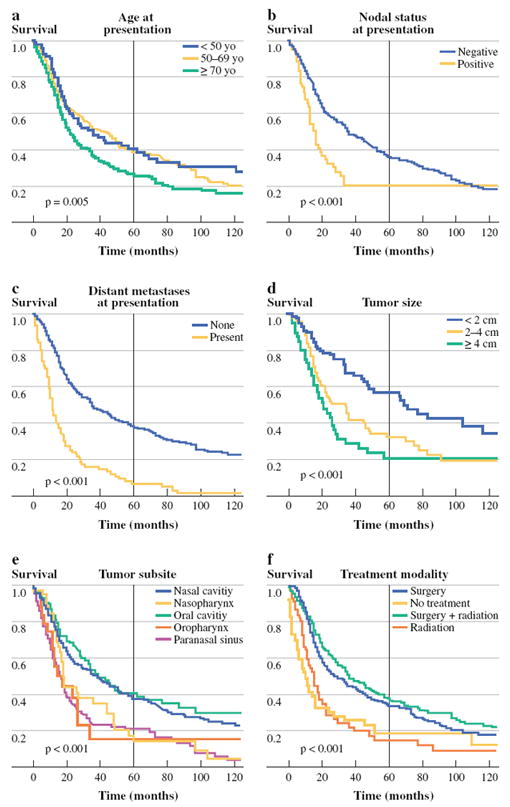
Factors associated with disease-specific survival, univariate analysis: a age at presentation, b nodal status at presentation, c distant metastases at presentation, d tumor size, e tumor subsite, and f treatment modality
On multivariable analysis, age carried significant prognostic value for OS for patients 50–69 years old [hazard ratio (HR) 1.52, 95% confidence interval (CI) 1.03–2.22] and patients > 70 years old (HR 2.65, 95% CI 2.37–2.98). Age > 70 years was significantly associated with poorer DSS as well (HR 1.57, 95% CI 1.44–1.75). Tumor size was a significant independent predictor of both OS and DSS, with the risk of death escalating for 2–4-cm tumors (OS: HR 1.43, 95%CI 1.13–1.82; DSS: HR 1.56, 95%CI 1.48–1.64) and > 4 cm tumors (OS: HR 1.49, 95%CI 1.14–1.75; DSS: 1.59, 95%CI 1.15–2.17). Patients with nodal metastases at presentation had poorer OS (HR 1.59, 95% CI 1.22–2.08) and DSS (HR 1.59, 95% CI 1.16–2.13), as did patients with distant metastases at presentation (OS: HR 1.75, 95% CI 1.33–2.27; DSS: HR 1.92, 95% CI 1.43–2.63).
Anatomic primary site was a significant predictor of survival on multivariable analysis (OS, P = 0.03; DSS, P < 0.001), with tumors arising from the nasal cavity and oral cavity generally associated with improved survival, compared with tumors arising in the nasopharynx and paranasal sinuses. Mean overall survival times ranged from 28.3 months for paranasal sinus tumors to 71 months for oral cavity tumors.
Treatment modality was categorized as radiation alone, surgery alone, both modalities or neither. On univariate analysis, patients receiving surgical treatment (surgery alone or surgery plus radiation) had the best survival rates, followed by patients triaged to radiation alone, or no treatment. On multivariable analysis, treatment modality was not a statistically significant prognostic factor for survival.
DISCUSSION
Mucosal melanomas of the head and neck are uncommon tumors carrying poor prognosis. Mucosal and cutaneous melanomas are known to be genetically distinct entities. Bastian et al. have reported that mucosal melanomas carry substantially more chromosomal aberrations and copy-number alterations than their cutaneous counterparts.13 Importantly, the tyrosine kinase c-kit is overexpressed and frequently mutated in mucosal melanomas, as opposed to rare c-kit alterations in cutaneous melanomas.14 In contrast to cutaneous melanoma, survival outcomes data for patients with MMHN remain limited to several small single-institutional series. Prognostic variables have not been well defined. Our objective is to systematically analyze survival data in a large US cohort of MMHN. Prognostic variables are most useful when identified in large sample sizes, subjected to multivariable analyses, and found to be consistent across datasets. In order to develop a robust set of prognostic variables for MMHN, we analyzed survival data in a large population-based cohort.
