Abstract
Background
Early diastolic myocardial tissue Doppler (TD) velocities have reported to be reduced in mutation-positive patients with HCM in some studies even in the absence of left ventricular hypertrophy (LVH). Strain is a sensitive tool in detecting early systolic abnormalities in patients with hypertrophic cardiomyopathy (HCM). Our goal is to examine novel echocardiographic characteristics of phenotype-negative carriers for a known sarcomeric gene mutation for HCM.
Methods
We evaluated 41 consecutive subjects with a known myosin binding protein C3 (MYBPC3) mutation (c.3330+2T>G). Subjects who were mutation-positive without LVH (G+/LVH−, n=35) were compared to healthy controls (n=30) regarding tissue Doppler and segmental longitudinal strain measures.
Results
The G+/LVH− group was similar to the normal controls with respect to chamber size, LV mass index, and most diastolic filling parameters, including tissue Doppler derived Ea. Global longitudinal strain was similar for both groups (20.3 ± 2.1 vs. 19.8 ± 1.8; p=0.36) although regional segment analysis showed a notable reduction in the basal septum (16.8 ± 3.1 vs. 19.0 ± 4.0%, p=0.02) and increase in the basal posterior (22.5 ± 5.2 vs. 17.9 ± 5.2, p=0.001) as well as mid posterior (21.8 ± 4.7 vs. 18.2 ± 3.0, p=0.001) walls.
Conclusions
In our cohort of phenotype-negative carriers of a specific MYBPC3 mutation, there were minimal differences in conventional 2-dimensional, Doppler, and speckle-tracking derived parameters of systolic and diastolic function compared to that of normal subjects. The presence of regional alterations in strain indicative of the presence of underlying subclinical disease requires further validation.
Keywords: hypertrophic cardiomyopathy, genetic heart disease, echocardiography, longitudinal strain
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a disease characterized by left ventricular hypertrophy (LVH) with septal predominance and several associated phenotypes1. Molecular genetic studies have identified over a hundred mutations that give rise to hypertrophy involving sarcomeric proteins2, 3. Mutations identified in the myosin-binding protein C3 (MYBPC3) gene account for 15% of all familial HCM cases4. As the clinical spectrum of phenotypes associated with HCM is increasing, it is becoming recognized that LVH may be absent in mutation carriers. MYBPC3 carriers demonstrate age related penetrance with hypertrophy occurring later in life than those with mutations involving cardiac beta-myosin heavy chain4.
Echocardiography remains a useful tool in the screening of family members of HCM5, 6. As a non-invasive test, echocardiography allows assessment of hypertrophy, valvular abnormalities, and diastolic dysfunction that may correlate with symptoms of syncope or breathlessness in some patients. Animal and human studies with sarcomeric gene mutations have demonstrated tissue Doppler (TD) imaging abnormalities in the absence of LVH, mostly characterized by reductions in early myocardial velocities7-10. However, this has been challenged by recent studies that observed preserved Ea velocities in some MYBPC3 mutation carriers, although these heterogeneous case series were small in sample sizes11.
Speckle tracking-derived longitudinal strain is a newer novel technology that assesses myocardial deformation using long axis images obtained by echocardiography12. Despite labor-intensive measurements in the current configuration, it offers several advantages over TD imaging particularly in the assessment of regional abnormalities and in the reduction of angle dependent errors of measurement. To date, several studies have demonstrated that early abnormalities in systolic function in patients with hypertrophied ventricles can be detected by strain analysis10, 12, 13, which may directly reflect underlying myocardial abnormalities. Herein, we aim to characterize the cardiac phenotypes of heterozygous carriers of the MYBPC3 gene (c.3320+2T>G), and particularly to assess the ability of TD imaging and longitudinal strain to distinguish these carriers from normal controls as well as to explore the heterogeneity of regional dysfunction in early forms of this inherited condition.
