Abstract
The National Comprehensive Cancer Network (NCCN) has published guidelines for hereditary breast and ovarian cancer syndrome (HBOCS) management. Little data exist on compliance with these guidelines among different physician specialties. We performed an on-line case-based survey by randomly sampling physicians from five specialties, Family Medicine (FM), Obstetrics and Gynecology (OG), General Surgery (GS), Internal Medicine (IM), and Hematology and Oncology (HO). The physicians (n = 225) were asked to provide HBOCS management of healthy women ages 40–42 in the presence of a familial BRCA1 mutation. For women negative for the BRCA1 mutation, 59% of the physicians recommended appropriate surveillance although with significant differences among specialties; P = 0.01. Using an aggregate screening intensity score, physicians clearly recommended more intense screening for mutation positive than negative women (P < 0.0001), but only 16% of physicians followed NCCN guidelines for BRCA1-positive women. Seventy-six percent of all physicians recommended breast MRI with significant variation among specialties ranging from 62% of FM to 89% of OG (P = 0.0020). Similarly, 63% of physicians recommended prophylactic oophorectomy, with 76 and 78% of GS and OG compared to 38% of IM (P < 0.0001) and 57% recommended prophylactic mastectomy ranging from 84% of HO to 32% of FM (P < 0.0001). Independent of specialty, respondents with BRCA testing experience recommended more intense management than those without; P = 0.021. Management recommendations of BRCA1 mutation carriers are not consistent with NCCN guidelines and vary by medical specialty and genetic testing experience. Targeted education of physicians by specialty is needed, so that optimal management is offered to these high-risk women.
Keywords: BRCA1, BRCA, Surveillance, Guidelines, Physicians
Introduction
Hereditary breast and ovarian cancer syndrome (HBOCS) is associated with mutations in two known tumor suppressor genes BRCA1 and BRCA2. Clinical testing for the BRCA1 and BRCA2 genes has been commercially available since 1995, and many thousands of women have undergone genetic testing to identify an inherited cancer predisposition. Mutation carrier status is important to determine further surveillance measures as well as management options. Recommendations for the clinical management of HBOCS are based on several key features of BRCA mutation carriers: (1) early onset of breast cancer, (2) increased risk of ovarian cancer, (3) risk of second primary breast cancer, and (4) risk for male breast cancer. Based on the best available evidence, the National Comprehensive Cancer Network (NCCN) has put forth practice guidelines for the appropriate management of BRCA1/2 mutation positive versus BRCA1/2 negative women (http://www.nccn.org; version 1.2010) [1] [2]. The following screening strategy for mutation-positive women is recommended for those who have not yet undergone risk-reducing surgery [3, 4].
Monthly breast self-examination (BSE) beginning at age 18 years
Clinical breast examination semi annually beginning at age 25 years
Annual mammography and breast MRI screening beginning at age 25 years or individualized based on the earliest age of onset in the family
Twice yearly ovarian cancer screening with ultrasound and serum CA-125 levels beginning at age 35 years
Benefits of risk-reduction surgeries and chemo-preventive therapies are also known and have become standard of care in the management of such patients. In both retrospective and prospective series, prophylactic mastectomy decreases the incidence of breast cancer by 90% or more in women at high risk [4, 5]. In women with BRCA1/2 mutations, bilateral oophorectomy reduces the risk of breast as well as ovarian cancer. The NCCN guidelines with regard to risk-reducing surgeries include [1]
Salpingo-oophorectomy, ideally between the ages of 35–40 years or upon completion of child bearing, or individualized based on earliest age of onset of ovarian cancer in the family [5, 6].
Discussion of risk-reducing mastectomy should be carried out on a case-by-case basis.
Although these guidelines have been available for many years, it is not clear as to what extent they are followed by physicians in clinical practice or how compliance may vary among different physician specialties. In this study, we present results from a survey of practicing physicians in Texas from five different specialties. We assessed their surveillance and management recommendations in simulated cases and assessed their experience with genetic testing in their practice. We report to what extent their recommendations were comparable to the NCCN guidelines.
Methods
Study participants and survey methods
This online survey of Texas physicians included sampling of 1000 practicing physicians from five specialties (200 physicians from each specialty were selected): Family Medicine (FM), General Surgery (GS), Internal Medicine (IM), Obstetrics and Gynecology (OG), and Hematology and Oncology (HO). Additional details about this survey are described in Plon et al. [7].