Outcomes of 815 patients from the SEER program were analyzed. The median age of our study population was 72 years with a wide range from 17 to 100 years, consistent with published literature showing that this disease affects adults of all ages but is most common in elderly patients.1,5,9 In partial contrast to prior institutional series, by far the most common anatomic tumor sites for MMHN in our population-based cohort were the nasal cavity and paranasal sinuses (72% of cases), with another 19% of cases arising in the oral cavity.1,15,16 Though the exact origin of sinonasal lesions can be difficult to identify in cases with local extension, up to 81% of sinonasal lesions originate from the nasal cavity as opposed to the sinuses and nasopharynx.1,5 In this SEER cohort, 49% of sinonasal lesions were attributed to nasal cavity primaries. These results are in line with described differences in the distribution of melanocytes across various head and neck mucosal sites. Fewer melanocytes migrate to endodermally derived tissue (in the oropharynx, nasopharynx, and larynx) as compared with tissue derived from ectoderm, including the nasal cavity and paranasal sinuses, which undergo a much higher amount of melanocyte migration during development.17 Overall survival at 5 years in the SEER cohort was 25.2%, consistent with rates reported in small MMHN series ranging from 17.1 to 35.1%.1,5 Five-year DSS of 34.4% is also consistent with previously published series ranging from 28.7 to 43.6%.5,9
There are several important limitations to this study. In general, outcomes analyses with large cancer registries tend to offer a trade-off, with large sample sizes offering excellent statistical power, in exchange for a smaller number of detailed covariates. First, the SEER program does not record recurrence data, preventing wider analysis of prognostic factors for recurrence. Accordingly, we have limited our analysis to factors associated with OS and DSS. Second, some pathologic details of interest in melanoma such as depth of invasion, vascular invasion, number of mitotic figures, and presence of melanosis were not available in our data, preventing incorporation of these covariates into our multivariable model. Third, because SEER is a population-based registry, cases reflect a range of treatment settings, from community to academic medical centers. This may introduce heterogeneity into pathologic and treatment details, which generally makes statistically significant associations harder to achieve. AJCC stage was not included in this dataset and analysis, as there was no dedicated staging system for mucosal melanoma until recently; this has now been addressed in the newest edition, in which the lowest T classification for mucosal melanoma is T3.18 Finally, this is a retrospective analysis, which makes any conclusions about treatment effectiveness potentially subject to bias.
Strengths of this study include a large sample size in comparison with existing single-institution series, which contributes statistical power to multivariable analysis. In addition, a high degree of detail in the SEER database regarding follow-up, survival outcome, and cause of death allows for robust analysis. Accordingly, we were able to identify important prognostic factors such as age, tumor size, and tumor subsite, which have not been identified in prior analyses to date, perhaps owing to insufficient statistical power in smaller institutional series.
Single-institution analyses to date have identified several factors associated with survival, including clinical stage and tumor pigmentation in one series, and clinical stage, tumor thickness, and vascular invasion in another large series.16,19 In our analysis, we identified poorer OS and DSS in patients older than 70 years, confirming age as an independent risk factor for MMHN-specific death. Larger tumor size was also an independent risk factor for overall death and disease-specific death, even when controlling for other factors such as nodal and distant metastasis. Interestingly, the anatomic location of MMHN by head and neck subsite was a strong predictor of both OS and DSS (Figs. 2e, 3e; Tables 2, 3). In general, tumors arising from the paranasal sinuses and nasopharynx had significantly poorer overall and disease-specific survival, as compared with more favorable survival for nasal cavity and oral cavity primary tumors. Previous analyses have not analyzed outcome by subsites within the sinonasal region, and the poorer survival for sinus tumors compared with nasal cavity tumors has not been previously reported. We attribute the association of primary site with survival to earlier symptomatic presentation (due to nasal congestion or bleeding) in the nasal cavity, and ease of examination in the oral cavity, as opposed to areas in which tumors may remain occult for longer periods of time, such as the nasopharynx and paranasal sinuses. Furthermore, on multivariable regression, survival with nasal cavity and oral cavity tumors remained significantly superior, even when controlling for factors such as tumor size, and nodal and distant metastases. Therefore, nasopharyngeal and sinus tumors may also exhibit poorer survival for reasons other than stage at presentation, perhaps due to a higher likelihood of proximity to the skull base or orbit, factors which would compromise effective extirpative surgery. The importance of subsite recapitulates the traditional concept of Ohngren’s line, the oblique plane running from medial canthus to mandibular angle that separates sinonasal tumors into favorable and unfavorable categories.
The effectiveness of treatment is difficult to assess in retrospective studies. In the SEER cohort, patients receiving surgery alone experienced survival similar to patients receiving surgery plus radiation, and survival superior to patients receiving radiation alone. Patients receiving no treatment had the poorest survival. These associations did not remain significant when nodal and distant metastasis status were controlled for in multivariable analysis, suggesting that poorer outcomes in patients receiving radiation or nonsurgical therapy are consistent with more advanced or unresectable disease in these patients. These data are consistent with the majority of the existing literature, where evidence supports improved locoregional control, but not improved survival, in patients receiving adjuvant radiation therapy.20-23 Retrospective analyses of adjuvant radiation in MMHN should be interpreted with caution, as patients triaged to postoperative radiation will generally have more advanced, more aggressive or less completely resected disease.