METHODS
Study Population
This is a prospective cohort outreach study evaluating subjects with an HCM-affected family member at the Geauga Amish community at the Das Deutsch Center Clinic for Special Needs Children in Middlefield, Ohio. A known MYBPC3 mutation (c.3330+2T>G) has been identified in this large Amish community based on homozygous mutation found in a number of children14, and family members without known cardiac diseases. All subjects provided informed consent approved by their local Institutional Review Board to undergo MYBPC3 gene testing for the specific mutation. All mutation carriers identified by genetic testing then underwent further evaluation including physical exams, 12-lead electrocardiograms, and transthoracic echocardiograms to identify underlying cardiac abnormalities. “Phenotype-negative” was defined as no clinical signs and symptoms consistent with obstructive HCM.
A separate control group of normals was assembled, age- and gender-matched based on the patient cohort. Healthy volunteers were recruited as controls after informed consent, and their echocardiographic examination reviewed by staff cardiologist to be deemed normal examination by standard measurements including TD parameters. All patients and controls had recorded height, weight, gender, blood pressure, and heart rate, and they had previously been scanned using a Vivid 7 machine with frame rates >50 fps so that appropriate longitudinal strain measurements could be made. The Cleveland Clinic Institutional Review Board approved this study whereby echocardiographic data from both cohorts were analyzed offline.
Echocardiography
All patients underwent standard transthoracic echocardiograms (Vivid i, GE Vingmed Ultrasound AS, Horten, Norway) using an ultrasound machine with a 3.5 MHz probe and digital storage capacity. Data was analyzed offline by a single observer who was blinded to patient factors. Standard views were taken and chamber dimensions were assessed using M-mode or 2D measurements based on current guidelines15. Left ventricular (LV) ejection fraction was calculated using the modified Simpson’s method15. LV mass and mass index were calculated based on established criteria. Mitral valve inflow using spectral Doppler displays were used to determine peak early (E) and late (A) trans-mitral filling velocities, E/A ratio, and deceleration time (DT) of E wave. Pulmonary vein flow was assessed for peak systolic velocity (S), peak diastolic velocity (D), atrial flow reversal velocity, and the duration of atrial flow reversal (Adur). Tissue Doppler imaging was used to measure septal and lateral mitral annular velocities, including those in systole (Sa), early (Ea), and late (Aa) diastole. Diastolic function was graded as stage I, II, or III based on the latest American Society of Echocardiography guideline recommendations16.
Longitudinal strain analysis (ε) was performed offline using dedicated software (EchoPac PC version BT06; GE Healthcare, Milwaukee, WI). Images were able to be analyzed in all patients with adequate frame rates (>50 fps). Two dimensional longitudinal strain was assessed in the apical 4, 2-, and 3- chamber views at 6 segments in each view17. For each apical view, sample points were placed along the endocardium at end systolic frames. The software package automatically constructed a region of interest along the length of the LV wall, after which individual speckles were tracked to generate strain curves. Global longitudinal strain was calculated as the average value of the total 18 segments. Measurements were made without knowledge of gene mutation carrier status.
Statistical Analysis
All data is expressed as mean and standard deviation or number and percentage. Mean measurements were compared by paired t-tests, with significance set at p<0.05. All statistics were done using JMP Statistical software for Macintosh (Version 7.0, SAS Institute Inc, Cary, North Carolina).