Survey instrument
The survey questionnaire consisted of four hypothetical case scenarios regarding genetic testing decisions and HBOCS cancer risk management of unaffected women ages 40–42 years in the presence of a relative affected with cancer. In this report, we focus on the two cases where healthy at-risk women were tested for the deleterious mutation in BRCA1 found in a first degree relative with cancer. Case 2 of survey: two daughters, ages 41 and 43 years, of a woman with ovarian cancer at age 65 years who is positive for the 4229delTG mutation in the BRCA1 gene. One daughter is found to be positive for the mutation while the other is negative. Case 4 of survey: 44-year-old daughter of a woman, who is positive for the Q1408X mutation and N810YVUS in the BRCA1 gene. The daughter was found to be positive for Q1408X and negative for VUS and 42-year-old niece of that woman was found to be negative for both BRCA1 changes. Only results from Case 2 are reported since the results of Case 4 were similar.
In each case the physician was provided the results of genetic testing for the at-risk relative and then asked the same set of cancer risk management questions (described below). In order to assess overall intensity of screening, we assigned a weighted score for each item. The questions and weighted score matrix are described in the Statistical analysis and Findings section.
Statistical analysis
Descriptive statistics were calculated to summarize the physicians’ choices to the survey questions and their characteristics. Chi-Square test was performed to examine the difference between the physician specialties and physicians’ BRCA1/2 testing experience. As described previously [7], we generate a HBOCS management intensity score by assigning points for the screening and surgery options selected and sum the total points for each case to develop a cancer risk management intensity score. However, in this report, we limited the items included in the score (Table 1) given that CA125 and transvaginal ultrasound are only recommended for women who decline oophorectomy and mastectomy should be discussed on a case-by-case basis. Therefore, we did not include those recommendations in this analysis, yielding a maximum score of 12. Distributions of scores for averages of positive cases and negative cases are shown graphically by histogram and kernel density curves and were compared by pairwise Wilcoxon signed-rank test. The difference between the scores of positive cases and negative cases was examined by general linear model with specialty and BRCA testing experience as the factors. The interaction term of specialty and testing experience was removed from the final model because it was not significant. P values of less than 5% were considered significant.
Table 1.
Cancer risk management questions included in the survey and the points allotted to each to generate HBOCS management intensity scores
| 6 months | 12 months | 24 months | Not appropriate | |
|---|---|---|---|---|
| Clinical breast exam | 3 |  |
1 | 0 |
| Mammogram | 3 |
|
1 | 0 |
| Breast MRI | 3 | 2 | 1 | 0 |
| Yes | No | |||
| Bilateral oophorectomy | 3 | 0 | ||
| Maximal Score | 12 | |||
| NCCN Score for BRCA1 negative |

|
|||
| NCCN Score for BRCA1 positive | 10 |
Role of the funding source
NHGRI supported the faculty, staff and costs of carrying out the online survey, the $50 incentive for physicians to complete the survey, and the analysis of the data. The funders had no role in the design of the study, data analysis, and interpretation in the writing of this report or the decision to submit for publication.
Findings
Physicians were randomly selected for participation from those for whom an email address was available in the Texas Medical Association database. Invitations were mailed to 200 individuals from each specialty group, and the response rate for all five specialty group samples combined was 23%. As described previously (Table 3 in previous publication by Plon et al.), detailed demographic and practice environment data were available for both responders and non-responders. Comparisons of the two groups on nine demographic variables revealed a significant difference only in the mean years in practice (14.2 years for responders vs. 16.7 years for non-responders; Kruskal–Wallis rank sum, P = 0.004).
Table 3.
Recommendations for breast MRI, prophylactic mastectomy, and prophylactic oophorectomy for BRCA-positive cases, based on having ever ordered the BRCA test versus not
| Ever ordered BRCA1/2 testing | |||
|---|---|---|---|
| Yes (n = 81) (%) |
No (n = 144) (%) |
P value* | |
| Clinical breast exam | 99 | 98 | – |
| Mammogram | 94 | 96 | – |
| Breast MRI | 83 | 73 | 0.015 |
| Bilateral mastectomy | 74 | 47 | <0.0001 |
| Bilateral oophorectomy | 78 | 54 | <0.0001 |
P values were calculated from Chi-square test
For each case, the physician was first provided the results of comprehensive BRCA1/2 genetic testing for the relative with cancer and then provided the results of genetic testing for the healthy at-risk relative (ages 40–43) with regard to whether the at-risk relative did or did not carry the deleterious BRCA1 mutation (test positive) found in the cancer patient. For each relative, the physicians were asked to make recommendations for management of the healthy woman’s cancer risk including surveillance and prophylactic surgery.
