Abstract
BACKGROUND
American Thyroid Association guidelines currently recommend the selective use of radioactive iodine (RAI) therapy in patients with well differentiated thyroid cancer (WDTC). Despite these guidelines, RAI ablation has been used routinely in all but the very lowest risk patients with thyroid cancer over the last 30 years. The objective of this study was to evaluate patterns of RAI use and elevated risk of secondary primary malignancies (SPM) in patients with low-risk (T1N0) WDTC.
METHODS
The Surveillance, Epidemiology, and End Results (SEER) database was used to analyze trends in RAI use over time in the United States. To determine the excess risk of SPM, the standardized incidence ratio (SIR) and excess absolute risk (EAR) of various cancers were calculated in the 2 cohorts. Between 1973 and 2007, 37,176 patients with WDTC were followed in the SEER Program, equating to 408,750 person-years at risk (PYR). In total, 14,589 patients received RAI, and SPMs were observed in 3223 patients.
RESULTS
During the study period, the rate of RAI use in patients with low-risk (T1N0) WDTC increased from 3.3% to 38.1%. For low-risk patients, the SIR of SPM was 1.21 (95% confidence interval [CI], 0.93-1.54), and the EAR was 4.6 excess cases per 10,000 PYR. SPM with significantly elevated risk because of RAI were salivary gland malignancies (SIR = 11.13; 95% CI, 1.35-40.2) and leukemia (SIR = 5.68; 95% CI, 2.09-12.37). The excess risk of leukemia was significantly greater in patients aged <45 years (SIR = 5.32; 95% CI, 2.75-9.30) compared with the excess risk in older patients (SIR = 2.26; 95% CI, 1.43-3.39).
CONCLUSIONS
The increased risk of a SPM in patients with low-risk (T1N0) WDTC, along with a lack of data demonstrating improved survival outcomes with adjuvant RAI, provide a compelling argument in favor of rationing the use of RAI in this patient population.
Keywords: Surveillance; Epidemiology, and End Results; second primary cancer; radioiodine; radioactive iodine; radiation-induced
The incidence of well differentiated thyroid cancers (WDTC) has increased substantially over the past 3 decades, and an estimated 37,200 cases were diagnosed in the United States in 2009. The majority of the increase is attributable to small (<2 cm) tumors (T1), a trend that has been ascribed mainly to improved detection of subclinical disease.1 The clinical relevance of these tumors is the subject of much debate, because a high prevalence of incidental microcarcinomas has been reported in autopsy series.2 Furthermore, the increased incidence in WDTC has not been associated with a concurrent increase in thyroid cancer mortality, suggesting that much of the disease being detected may be clinically insignificant.
Despite the indolent nature of small WDTCs, few clinicians are willing to conservatively manage these malignancies. Treatment usually comprises thyroidectomy, and, when indicated, adjuvant radioactive iodine (RAI) therapy. Decision-making regarding the extent of surgery remains a subject of debate, reflecting a lack of high-quality data to support management decisions. Similar levels of controversy exist regarding the role of adjuvant RAI therapy. The administration of adjuvant RAI has 2 main objectives: first, to ablate occult microscopic foci of WDTC, reducing the risk of tumor recurrence, and, second, to ablate any remaining normal thyroid tissue, thereby facilitating surveillance with serum thyroglobulin or radioiodine whole-body scintigraphy.3 On the basis of these objectives, the decision to use RAI depends on the risk of the original thyroid cancer and the completeness of surgery in removing malignant and normal thyroid tissue.