Sources of Funding
This research is supported by a Pilot Research Grants Award from the Cleveland Clinical and Translational Sciences Collaborative (CTSA Award, NCRR/NIH UL1 RR024989). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Baseline characteristics
A total of 41 consecutive mutation carriers for the specific MYBPC3 mutation were identified as part of the screening study. Of these, 6 were felt to have possible phenotype expression, with 4 having echocardiographic features of HCM and 2 having concentric LVH. The phenotypes identified in these subjects present with classic septal hypertrophy as described in the Supplementary Files. The remaining 35 patients were identified as genotype-positive/phenotype-negative (“G+/LVH−“’). Comparing subjects in the G+/LVH− and control groups, mean age (30 ±14 and 35 ±12 years, respective) and gender (51% and 47% males, respectively) were similar. They also had similar body mass indices (24.8 ± 5.9 vs 23.9 ± 3.0 kg/m2), and all blood pressures and heart rates were within normal limits. All subjects were noted to be in sinus rhythm with no evidence of LVH or conduction block by 12-lead electrocardiogram. Nonspecific T-wave abnormalities were evident in 12 G+/LVH− subjects, while 11 showed an RSR’ or incomplete right bundle branch block patterns in lead V1.
2D Echocardiographic and Doppler Indices
Subjects in both groups had similar LV dimensions, mass, and left atrial areas (Table 1). All subjects have preserved LV ejection fraction, even though G+/LVH− subjects demonstrated statistically significant higher LV ejection fraction compared to normal control subjects. No subject had evidence of significant valvular lesions, systolic anterior motion (SAM) of the mitral valve, papillary muscle abnormalities, pulmonary hypertension estimated by tricuspid regurgitant velocities, gradients across any valve or left ventricular outflow tract (LVOT). For G+/LVH− subjects, septal thicknesses ranged from 7 to 12 mm and posterior wall thicknesses ranged from 7 to 12 mm with the criteria for LVH based on current ASE guidelines. LV mass was further indexed to height^2.7 for 10 pediatric aged patients (0-18years) to further ensure that none had echocardiographic evidence of LVH.
Table 1.
Standard Echocardiographic and Doppler Indices between Genotype-positive/phenotype-negative and normal control subjects
| G+/LVH− (n=35) |
Normal Controls (n=30) |
p-value | |
|---|---|---|---|
| Cardiac Structure and Function | |||
|
| |||
| Septal wall thickness (cm) | 1.0 ± 0.2 | 0.9 ± 0.1 | NS |
|
| |||
| Posterior wall thickness (cm) | 0.9 ± 0.2 | 0.9 ± 0.1 | NS |
|
| |||
| LV mass (g) | 143 ± 50 | 146 ± 41 | NS |
|
| |||
| LV mass index (g/m2) | 80 ± 19 | 80 ± 18 | NS |
|
| |||
| LV ejection fraction (%) | 61 ± 4 | 58 ± 4 | p<0.05 |
|
| |||
| Doppler Indices | |||