As described in methods, we generated a limited HBOCS management intensity score by summing the assigned points for the most straightforward screening and surgery options in the NCCN guidelines; clinical breast exam, mammography, breast MRI, and prophylactic oophorectomy (Table 1). Based on NCCN guidelines, for a woman found to carry a deleterious BRCA1 mutation, the optimal score was 10 and 4 for a non-carrier as screening is similar to general population recommendations predominantly focusing on routine annual mammography and annual clinical breast exam (Table 1).
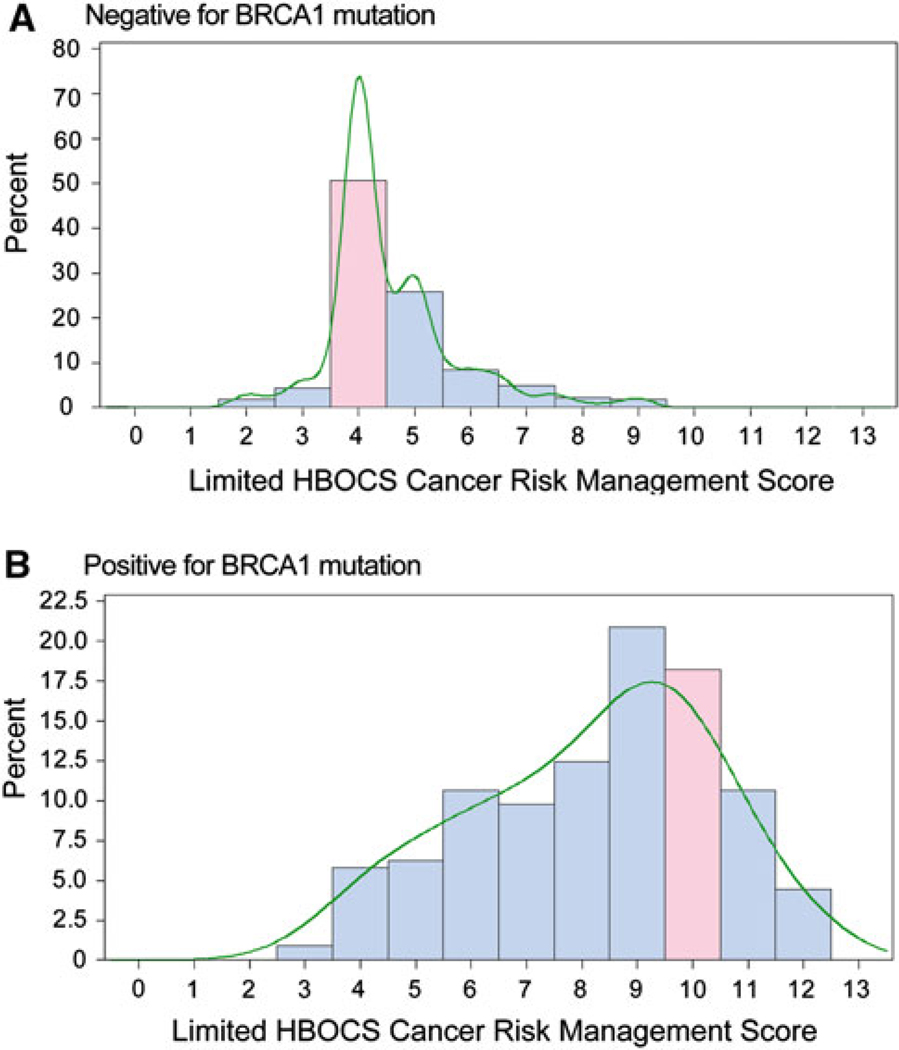
For the healthy daughter found to be negative for the deleterious BRCA1 mutation, 59% of the physicians recommended surveillance as set forth by NCCN (Fig. 1a). There were significant differences among specialties; only 43% of GS and HO physicians recommended appropriate surveillance compared to 75% of OG, 64% of FM, and 60% of IM (Chi-Square test, P = 0.01). This likely reflects that HO and GS typically manage high-risk patients.