Most studies report little benefit from RAI in low-risk patients.4,5 Even in intermediate-risk patients, RAI administration confers only minor benefit in reducing the risk of recurrence or death.6 Existing guidelines from the American Thyroid Association (ATA) cite these factors.7 Notwithstanding available data and guidelines, there is widespread use of RAI in patients who have a low risk of recurrence. The decision to treat patients with RAI should be undertaken carefully. Although the side-effect profile is superior to those of other commonly used adjuvant modalities, such as external-beam radiation or cytotoxic chemotherapy, currently, there are ample data to suggest that RAI is not altogether benign.8,9 Complications range from relatively minor salivary gland dysfunction (short-term and long-term) to more serious but rare complications, such as myelosuppression and aplastic anemia.10 Furthermore, several studies have documented an increase in the incidence of second primary malignancies (SPM) in organs known to concentrate RAI.11-14 In 1 of those studies, the authors established a clear-cut dose-dependent effect, which implies causality.13 However, those analyses included patients with locally advanced and/or metastatic disease, who have few effective options apart from RAI therapy. In fact, there is no debate on the utility of RAI for patients who fall into the high-risk group, such as patients with gross extrathyroid extension or distant metastases, for whom the risk-benefit ratio favors RAI.7 However, the risk-benefit ratio may be far less favorable in low-risk patients. To our knowledge, there are no reports specifically focusing on the risk of SPM after RAI in patients with low-risk WDTC. Because this is the cohort of patients that now forms the majority of thyroid cancer cases, it is important to comprehensively define the risk of SPM caused by RAI in these patients. Therefore, the objectives of the current study were to report the use patterns of adjuvant RAI in patients with low-risk WDTC over time using a population-based registry, to determine the excess risk of SPM in the same cohort of patients, and to determine whether there is any correlation between SPM incidence and trends in RAI use.
MATERIALS AND METHODS
Patients in the Surveillance, Epidemiology and End Results Program
The National Cancer Institute’s (NCI) Surveillance, Epidemiology and End Results (SEER) Program is a population-based registry that captures 26% of cancers diagnosed in the United States. Quality control is an integral part of SEER, and comparison studies confirm that pathologic, surgical, and radiation data are recorded accurately.15,16 Information on thyroid tumor size has been recorded in the database consistently since 1983. RAI therapy is captured reliably provided that it is administered as part of the first course of therapy or within 1 year of surgery.
All patients who had an index WDTC, which we defined as classical papillary carcinoma, variants of papillary carcinoma, and follicular carcinoma (International Classification of Diseases for Oncology, third edition histology codes 8050, 8052, 8130, 8260, 8290, 8330-8332, 8335, 8340-8344, 8450, and 8452) were included. Additional covariates of interest were age, sex, year of diagnosis, tumor size (recorded since 1983), lymph node and distant metastasis status, extrathyroid extension, multicentricity (recorded since 2004), and administration of RAI. The SEER Program classifies patients as N0 based on pathologic analysis or on clinical and radiographic data if patients do not undergo lymph node dissection.
In total, 78,864 patients were identified in the SEER 17 cohort (1973-2006), and 9385 patients (11.9%) were excluded because of missing data regarding tumor size, extra-thyroid extension, lymph node status, metastasis status, or RAI administration. Low-risk tumors were defined as those <2 cm in size without extrathyroid extension, lymph node metastases, or distant metastases (ie, essentially intrathyroid T1N0M0 tumors) in patients aged <45 years.
Definition of SPM Risk
An SPM was defined as an invasive solid or hematologic cancer that developed >6 months after the index WDTC. SPMs were classified according to standard Warren and Gates criteria modified by the NCI.17,18 The risk of SPM was defined as the standardized incidence ratio (SIR) adapted for cancer registry analysis.19,20 The SIR is the ratio of observed to expected (O/E) second cancers, in which the expected number is calculated for a reference cohort of identical age, sex, race, and time. The excess absolute risk (EAR) represents the absolute number of additional second cancers attributable to the index WDTC and is calculated as the excess (O/E) number of second cancers in patients with an index WDTC diagnosis per 10,000 person-years at risk (PYR).18
SPM analysis was conducted in the SEER 13 cohort (1973-2006), which comprises 37,176 actively followed patients with WDTC, equal to 408,750 PYR. SPMs with a meaningfully elevated risk after WDTC diagnosis and RAI treatment were identified as follows. First, SIR and EAR statistics were generated for 194 SPM sites in cohorts of patients with WDTC who received RAI (RAI-positive) and did not receive RAI (RAI-negative). SPM sites were then filtered to include sites with an SIR significantly >1.0 (at P < .05) in only the RAI-positive cohort, but not in the RAI-negative cohort, thereby identifying second cancer sites that were at elevated risk specifically associated with RAI administration. Finally, SPM sites were limited to include only those for which the EAR was ≥0.5, because only SPMs with ≥0.5 excess cases per 10,000 PYR were deemed clinically meaningful.