|
| |||
| Mitral valve E wave (cm/s) | 84 ± 15 | 77 ± 13 | NS |
|
| |||
| Mitral valve A wave (cm/s) | 58 ± 18 | 45 ± 12 | p<0.05 |
|
| |||
| Mitral valve E/A ratio | 1.6 ± 0.6 | 1.8 ± 0.5 | NS |
|
| |||
| Deceleration time (ms) | 185 ± 38 | 186 ± 24 | NS |
|
| |||
| Pulmonary vein S wave (cm/s) | 51 ± 15 | 49 ± 10 | NS |
|
| |||
| Pulmonary vein D wave (cm/s) | 56 ± 16 | 52 ± 12 | NS |
|
| |||
| PV A wave (cm/s) | 25 ± 10 | 27 ± 5 | NS |
|
| |||
| PV Adur (cm) | 115 ± 18 | 105 ± 17 | NS |
|
| |||
| Mitral Annular Tissue Doppler Velocities | |||
|
| |||
| Lateral Wall (cm/s) | |||
| Sa | 10.2 ± 2 | 9.1 ± 2.1 | p<0.05 |
| Ea | 16.4 ± 4.9 | 14.1 ± 3.0 | NS |
| Aa | 8.8 ± 3.0 | 8.1 ± 1.4 | NS |
|
| |||
| Septal Wall (cm/s) | |||
| Sa | 7.9 ± 1.2 | 8.0 ± 1.6 | NS |
| Ea | 11.7 ± 3.2 | 11.0 ± 2.6 | NS |
| Aa | 7.5 ± 2.2 | 8.1 ± 1.4 | NS |
|
| |||
| Averaged (cm/s) | |||
| Sa | 9.0 ± 1.3 | 8.5 ± 1.6 | NS |
| Ea | 13.9 ± 3.8 | 12.0 ± 2.7 | NS |
Abbreviations: E: early rapid filling wave, A: filling wave due to atrial contraction, S: systolic peak velocity, D: diastolic peak velocity, PV A: pulmonary vein atrial flow reversal velocity, PV Adur: duration of pulmonary vein atrial flow reversal, Sa: systolic annular velocities, Ea: early diastolic annular velocities, Aa: late diastolic annular velocities
Based on the latest guidelines, diastolic function was normal in all control patients and 89% of G+/LVH− subjects, while 4 G+/LVH− subjects showed evidence of Grade I diastolic dysfunction. Mean Doppler variables based on mitral valve inflow and pulmonary vein flow were similar in both groups with the only difference being noted the A-wave amplitude across the mitral valve (Table 1). Mean TDI septal Sa, Ea, and Aa velocities were comparable between G+/LVH− subjects and normal controls. In contrast, there was a statistically significantly higher lateral annular Sa and a trend towards higher lateral annular Ea in G+/LVH− subjects compared to controls, but no difference were noted in Aa between the groups (Table 1). When averaging septal and lateral Sa and Ea, there were no difference between G+/LVH− subjects and normal controls.
Longitudinal Strain
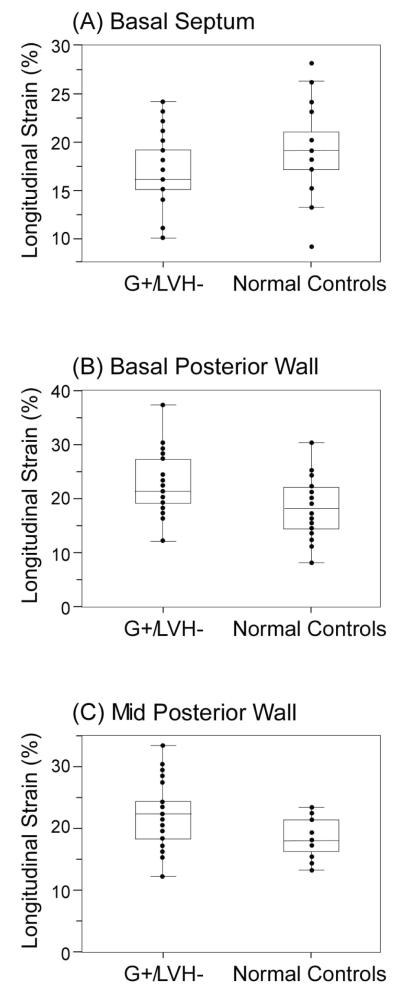
Table 2 illustrates the longitudinal strain patterns between G+/LVH− subjects and normal controls. There was no significant difference noted in mean global longitudinal strain between the two groups. Overall, the mean of segments taken in the apical 3-chamber view was higher in the mutation positive group compared to normal controls. For mutation positive patients, the segment associated with the lowest value of all 18 segments was the basal septum (Figure 1), whereas the basal and mid posterior wall demonstrated increased strain compared to normal controls, all statistically significantly different.
Table 2.