Fig. 1.
Distribution of HBOCS surveillance scores from all respondents using the weighted screening score for healthy women in their early forties who are a negative and b positive for the BRCA1 mutation found in their mother. The score in pink is consistent with NCCN guidelines. The curves show the non-parametric kernel density estimation
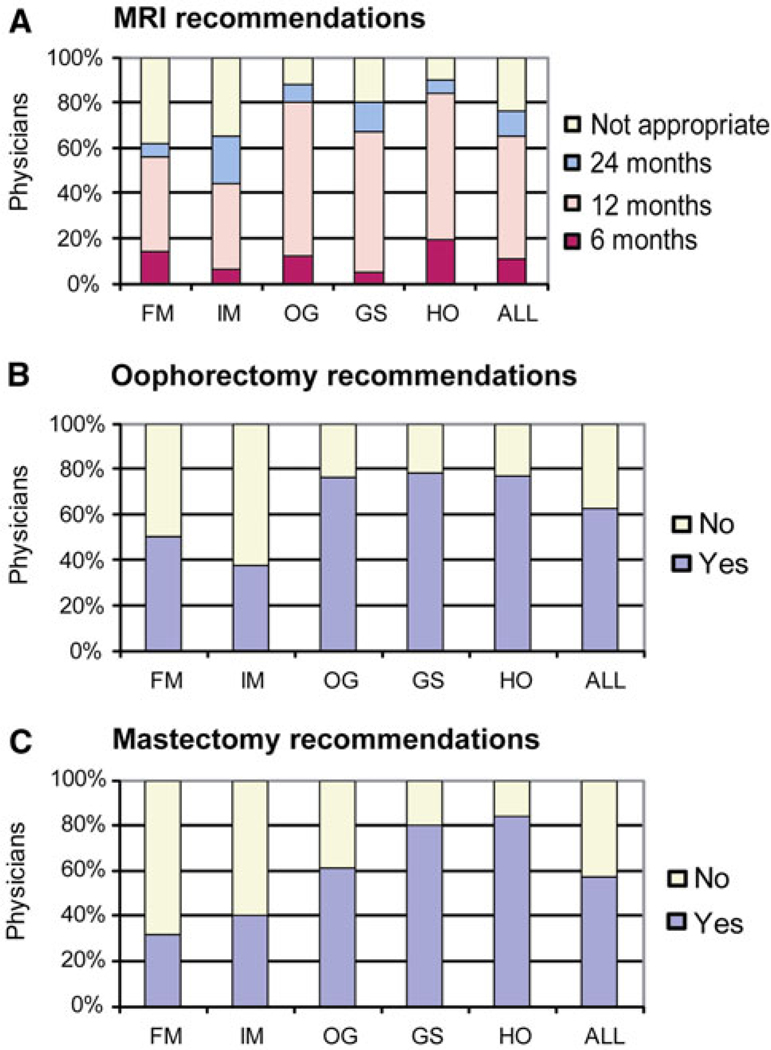
Compared to the daughter with a negative result the intensity of screening was clearly increased for the daughter found to carry the deleterious BRCA1 mutation with a median of 9 (range 2–12) (Wilcoxon signed-rank test, P < 0.0001) (Fig. 1b). However, only approximately 16% (n = 37) of physicians made recommendations consistent with the NCCN guidelines. Overall, IM and FM recommended less intense screening. For example, only 65% of IM and 62% of FM recommended breast MRI for mutation-positive women compared to 89% of OG and 90% of HO (Chi-Square test, P < 0.0001) (Fig. 2a). With regard to recommendations for prophylactic surgery 78, 77, and 76% of the GS, HO, and OG specialty groups respectively, recommended prophylactic oophorectomy compared with 38% of IM and 50% of FM (Chi-Square test, P < 0.0001) (Fig. 2b). Similar differences among specialties were seen for prophylactic mastectomy recommendations (Fig. 2c).
Fig. 2.