Statistical Methods
There are 2 possible sources of misclassification error in SEER RAI data that deserve mention, both of which would lead to under counting of RAI administration. First, before 1987, RAI therapy was coded as “other radiation,” a category that potentially could include other modalities, such as brachytherapy. Because brachytherapy has not been used commonly in thyroid cancer, and because there have been no cases in the database of “other radiation” for thyroid cancer recorded since 1988, when the classification for RAI was introduced, we considered cases of “other radiation” before 1987 to represent RAI. Second, it is possible that the administration of RAI may be missed by SEER registrars, leading to under counting. Although RAI is recorded reliably in the first postoperative year, patients are not followed specifically for RAI status after 1 year. In both instances, these possible misclassification errors would have conservative effects on our analyses, in that they would dilute the trend in RAI use and would attenuate the excess risk of SPM in the RAI-positive cohort.
Thyroid cancer incidence and RAI use were regressed over time using linear and polynomial least-squares regression models. SIR confidence intervals (CIs) were calculated using the Byar approximation to Poisson distribution.18 To determine changes in the risk of SPM over time, the trend in SIR was analyzed across 4 time periods, binned by year of diagnosis (1973-81, 1982-89, 1990-98, and 1999-2006). Overall survival rates were calculated using the Kaplan-Meier method.
SIR and EAR values were calculated using the SEER*Stat software package (release 6.6.2, March 2010; NCI Cancer Statistics Branch, Bethesda, Md). Additional statistical analyses were performed using the SAS statistical software package (version 9.2, March 2008; SAS Institute Inc., Cary, NC).
RESULTS
Trends in WDTC Incidence and RAI Use
Over the past 3 decades, there has been a dramatic increase in the incidence of WDTC, from 3.5 per 100,000 in 1973 to 11.4 per 100,000 in 2007. Thyroid tumor size has been recorded since 1983. In 1983, low-risk WDTC (intrathyroid T1N0M0 tumors <2 cm) accounted for 28.6% of all WDTC. Since 1983, the incidence of low-risk WDTC has increased from 1.2 to 5.2 per 100,000 (Fig. 1). This accounts for the majority (55.6%) of the increased incidence of WDTC overall.
Figure 1.
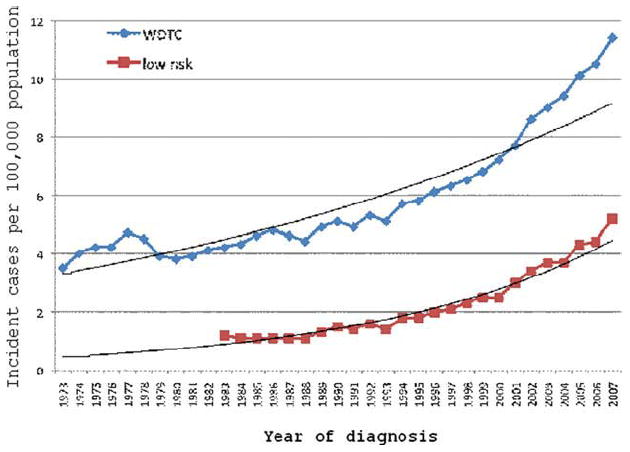
The incidence of well differentiated thyroid cancer (WDTC) over time is illustrated for all patients and for low-risk patients.
Therefore, the proportion of WDTCs that are low-risk tumors has steadily increased to 45.6% of all WDTC in 2007. Between 1983 and 2007, the percentage of tumors <2 cm increased from 47.1% to 60%, the percentage without gross extrathyroid extension increased from 80.5% to 83%, and the percentage without cervical metastases increased from 71.9% to 79.4%. Multicentricity has been recorded since 2004, and the data indicated that 38.5% of all tumors and 27.2% of microcarcinomas were multicentric.
Despite the increasing proportion of low-risk tumors, the use of RAI for WDTC has increased dramatically since 1973. Between 1973 and 2006, RAI was received by 14,589 patients. The percentage of patients with WDTC who received RAI as part of the first course of therapy overall has increased from 6.1% to 48.7% (P < .0001). Among patients aged <45 years with low-risk tumors, the receipt of RAI increased from 3.3% in 1973 to 38.1% in 2006 (P < .0001). Despite this increase, the rate of overall survival among patients with low-risk WDTC has remained constant (Fig. 2). The rate of RAI administration was associated with multicentricity: 45.9% of patients who had microcarcinomas with microscopic multicentricity received RAI compared with 18.1% of patients who had unifocal microcarcinomas (P < .0001).