Longitudinal Strain Assessment of Individual Segments Based Comprising Apical 2-, 3-, and 4- Views by Echocardiography
| G+/LVH− (n=35) |
Normal Controls (n=30) |
p value | |
|---|---|---|---|
| Apical 4-chamber | |||
| Basal Septum (%) | 16.8 ± 3.1 | 19.0 ± 4.0 | 0.02 |
| Mid Septum (%) | 20.5 ± 4.3 | 21.5 ± 3.4 | NS |
| Apical Septum (%) | 23.1 ± 6.2 | 23.1 ± 4.2 | NS |
| Basal Lateral (%) | 21.3 ± 3.7 | 21.5 ± 6.9 | NS |
| Mid Lateral (%) | 19.8 ± 3.6 | 18.8 ± 4.0 | NS |
| Apical Lateral (%) | 19.2 ± 5.1 | 19.0 ± 8.5 | NS |
| Apical 2-chamber | |||
| Basal Inferior (%) | 21.1 ± 4.5 | 20.1 ± 6.6 | NS |
| Mid Inferior (%) | 22.0 ± 3.6 | 20.5 ± 3.9 | NS |
| Apical Inferior (%) | 22.6 ± 4.8 | 23.2 ± 4.7 | NS |
| Basal Anterior (%) | 18.9 ± 4.8 | 19.0 ± 9.3 | NS |
| Mid Anterior (%) | 20.4 ± 3.2 | 18.7 ± 5.3 | NS |
| Apical Anterior (%) | 18.7 ± 6.1 | 19.2 ± 6.4 | NS |
| Apical 3-chamber | |||
| Basal Posterior (%) | 22.5 ± 5.2 | 17.9 ± 5.2 | 0.002 |
| Mid Posterior (%) | 21.8 ± 4.7 | 18.2 ± 3.0 | 0.001 |
| Apical Posterior (%) | 20.6 ± 5.3 | 20.1 ± 5.1 | NS |
| Basal Antero-septum (%) | 17.8 ± 5.4 | 17.2 ± 4.6 | NS |
| Mid Antero-septum (%) | 21.3 ± 3.9 | 20.2 ± 3.7 | NS |
| Apical Antero-septum (%) | 20.6 ± 5.3 | 22.3 ± 6.1 | NS |
| Global Strain (%) | 20.3 ± 2.1 | 19.8 ± 1.8 | NS |
Figure 1.
Segments associated with regional differences in longitudinal strain (A) Basal septum (p=0.02) (B) Basal posterior (p=0.001) and (C) Mid posterior (p=0.001) walls. Abbreviation: G+/LVH−: gene mutation positive, no left ventricular hypertrophy
DISCUSSION
This is the first study to our knowledge to not only carefully examine typical systolic and diastolic echocardiographic markers of cardiac performance in a large group of asymptomatic carriers with the same MYBPC3 mutation, but also to utilize novel speckle tracking techniques that examine the tissue deformation and regional abnormalities to further delineate the presence of early subclinical disease. We observed that in the absence of cardiac hypertrophy, TDI indices were relatively preserved in our specific cohort of mutation carriers. These findings suggested that prior reports of diminished TDI indices in HCM mutation carriers may not universally apply to all sarcomeric mutations, or may imply that TDI indices may vary depending on the different stages of disease progression. In other words, the absence of TDI abnormalities does not exclude the presence of underlying sarcomeric gene mutation. Another important observation is that despite similar degrees of global longitudinal strain compared to healthy controls, we observed a consistent decrease in longitudinal strain at the basal septum accompanied by a consistent increase in longitudinal strain at the basal and mid segment of the posterior wall in our cohort of MYBPC3 mutation carriers. These findings demonstrate the presence of early subclinical regional (but not global) deformities in this particular cohort of phenotype-negative MYBPC3 mutation carriers, and provide potential clues to localization of the fundamental defects leading to the development of the HCM phenotype.