Recommendations for a breast MRI, b prophylactic salpingooophorectomy, and c prophylactic mastectomy for BRCA1 mutation-positive individuals, by different specialties
One explanation for the differences among specialties in screening recommendations may relate to the physician experience with genetic testing in their practice. After completing the questions with regard to the cases each physician was asked about whether they had ever ordered BRCA1/2 testing and approximately how many times in the last 6 months. IM and FM physicians were less likely to have ordered BRCA testing in their own practice (Chi-Square test, P < 0.0001) (Table 2). We then analyzed the management recommendations based on experience and specialty. Overall, physicians who had ordered BRCA testing in their practice recommended more intense management of mutation-positive women than those that had not (general linear model, P = 0.021). Regardless of testing experience, FM and IM still recommended less intense management of mutation-positive women (contrast in general linear model, P = 0.013). Bilateral mastectomy was recommended by 74% of physicians who had ever ordered BRCA testing compared to 47% who had never ordered the test (P < 0.0001) (Table 3). Similarly, 78% of physicians who had ordered BRCA testing had recommended bilateral oophorectomy compared to 54% of those that had never ordered the test (P < 0.0001). No significant differences in recommendations for breast MRI were found among physicians who had ordered the test versus those that had not. This demonstrates that physicians who have experience in ordering the BRCA test were also more comfortable in recommending life altering management options such as prophylactic surgeries.
Table 2.
Distribution of physician specialties response to question about ever having ordered BRCA testing
| Ever ordered BRCA | FM (%) | IM (%) | OG (%) | GS (%) | HO (%) | ALL (%) | P value |
|---|---|---|---|---|---|---|---|
| Yes | 16 | 10 | 49 | 36 | 87 | 36 | <0.0001 |
| No | 84 | 90 | 51 | 64 | 13 | 64 |
Discussion
Genetic testing for BRCA1 and BRCA2 mutations is widely accepted as standard of care in patients with known or suspected diagnosis of HBOCS, and cancer risk management strategies have been established for mutation carriers. We surveyed Texas physicians from five specialty groups as to their recommendations for cancer risk management of unaffected women who have undergone testing for a deleterious BRCA1 mutation found in a close family member with cancer. The response rate in this survey was low, which we understand is one limitation of this study; however, as previously described we have detailed information on both responders and non-responders and found only time in practice as significantly different [8]. The physician respondents are highly representative of the physicians in these specialty groups in the Texas Medical Association database. The respondents were clinically active physicians participating in direct patient care.
Not surprisingly physicians clearly recommended more intense cancer screening when a woman was found to carry a BRCA1 deleterious mutation, compared to a non-carrier, consistent with the increased risk of early-onset cancer. However, when each physician response was analyzed individually, only 17% of the physicians followed all the NCCN recommendations. It is important to note that the degree of intensity was clearly a function of physician specialty. The current NCCN recommendations for breast MRI [2] have also been adopted by other organizations including the American Cancer Society who recommend annual MRI, in addition to mammography for screening women with a 20–25% or greater lifetime risk of breast cancer from the age of 30 years [3]. The National Institute for Health and Clinical Excellence has published guidelines that advocate breast MRI for those women with a strong family history or those with BRCA1/2 mutations, as clinical breast exams and conventional imaging techniques such as mammography and breast USG are less effective in this group [9]. Thus, despite the consensus of recommendations for breast MRI as a breast cancer screening modality based on both family history and genetic testing status, it is disconcerting that a significantly lower percentage of FM and IM physicians recommended breast MRI for a BRCA1 mutation carrier compared with other specialties. Given that FM/IM physicians often recommend breast cancer surveillance for their patients there is a need for better education on the indications for breast MRI. The FM/IM specialty groups were also less likely to recommend prophylactic salpingo-oophorectomy for mutation carriers, another NCCN recommendation.
Older studies carried out when BRCA testing was first available demonstrated that few non-geneticist physicians have discussed or ordered BRCA testing [10, 11]. As we found, oncologists are more likely than primary care specialists (IM and FM) to have discussed and/or ordered BRCA testing [12–14]. In another study, 38% of oncologists had ever ordered a BRCA test compared with only 20% of OG and 11% of IM [8]. However, it is important to note that 36% of the randomly selected physicians in this study who were surveyed in late 2008 and early 2009 had ordered BRCA1/2 testing in their own practice. A similar survey performed by our group in late 2009 found that 43% had ordered testing (data not shown). There is clearly increased ordering of genetic testing by physicians without genetics specialty training. Physicians who have ordered the test in their practice might be assumed to be more aware and cognizant of management strategies and as shown in Table 3, breast MRI and prophylactic surgeries were recommended significantly more by physicians familiar with the test. In some cases, physicians with testing experience recommended more intense management than was recommended by the NCCN guidelines, e.g., with regard to frequency of breast MRI. Conversely among physicians without testing experience a small subset recommended surveillance of a BRCA1-positive woman comparable to general population recommendations.