Figure 2.
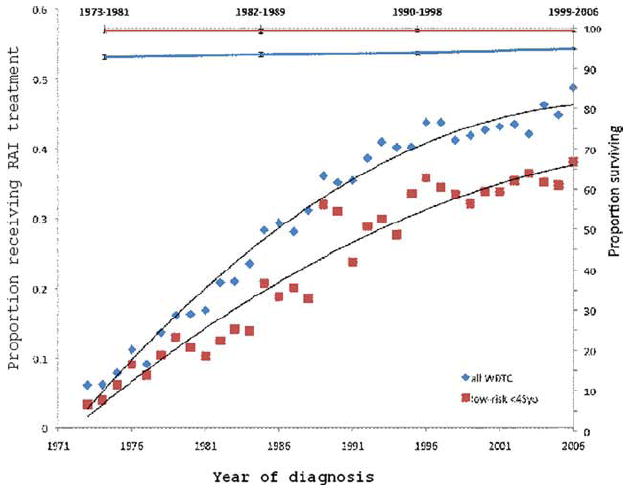
The proportion of patients receiving radioactive iodine (RAI) treatment in illustrated for all patients and for patients aged <45 years with low-risk well differentiated thyroid cancer (WDTC) (left; y-axis), and overall survival is illustrated (right; y-axis) for the period from 1973 and 2006. Tumor size was recorded after 1983. For the purposes of this illustration, before 1983, low-risk disease was defined as intrathyroid N0M0 tumors; after 1983, low-risk was defined as T1N0M0 tumors.
Elevated SPM Risk After WDTC
During the study period, an elevated risk of SPM after a diagnosis of WDTC emerged. SPMs were observed in 3223 patients. The expected number of SPMs in a reference population was 3029.
These trends have occurred concurrently with increasing use of RAI. During the era when RAI use was uncommon, there was no statistically significant elevated risk of SPM among patients who were diagnosed with WDTC. However, the risk of SPM subsequently has increased in parallel with the escalating frequency of RAI use. For all patients with WDTC, the overall risk of SPM at any site has increased from 1.00 (95% CI, 0.94-1.16) during 1973 to 1981 to 1.22 (95% CI, 1.10-1.35) during 1999 to 2006 (Fig. 3). This corresponds to an EAR that has increased from 0 to 14.3 excess cases per 10,000 PYR. The increased risk is most pronounced in hematologic malignancies. For leukemia, the SIR has increased from 1.09 (95% CI, 0.72-1.57) during 1973 to 1981 to 2.40 (95% CI, 1.40-3.85) during 1999 to 2006. This corresponds to an EAR that has increased from 0.2 to 2.0 excess cases per 10,000 PYR.
Figure 3.
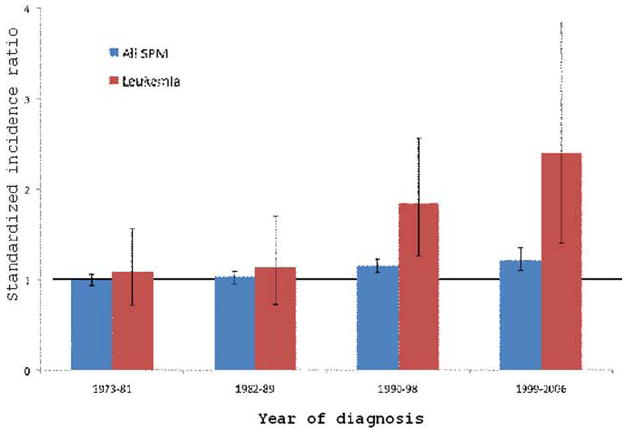
Increasing second primary malignancy (SPM) rates with time for all malignancies and leukemias are illustrated in patients with well differentiated thyroid cancer (WDTC). The horizontal line indicates a standardized incidence ratio (SIR) of 1.0; error bars indicate 95% confidence intervals.