Clinicians have relied heavily on echocardiographic screening as the primary modality in identifying affected individuals with clinically significant HCM5. Despite relatively preserved cardiac structure and LV systolic function being observed in many affected but asymptomatic HCM mutation carriers, the sarcomeric gene mutation itself is believed to contribute to a fundamental global myocardial defect. Such subclinical abnormalities can therefore lead to progressive diastolic dysfunction or microvascular dysfunction, thereby producing pathologic findings of fibrosis and myofibril disarray. This hypothesis is supported by previous observations whereby septal and lateral Ea measurements at the mitral annulus were significantly reduced in G+/LVH− subjects when compared to non-affected individuals (some also affected by various forms of MYBPC3 mutations)7, 9. However, such findings were not replicated in our cohort, nor identified in other contemporary series11. Even with the use of speckle-tracking techniques to determine global longitudinal strain, the overall G+/LVH− population demonstrated similar global strain levels than that of normal controls. There may be several explanations for this discrepancy. First, a referral bias in those undergoing genetic testing at tertiary medical centers in other reports may favor individuals with more “malignant” mutations, and hence with genotype-positive individuals having more prominent phenotypes. Second, most reports have combined different mutations in their analysis, whereas our study population all shared the same MYBPC3 gene mutation. We also cannot exclude that the specific mutation affected this particular Amish population may have a more benign clinical manifestation, even though homogenous mutations are generally lethal in early life and some individuals have echocardiographic characteristics (see Supplemental Files)14. It is therefore important to emphasize that TDI abnormalities observed in prior reports may represent only a proportion of sarcomeric gene mutation carriers, and absence of TDI abnormalities (or with lack of abnormal global longitudinal strain) do not preclude the presence of underlying sarcomeric gene mutations even in a young adult population.
Our study cohort did not identify any overt hypertrophic phenotypes in the majority of MYBPC3 mutation carriers. In particular, we identified lower percentages of echocardiographic abnormalities than reported in previous studies11, with only 6 of the initial 42 individuals screened (15%) showing any evidence of cardiac hypertrophy. While it is believed that 60% of patients with the MYBPC3 mutation will eventually develop some form of hypertrophy, there are many environmental and genetic factors that may contribute to incomplete penetrance of the gene mutation. Nevertheless in our G+/LVH− cohort, 11% showed unexplained stage I diastolic dysfunction, and an incomplete right bundle branch block or RSR’ was observed in 30% of subjects. Meanwhile in our G+/LVH−, TDI-derived Sa and Ea velocities were well above published normal values16 as well as above those observed in our healthy controls. Instead of implying preclinical diastolic dysfunction in this population, our observations may suggest that MYBPC3 mutation carriers in our cohort exhibit “supra-normal” myocardial contractile function, with regards to annular motion both in systole and diastole. This may paradoxically precede (or may even be independent of) the development of left ventricular hypertrophy and overt diastolic dysfunction, and consistent with the notion that compensatory mechanisms to sarcomeric mutations may play a role in the development of asymmetric hypertrophy.
Speckle tracking overcomes difficulties in angle dependence, and provides information on strain, a more direct measurement of myocardial deformation12. Prior strain studies have shown the presence of reduced septal strain being more pronounced in the mid-septum17, and reductions in global strain were observed in patients with overt HCM18. This is to our knowledge the first report to assess longitudinal strain for regional myocardial abnormalities in a homogenous asymptomatic carrier population with a single MYBPC3 mutation. Assessment of regional strain identified a significantly lower strain at the basal septum (despite being within the reported “normal range”18), and a significantly higher strain in the basal and mid posterior wall. These regional deformities occur in the approximate location where hypertrophy may most commonly manifest. It is interesting to note that the six patients identified with abnormal phenotypes showed marked reduced global strain, with an average value of 17.5 ±1.2%, with the lowest strain also noted at the basal septum (group mean 11.3 ±4.5%), also predominantly at the site of regional hypertrophy. The precise mechanism of MYBPC3 mutation leading to a regional abnormality of longitudinal strain remains unclear. Whether regions experiencing higher longitudinal strain occur as a cause or effect of adjacent basal septal deformity (with lower strain) remains to be determined and such observations may not be applicable to a broad heterogeneous HCM population. Even thought the absolute values of these measurements were different, true differences between the two groups may not be apparent in larger populations or in different mutation carriers. Further investigations are warranted to better explain why a genetic mutation implicated in all sarcomeric apparatus may lead to a regional hypertrophy and pathologic changes.