Other studies have demonstrated that risk-reducing surgeries such as prophylactic mastectomies and salpingooophorectomies are less accepted than other interventions. In a French study of surgeons and gynecologists and obstetricians’ attitudes only 10.9 and 22.9% said they would find it acceptable to recommend mastectomy and oophorectomy respectively [15]. In one US survey of surgeons, 85% of plastic surgeons compared to 47% of general surgeons and 38% of gynecologists agreed that prophylactic mastectomy had a role in the care of high-risk women [16]. Prophylactic oophorectomy is more widely accepted than mastectomy [17, 18]. We found that recommendations for prophylactic surgery are not limited to the physician groups performing the operation as GS and OG were second to HO in recommending prophylactic surgeries potentially reflecting experience and/or specialized knowledge of the HO physicians with the NCCN guidelines [19, 20].
Genetics knowledge is not uniform across medical specialties. The greatest knowledge about specific pathologies is found among physicians caring for affected persons [21]. Several studies have shown that specialists were more knowledgeable about cancer genetics than general and family practitioners [22]. Consistent with our findings on genetic testing experience, Doksum et al. [8] showed that knowledge of genetics of breast cancer was greater among oncologists who were familiar with the test compared to oncologists who had never discussed the test. On the other hand, direct to consumer marketing has increased patient awareness about HBOCS and may lead to an increasing burden on FM and IM who are faced with patient inquiries from healthy at-risk relatives of cancer patients about testing and the appropriate cancer risk-reduction strategies. This study demonstrates that these two specialty groups are likely to recommend less intense risk-reduction strategies even in the face of a BRCA1-positive test compared to national guidelines.
Knowledge of national guidelines and physician recommendation is important for optimal patient care. In one recent report, lack of physician recommendation was the most frequently cited reason by at-risk women for not having surveillance procedures [23]. Our study also portrays varying concordance of recommendations with NCCN guidelines among physician specialties with less intense management by FM and IM physicians and more intense cancer risk management (such as semi-annual breast MRI or mammograms) by other specialties. There is a need for a large educational effort to prepare non-geneticist physicians for the challenges as genetic testing becomes available in many different areas of medicine [24, 25]. This study highlights that there is a need to tailor this physician education to each specialty so that educational issues can be addressed that allows the integration of genetic testing and appropriate subsequent cancer risk management into clinical practice.
Footnotes
Financial disclosures None reported.
Contributor Information
S. U. Dhar, Department of Molecular & Human Genetics, Baylor College of Medicine, Houston, TX, USA
H. P. Cooper, Department of Medicine, Baylor College of Medicine, Houston, TX, USA
T. Wang, Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA
B. Parks, Department of Molecular & Human Genetics, Baylor College of Medicine, Feigin Center, Room 1200.18, 1102 Bates Street, Houston, TX 77030, USA
S. A. Staggs, Physician Oncology Education Program, Texas Medical Association, Austin, TX, USA
S. Hilsenbeck, Department of Medicine, Baylor College of Medicine, Houston, TX, USA Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA.