CONCLUSIONS
We report survival outcomes of MMHN in the largest cohort systematically analyzed to date, drawn from a population-based registry. In the USA, survival with MMHN remains poor, with five-year overall survival of 25%.9,16,19 This analysis has identified several novel factors with independent prognostic value for survival in patients with MMHN—head and neck subsite and tumor size—in addition to other factors such as age, nodal status, and distant metastasis status that have been identified in independent datasets. These findings will be helpful in contributing to efforts to personalize therapy for MMHN, by generating hypotheses for further research, assisting with risk stratification, and informing therapeutic decision-making.
Acknowledgments
This work was supported by NIH grant T32 CA009685 (to LGTM).
References
- 1.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997;80:1373–1386. doi: 10.1002/(sici)1097-0142(19971015)80:8<1373::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma—a summary of 84,836 cases from the past decade. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LJ, Berthelsen A, Hansen HS. Malignant melanoma of the upper respiratory tract and the oral cavity. J Otolaryngol. 1992;21:180–185. [PubMed] [Google Scholar]
- 4.Temam S, Mamelle G, Marandas P, Wibault P, Avril MF, Janot F, et al. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005;103:313–319. doi: 10.1002/cncr.20775. [DOI] [PubMed] [Google Scholar]
- 5.Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 6.Loree TR, Mullins AP, Spellman J, North JH, Hicks WL. Head and neck mucosal melanoma: a 32-year review. Ear Nose Throat J. 1999;78:372–375. [PubMed] [Google Scholar]
- 7.Lee SP, Shimizu KT, Tran LM, Juillard G, Calcaterra TC. Mucosal melanoma of the head and neck—the impact of local control on survival. Laryngoscope. 1994;104:121–126. doi: 10.1288/00005537-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 8.NCI. SEER Cancer Statistics Review—SEER Cancer Statistics. [15 Dec 2010];2010 Available at: http://seer.cancer.gov/csr/1975_2007/about.html.
- 9.Bachar G, Loh KS, O’Sullivan B, Goldstein D, Wood S, Brown D, et al. Mucosal melanomas of the head and neck: The Princess Margaret Hospital experience. Head Neck J Sci Spec. 2008;30:1325–1331. doi: 10.1002/hed.20878. [DOI] [PubMed] [Google Scholar]
- 10.Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Survival differences between patients with scalp or neck melanoma and those with melanoma of other sites in the surveillance, epidemiology, and end results (SEER) program. Arch Dermatol. 2008;144:515–521. doi: 10.1001/archderm.144.4.515. [DOI] [PubMed] [Google Scholar]
- 11.NCI. Number of persons by race and hispanic ethnicity for SEER participants. [8 Oct 2010];2010 Available at http://seer.cancer.gov/registries/data.html.
- 12.WHO. Edition 2000. Geneva: World Health Organization; 2000. International classification of diseases for oncology, 3rd ed (ICD-O-3) [Google Scholar]
- 13.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 14.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 15.Medina JE, Ferlito A, Pellitteri PK, Shaha AR, Khafif A, Devaney KO, et al. Current management of mucosal melanoma of the head and neck. J Surg Oncol. 2003;83:116–122. doi: 10.1002/jso.10247. [DOI] [PubMed] [Google Scholar]
- 16.Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck J Sci Spec. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 17.Goldman JL, Roffman JD, Zak FG, Lawson W. The presence of melanocytes in the human larynx. Laryngoscope. 1972;82:824–835. doi: 10.1288/00005537-197205000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 19.Moreno MA, Roberts DB, Kupferman ME, Demonte F, El-Naggar AK, Williams M, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M.D. Anderson Cancer Center. Cancer. 2010;116:2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 20.Wagner M, Morris CG, Werning JW, Mendenhall WM. Mucosal melanoma of the head and neck. Am J Clin Oncol Cancer Clin Trials. 2008;31:43–48. doi: 10.1097/COC.0b013e318134ee88. [DOI] [PubMed] [Google Scholar]
- 21.Douglas CM, Malik T, Swindell R, Lorrigan P, Slevin NJ, Homer JJ. Mucosal melanoma of the head and neck: radiotherapy or surgery? J Otolaryngol Head Neck Surg. 2010;39:385–392. [PubMed] [Google Scholar]
- 22.Wu AJ, Gomez J, Zhung JE, Chan K, Gomez DR, Wolden SL, et al. Radiotherapy after surgical resection for head and neck mucosal melanoma. Am J Clin Oncol Cancer Clin Trials. 2010;33:281–285. doi: 10.1097/COC.0b013e3181a879f5. [DOI] [PubMed] [Google Scholar]
- 23.Krengli M, Masini L, Kaanders J, et al. Radiotherapy in the treatment of mucosal melanoma of the upper aerodigestive tract: analysis of 74 cases. A rare cancer network study. Int J Radiat Oncol Biol Phys. 2006;65:751–759. doi: 10.1016/j.ijrobp.2006.01.016. [DOI] [PubMed] [Google Scholar]