CONCLUSIONS
We observed minimal differences between phenotype-negative subjects with the MYBPC3 gene mutation without LVH and normal controls based on conventional 2D echocardiographic and tissue Doppler indices as well as with global longitudinal strain measurements. Our data do not support prior reports regarding the presence of subclinical echocardiography abnormalities found in phenotype-negative subjects.
Supplementary Material
Acknowledgments
FUNDING SOURCES This publication was made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH
Footnotes
DISCLOSURES No relationships to disclose for all authors related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marian AJ. Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet. 2000;355(9197):58–60. doi: 10.1016/s0140-6736(99)06187-5. [DOI] [PubMed] [Google Scholar]
- 2.Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hainque B, et al. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat Genet. 1995;11(4):438–40. doi: 10.1038/ng1295-438. [DOI] [PubMed] [Google Scholar]
- 3.Watkins H, Conner D, Thierfelder L, Jarcho JA, MacRae C, McKenna WJ, et al. Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy. Nat Genet. 1995;11(4):434–7. doi: 10.1038/ng1295-434. [DOI] [PubMed] [Google Scholar]
- 4.Niimura H, Bachinski LL, Sangwatanaroj S, Watkins H, Chudley AE, McKenna W, et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338(18):1248–57. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42(9):1687–713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 6.Michels M, Hoedemaekers YM, Kofflard MJ, Frohn-Mulder I, Dooijes D, Majoor-Krakauer D, et al. Familial screening and genetic counselling in hypertrophic cardiomyopathy: the Rotterdam experience. Neth Heart J. 2007;15(5):184–90. doi: 10.1007/BF03085978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho CY, Sweitzer NK, McDonough B, Maron BJ, Casey SA, Seidman JG, et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation. 2002;105(25):2992–7. doi: 10.1161/01.cir.0000019070.70491.6d. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Bachinski LL, Meyer D, Hill R, Zoghbi WA, Tam JW, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104(2):128–30. doi: 10.1161/01.cir.104.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagueh SF, Kopelen HA, Lim DS, Zoghbi WA, Quinones MA, Roberts R, et al. Tissue Doppler imaging consistently detects myocardial contraction and relaxation abnormalities, irrespective of cardiac hypertrophy, in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2000;102(12):1346–50. doi: 10.1161/01.cir.102.12.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serri K, Reant P, Lafitte M, Berhouet M, Le Bouffos V, Roudaut R, et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47(6):1175–81. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Michels M, Soliman OI, Kofflard MJ, Hoedemaekers YM, Dooijes D, Majoor-Krakauer D, et al. Diastolic abnormalities as the first feature of hypertrophic cardiomyopathy in Dutch myosin-binding protein C founder mutations. JACC Cardiovasc Imaging. 2009;2(1):58–64. doi: 10.1016/j.jcmg.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17(10):1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Afonso LC, Bernal J, Bax JJ, Abraham TP. Echocardiography in hypertrophic cardiomyopathy: the role of conventional and emerging technologies. JACC Cardiovasc Imaging. 2008;1(6):787–800. doi: 10.1016/j.jcmg.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Xin B, Puffenberger E, Tumbush J, Bockoven JR, Wang H. Homozygosity for a novel splice site mutation in the cardiac myosin-binding protein C gene causes severe neonatal hypertrophic cardiomyopathy. Am J Med Genet A. 2007;143A(22):2662–7. doi: 10.1002/ajmg.a.31981. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Sun JP, Lever HM, Popovic ZB, Drinko JK, Greenberg NL, et al. Use of strain imaging in detecting segmental dysfunction in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2003;16(3):233–9. doi: 10.1067/mje.2003.60. [DOI] [PubMed] [Google Scholar]
- 18.Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM, et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol. 2009;103(3):411–5. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.