S. E. Plon, Email: splon@bcm.edu, Department of Molecular & Human Genetics, Baylor College of Medicine, Feigin Center, Room 1200.18, 1102 Bates Street, Houston, TX 77030, USA; Dan L. Duncan Cancer Center, Baylor College of Medicine, Houston, TX, USA; Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
References
- 1.Daly MB, Axilbund JE, Buys S, Crawford B, Farrell CD, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 2.Burke W, Daly M, Garber J, Botkin J, Kahn MJ, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA. 1997;277:997–1003. [PubMed] [Google Scholar]
- 3.Saslow D, Boetes C, Burke W, Harms S, Leach MO, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 4.Scheuer L, Kauff N, Robson M, Kelly B, Barakat R, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–1268. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 5.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van‘t Veer L, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 7.Plon SE, Cooper PH, Parks B, Dhar S, Kelly A, Weinberg A, Staggs S, Wang T, Hilsenbeck S. Genetic testing and cancer risk management recommendations by Physicians for at risk relatives. Genet Med. 2011;13(2):148–154. doi: 10.1097/GIM.0b013e318207f564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doksum T, Bernhardt BA, Holtzman NA. Does knowledge about the genetics of breast cancer differ between nongeneticist physicians who do or do not discuss or order BRCA testing? Genet Med. 2003;5:99–105. doi: 10.1097/01.GIM.0000055198.63593.32. [DOI] [PubMed] [Google Scholar]
- 9.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, et al. Efficacy of MRI and mammography for breastcancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 10.Rowley PT, Loader S. Attitudes of obstetrician–gynecologists toward DNA testing for a genetic susceptibility to breast cancer. Obstet Gynecol. 1996;88:611–615. doi: 10.1016/0029-7844(96)00199-8. [DOI] [PubMed] [Google Scholar]
- 11.O’Malley MS, Klabunde CN, McKinley ED, Newman B. Should we test women for inherited susceptibility to breast cancer? What do NC primary care physicians think. N C Med J. 1997;58:176–180. [PubMed] [Google Scholar]
- 12.Cho MK, Sankar P, Wolpe PR, Godmilow L. Commercialization of BRCA1/2 testing: practitioner awareness and use of a new genetic test. Am J Med Genet. 1999;83:157–163. doi: 10.1002/(sici)1096-8628(19990319)83:3<157::aid-ajmg4>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polednak AP. Do physicians discuss genetic testing with family-history-positive breast cancer patients? Conn Med. 1998;62:3–7. [PubMed] [Google Scholar]
- 14.Wilkins-Haug L, Hill LD, Power ML, Holzman GB, Schulkin J. Gynecologists’ training, knowledge, and experiences in genetics: a survey. Obstet Gynecol. 2000;95:421–424. doi: 10.1016/s0029-7844(99)00581-5. [DOI] [PubMed] [Google Scholar]
- 15.Julian-Reynier C, Eisinger F, Moatti JP, Sobol H. Physicians’ attitudes towards mammography and prophylactic surgery for hereditary breast/ovarian cancer risk and subsequently published guidelines. Eur J Hum Genet. 2000;8:204–208. doi: 10.1038/sj.ejhg.5200418. [DOI] [PubMed] [Google Scholar]
- 16.Houn F, Helzlsouer KJ, Friedman NB, Stefanek ME. The practice of prophylactic mastectomy: a survey of Maryland surgeons. Am J Public Health. 1995;85:801–805. doi: 10.2105/ajph.85.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisinger F, Stoppa-Lyonnet D, Lasset C, Vennin P, Chabal F, et al. Comparison of physicians’ and cancer prone women’s attitudes about breast/ovarian prophylactic surgery. Results from two national surveys. Fam Cancer. 2001;1:157–162. doi: 10.1023/a:1021113715998. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe KA, Lubinski J, Ghadirian P, Lynch H, Kim-Sing C, et al. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J Clin Oncol. 2008;26:1093–1097. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- 19.Patani N, Mokbel K. The utility of MRI for the screening and staging of breast cancer. Int J Clin Pract. 2008;62:450–453. doi: 10.1111/j.1742-1241.2007.01677.x. [DOI] [PubMed] [Google Scholar]
- 20.Granader EJ, Dwamena B, Carlos RC. MRI and mammography surveillance of women at increased risk for breast cancer: recommendations using an evidence-based approach. Acad Radiol. 2008;15:1590–1595. doi: 10.1016/j.acra.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Hunter A, Wright P, Cappelli M, Kasaboski A, Surh L. Physician knowledge and attitudes towards molecular genetic (DNA) testing of their patients. Clin Genet. 1998;53:447–455. doi: 10.1111/j.1399-0004.1998.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 22.Wideroff L, Vadaparampil ST, Greene MH, Taplin S, Olson L, et al. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42:749–755. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loescher LJ, Lim KH, Leitner O, Ray J, D’Souza J, et al. Cancer surveillance behaviors in women presenting for clinical BRCA genetic susceptibility testing. Oncol Nurs Forum. 2009;36:E57–E67. doi: 10.1188/09.onf.e57-e67. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JC, Brown JB, Blaine S, Glendon G, Pugh P, et al. Genetic susceptibility to cancer. Family physicians’ experience. Can Fam Physician. 2003;49:45–52. [PMC free article] [PubMed] [Google Scholar]
- 25.Watson EK, Shickle D, Qureshi N, Emery J, Austoker J. The ‘new genetics’ and primary care: GPs’ views on their role and their educational needs. Fam Pract. 1999;16:420–425. doi: 10.1093/fampra/16.4.420. [DOI] [PubMed] [Google Scholar]