Elevated SPM Risk After RAI for All WDTC
Among all patients with WDTC who received RAI between 1973 and 2007, the SIR of SPM at any site was 1.18 (95% CI, 1.10-1.25). In contrast, there was no increased risk of SPM among patients who did not receive RAI (SIR = 1.02; 95% CI, 0.98-1.06). Patients with WDTC who received RAI experienced an EAR of 11.9 excess cancers per 10,000 PYR.
Among all patients with WDTC, increased risks of SPM caused by WDTC and RAI treatment were observed for cancers of the salivary glands (SIR = 3.84; 95% CI, 1.66-7.56), skin (melanoma; SIR = 1.61; 95% CI, 1.2-2.1), and kidneys (SIR = 2.62; 95% CI, 1.94-3.47) along with increased risks of lymphoma (SIR = 1.44; 95% CI, 1.07-1.88) and leukemia (SIR = 2.09; 95% CI, 1.49-2.86) (Table 1). The EAR of leukemia in patients with WDTC who received RAI was 1.6 cases per 10,000 PYR.
Table 1.
Rates of Second Primary Malignancies and Anatomic Distribution in All Patients With Well Differentiated Thyroid Cancer (WDTC) and in Patients With Low-Risk WDTC Who Received Radioactive Iodine
| All WDTC/RAI1+ | Low-Risk WDTC/RAI1+ | |||
|---|---|---|---|---|
| SPM Site | SIR (95% CI | EAR | SIR (95% CI) | EAR |
| All sites | 1.18 (1.10-1.25) | 11.9 | 1.21 (0.93-1.54) | 4.6 |
| All solid tumors | 1.12 (1.05-1.20) | 7.4 | 1.13 (0.85-1.46) | 2.5 |
| Salivary gland | 3.84 (1.66-7.56) | 0.5 | 11.13 (1.35-40.2) | 0.7 |
| Melanoma | 1.61 (1.21-2.10) | 1.6 | 1.63 (0.66-3.66) | 1.1 |
| Kidney | 2.62 (1.94-3.47) | 2.4 | 0 (0-3.3) | 0.0 |
| All lymphatic/hematologic | 1.70 (1.40-2.05) | 3.6 | 2.18 (1.00-4.13) | 2.0 |
| Lymphoma | 1.44 (1.07-1.88) | 1.3 | 0.72 (0.09-2.60) | 0.0 |
| Leukemia | 2.09 (1.49-2.86) | 1.6 | 5.68 (2.09-12.37) | 2.0 |
RAI+ indicates, patients who received radioactive iodine; SPM, second primary malignancy; SIR, standardized incidence ratio; CI, confidence interval; EAR, excess absolute risk.
Elevated SPM Risk in Young Patients Receiving RAI for Low-Risk WDTC
A similar pattern was observed among patients aged <45 years who had low-risk WDTC (intrathyroid T1N0M0 tumors). In this cohort, the overall risk of SPM was 1.21 (95% CI, 0.93-1.54), corresponding to 4.6 excess cancers per 10,000 PYR. Because of the smaller size of this subgroup, the CIs were wider, and the risk was significantly elevated only for cancers of the salivary gland (SIR = 11.13; 95% CI, 1.35-40.2) and leukemia (SIR = 5.68; 95% CI, 2.09-12.37). The administration of RAI to patients with low-risk WDTC caused an estimated 2.0 excess cases of leukemia per 10,000 PYR (Table 1).
When comparing the risk of leukemia after RAI in younger patients (aged <45 years) versus older patients (aged ≥45 years) with WDTC, the risk of leukemia was elevated in both groups but was elevated to a greater degree in younger patients (SIR = 5.32; 95% CI, 2.75-9.30) than in older patients (SIR = 2.26; 95% CI, 1.43-3.39). Further analyses were performed by sex and age group. There was no difference in the risk of SPM among men and women who received RAI. In men, the SIR of SPM was 1.23 (95% CI, 1.08-1.40), whereas, in women, the SIR of SPM was 1.18 (95% CI, 1.07-1.29). Similarly, there was no difference in the risk of leukemia after RAI: The SIR was 2.28 (95% CI, 1.18-3.99) for men and 3.20 (95% CI, 2.03-4.80) for women.
DISCUSSION
Although several studies (both population-based and cohort) have reported an elevated risk of SPM from the use of RAI therapy for thyroid cancer,11-14 to our knowledge, no reports have specifically addressed this issue in patients with low-risk WDTC, for whom the benefits of RAI are less clear. Therefore, the objective of the current study was to specifically determine trends in RAI use and the associated risk of SPM in patients with low-risk WDTC. These patients account for the majority of the increased incidence of thyroid cancer observed in the United States over the past decade.
There are limited data indicating any efficacy of RAI as adjuvant treatment to prevent recurrences or prolong survival in patients who fall into the low-risk WDTC category, which led the ATA to revise their guidelines for RAI ablation in 2009.7 These recommendations now advise that there are no data to support the routine use of RAI in patients with intrathyroid tumors with or without multifocality that measure <1 cm. Because there is conflicting evidence on the benefits of RAI for patients who have tumors that measure >1 cm, the ATA suggests limiting its use to patients who have intermediate-risk or high-risk features, such as extrathyroid extension, aggressive histologic variants, or the presence of cervical lymph adenopathy. On the basis of the guidelines outlined above, there is probably minimal valued-added benefit to the use of adjuvant RAI in young patients with low-risk tumors; ie, patients aged <45 years with intrathyroid T1 tumors (<2 cm) and without extrathyroid extension or lymph node metastasis. These patients are considered “low risk” in the current ATA guidelines7 and were categorized as “very low risk” in a recent systematic analysis of the literature.21 Despite this, 38% of patients who fall into this low-risk category currently receive adjuvant RAI in the United States. For patients with microcarcinomas, the presence of microscopic multicentricity in recent years nearly tripled the likelihood of RAI use; although, currently, this factor is not considered an indication for adjuvant RAI.7 More important, time trends demonstrate that the receipt of RAI by low-risk patients continues to increase with time in an almost linear manner despite the absence of any evidence for an impact on survival. It has been suggested that RAI should be used in all but the most microscopic of tumors, and some have advocated adjuvant RAI for tumors that measure >0.5 cm.22 However, these statements are based on “expert opinion” and, thus, should be viewed with scrutiny. Furthermore, many of these tumors are incidental, subclinical tumors that probably would remain asymptomatic and, in an earlier era, would have been diagnosed only at autopsy.2
The decision to use RAI should be based on a balance between its risks and benefits. The side-effect profile of RAI has been underplayed as minor compared with external-beam radiation, and this view often is passed on to patients. Short-term side effects of RAI include salivary gland dysfunction (sialadenitis, altered taste), which is observed in >40% of patients; xerophthalmia (25%); transient fertility reduction (20%); transient leukopenia; and thrombocytopenia.8-10,23,24 Fortunately, life-threatening, short-term risks, such as acute bone marrow failure, are rare. Delayed toxicities are less well appreciated and are not mentioned routinely, such as xerostomia (40% partial, 4% complete) or reduced pulmonary function secondary to radiation pneumonitis (6%). In a quality-of-life analysis, Almeida et al reported that, in patients with low-risk thyroid cancer, treatment with RAI was the only factor on multivariate analysis associated with poorer quality-of-life scores.25
The development of SPM attributable to RAI is viewed as 1 of the most serious sequelae of treatment.9,26 However, it is generally believed that this is a rare outcome; therefore, it is not emphasized when recommending adjuvant treatment. Several studies, both cohort and population-based, have demonstrated a significant increase in the incidence of SPM among patients with WDTC who received RAI compared with their counterparts who did not receive RAI.11-14 It is estimated that this increased risk ranges from 20% to 30% compared with a matched population. Undoubtedly, in patients who have high-risk or metastatic disease, there are limited options available, and the comparatively low SPM risk can be justified. Although some authors suggest that low-dose RAI does not increase SPM risk, our data may not be consistent with that suggestion. In our current study cohort, most patients deemed as low-risk would have received a lower cumulative dose of RAI compared with patients who had recurrences or distant metastasis, yet this low-risk cohort experienced a 21% excess SPM risk attributable to the receipt of RAI. The SPM sites at elevated risk encompassed diverse malignancies, but hematologic cancers carried the most pronounced elevation in risk.
There are important limitations to our data that deserve mention. Although few patients had missing data, the SEER Program does not record certain covariates of interest, such as completeness of resection, tumor multicentricity (before 2004), or data from postoperative thyroglobulin or whole-body scanning, some of which potentially may explain the decision to use adjuvant RAI. RAI administration is recorded reliably in the SEER Program only in the adjuvant setting, as outlined above (see Statistical Methods), and may not be recorded if RAI is given at a later date for recurrent or new disease. This may lead to misclassification of RAI-positive patients into the RAI-negative cohort, attenuating the measured differences between cohorts, and leading to an under estimation of the elevated risk attributable to RAI. Second, the SEER Program does not include information on RAI dose; therefore, we were unable to correlate RAI dose with the risk of SPM.
Nonetheless, we report a striking increase in the risk of all malignancies and hematologic cancers among patients with WDTC in parallel with the increasing receipt of RAI by low-risk patients. There may be a mechanistic explanation with regard to cancers arising from the salivary glands, hematologic system, and kidney, because it is known that RAI concentrates in the salivary glands and bone marrow and is excreted through the kidneys. There are data to suggest that expression of the Na+/I− symporter in extrathyroid tissue, like that of the salivary gland, may be responsible for concentrating RAI in these cells, driving carcinogenesis.27 To our knowledge, our finding of an increase in the incidence of melanoma has not been reported previously, and we know of no mechanistic explanation for this. The incidence of both melanoma and renal cell cancers has been increasing in recent years, and both have been described by Welch and Black as cancers subject to overdiagnosis in the setting of increased surveillance. Therefore, it is possible that the elevated risk of second cancers in these sites simply may represent increased ascertainment in a population undergoing rigorous cancer surveillance.28 Similarly, using data from the SEER database, previous studies have demonstrated an association between thyroid cancer and breast cancer in women.29 Although we did observe a significant increase in the number of breast cancers as SPMs in patients with thyroid cancer (all patients and low-risk patients), our analyses indicated no difference in the rates between the RAI-positive and RAI-negative patients (SIR of breast cancer after WDTC: RAI-positive cohort, 1.12 (95% CI, 0.97-1.29); RAI-negative cohort, 1.15 (95% CI, 1.06-1.25).
Previous data have suggested that younger patients are unlikely to develop hematologic malignancies after RAI, which is a critical consideration given the excellent prognosis for these patients, who essentially have no mortality risk from low-risk WDTC. In our analysis, the EAR was 4.6 patients per 10,000 PYR, approximating an incidence of 0.05% per year. It is worth noting that this risk is similar to rates of radiation-induced sarcomas attributed to external-beam radiation therapy, which is estimated between 0.03% and 0.2% per year.30-32 The latter attributable risk has always been included in decision-making algorithms by radiation oncologists when advocating adjuvant treatment for young patients, in whom a balance needs to be struck between the estimated benefits of adjuvant external-beam radiation versus the risk of a radiation-induced second cancer. Perhaps it is worthwhile to consider a similar decision-making algorithm in low-risk patients with WDTC and to ask whether existing data on the benefit of RAI in these low-risk patients justify the elevated risk of an SPM across the lifetime of young patients. Clearly, these data should be incorporated into future recommendations of RAI treatment, especially when dealing with low-risk patients who are unlikely to die from WDTC. In conclusion, the increased risk of a second primary cancer in patients with low-risk WDTC, along with the lack of any data demonstrating improved survival outcomes with adjuvant RAI, provides a compelling argument for rationing the use of RAI in this patient population.
Acknowledgments
FUNDING SOURCES No specific funding sources were disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES The authors made no disclosures.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Iyer NG, Shaha AR, Silver CE, et al. Thyroid incidentalomas: to treat or not to treat. Eur Arch Otorhinolaryngol. 2010;267:1019–1026. doi: 10.1007/s00405-010-1207-1. [DOI] [PubMed] [Google Scholar]
- 3.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 4.Tuttle RM, Leboeuf R, Shaha AR. Medical management of thyroid cancer: a risk adapted approach. J Surg Oncol. 2008;97:712–716. doi: 10.1002/jso.21010. [DOI] [PubMed] [Google Scholar]
- 5.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during 6 decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, McDougall IR, Sisson JC. A proposition for the use of radioiodine in WDTC management [published online ahead of print January 21, 2009] J Nucl Med. 2009 [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Chow SM. Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid cancer. J HK Coll Radiol. 2005;8:127–135. [Google Scholar]
- 9.Van Nostrand D. The benefits and risks of I-131 therapy in patients with well differentiated thyroid cancer. Thyroid. 2009;19:1381–1391. doi: 10.1089/thy.2009.1611. [DOI] [PubMed] [Google Scholar]
- 10.Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med. 1998;39:1551–1554. [PubMed] [Google Scholar]
- 11.Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–457. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 12.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to 3 decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93:504–515. doi: 10.1210/jc.2007-1154. [DOI] [PubMed] [Google Scholar]
- 13.Rubino C, de Vathaire F, Dottorini ME, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89:1638–1644. doi: 10.1038/sj.bjc.6601319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandeep TC, Strachan MW, Reynolds RM, et al. Second primary cancers in thyroid cancer patients: a multinational record linkage study. J Clin Endocrinol Metab. 2006;91:1819–1825. doi: 10.1210/jc.2005-2009. [DOI] [PubMed] [Google Scholar]
- 15.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlan LC, Hankey BF. The Surveillance, Epidemiology, and End Results Program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21:2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 17.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Cancer. 1932;16:1358–1414. [Google Scholar]
- 18.Curtis RE, Ries LA. Methods. In: Curtis RE, Freedman DM, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute; 2006. pp. 9–14. [Google Scholar]
- 19.Schoenberg BS, Myers MH. Statistical methods for studying multiple primary malignant neoplasms. Cancer. 1977;40(4 suppl):1892–1898. doi: 10.1002/1097-0142(197710)40:4+<1892::aid-cncr2820400820>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Begg CB, Zhang ZF, Sun M, Herr HW, Schantz SP. Methodology for evaluating the incidence of second primary cancers with application to smoking-related cancers from the Surveillance, Epidemiology, and End Results (SEER) Program. Am J Epidemiol. 1995;142:653–665. doi: 10.1093/oxfordjournals.aje.a117689. [DOI] [PubMed] [Google Scholar]
- 21.Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–1245. doi: 10.1089/thy.2009.0455. [DOI] [PubMed] [Google Scholar]
- 22.Luster M, Clarke SE, Dietlein M, et al. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 23.Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid. 2003;13:265–271. doi: 10.1089/105072503321582060. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 25.Almeida JP, Vartanian JG, Kowalski LP. Clinical predictors of quality of life in patients with initial differentiated thyroid cancers. Arch Otolaryngol Head Neck Surg. 2009;135:342–346. doi: 10.1001/archoto.2009.16. [DOI] [PubMed] [Google Scholar]
- 26.de Vathaire F. The carcinogenic effects of radioiodine therapy for thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4:180–181. doi: 10.1038/ncpendmet0761. [DOI] [PubMed] [Google Scholar]
- 27.Dohan O, Carrasco N. Advances in Na(+)/I(−) symporter (NIS) research in the thyroid and beyond. Mol Cell Endocrinol 1. 2003;213:59–70. doi: 10.1016/j.mce.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 28.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 29.Chen AY, Levy L, Goepfert H, Brown BW, Spitz MR, Vassilopoulou-Sellin R. The development of breast carcinoma in women with thyroid carcinoma. Cancer. 2001;92:225–231. doi: 10.1002/1097-0142(20010715)92:2<225::aid-cncr1313>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Wiklund TA, Blomqvist CP, Raty J, Elomaa I, Rissanen P, Miettinen M. Postirradiation sarcoma Analysis of a nationwide cancer registry material. Cancer. 1991;68:524–531. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 31.Brady MS, Gaynor JJ, Brennan MF. Radiation-associated sarcoma of bone and soft tissue. Arch Surg. 1992;127:1379–1385. doi: 10.1001/archsurg.1992.01420120013002. [DOI] [PubMed] [Google Scholar]
- 32.Robinson E, Neugut AI, Wylie P. Clinical aspects of postirradiation sarcomas. J Natl Cancer Inst. 1988;80:233–240. doi: 10.1093/jnci/80.4.233. [DOI] [PubMed] [Google Scholar]


