Abstract
Background: Despite the well-known recidivism of obesity, surprisingly little is known about the composition of body weight during weight regain.
Objective: The objective of this study was to determine whether the composition of body weight regained after intentional weight loss is similar to the composition of body weight lost.
Design: The design was a follow-up to a randomized controlled trial of weight loss in which body composition was analyzed and compared in 78 postmenopausal women before the intervention, immediately after the intervention, and 6 and 12 mo after the intervention.
Results: All body mass and composition variables were lower immediately after weight loss than at baseline (all P < 0.05). More fat than lean mass was lost with weight loss, which resulted in body-composition changes favoring a lower percentage of body fat and a higher lean-to-fat mass ratio (P < 0.001). Considerable interindividual variability in weight regain was noted (CV = 1.07). In women who regained ≥2 kg body weight, a decreasing trend in the lean-to-fat mass ratio was observed, which indicated greater fat mass accretion than lean mass accretion (P < 0.001). Specifically, for every 1 kg fat lost during the weight-loss intervention, 0.26 kg lean tissue was lost; for every 1 kg fat regained over the following year, only 0.12 kg lean tissue was regained.
Conclusions: Although not all postmenopausal women who intentionally lose weight will regain it within 1 y, the data suggest that fat mass is regained to a greater degree than is lean mass in those who do experience some weight regain. The health ramifications of our findings remain to be seen.
INTRODUCTION
Many health complications associated with overweight and obesity are improved with weight loss. However, negative consequences (such as loss of muscle mass and bone density) are also associated with weight loss and are detrimental for older adults, which results in a reluctance to recommend intentional weight loss in this population (1–3). Notably, caloric restriction simultaneously results in reductions in both fat and lean tissue, with lean mass loss ranging from 14% to 23% of total weight lost (4). Because lean mass loss in older adults may be associated with the development of adverse health events and disability (5, 6), it is important to examine whether the benefits of weight loss outweigh the risks in this population.
Another factor complicating the promotion of weight loss among older adults is that weight regain is an intractable problem. Only 20% of people in the general population are successful at long-term weight-loss maintenance (7). Most individuals who voluntarily lose weight regain ≈30% of their lost weight within 1 y, and ≈95% within 5 y (8), independent of initial weight loss (9). Although therapeutic factors in the long-term treatment of obesity have been identified (10), efforts to maintain clinically significant weight loss (11) remain largely unsuccessful. Therefore, for most, weight regain in the setting of intentional weight loss is an expected occurrence.
Given the well-known recidivism of obesity, surprisingly little is known about the composition of body weight with weight regain. Most studies on weight-reduction interventions have relatively short follow-up periods and do not include information on body composition. As such, there is limited and conflicting information on changes in body composition with weight regain after successful weight loss. Data from a small prospective study in obese, middle-aged women found that 4 y after a successful weight-loss intervention, mean overall weight was not significantly different from baseline; however, lean mass was significantly greater than the mean baseline value (12). In contrast, an observational study showed that, among community-dwelling older adults, significantly more lean mass was lost with overall weight loss than was gained with overall weight gain over 2- and 4-y periods, both as a function of age (13) and weight cycling (14). Given these discrepant results, and the lack of knowledge regarding body-composition changes with weight regain in older adults, the purpose of this study was to examine whether the composition of body weight gained in the 6 and 12 mo subsequent to a successful weight-loss intervention is similar to the composition of weight lost in postmenopausal women. A secondary purpose was to examine whether changes in body-fat distribution, measured via waist and hip size, were similar during weight loss and weight regain.
SUBJECTS AND METHODS
Design overview
This article presents data from a randomized clinical trial originally designed to determine whether the intensity of aerobic exercise affects the loss of abdominal adipose tissue in abdominally obese postmenopausal women, under controlled conditions of isocaloric deficit. Study design and recruitment details were previously published (15). Briefly, this study was a 5-mo trial that compared the following treatment arms: 1) caloric restriction alone, 2) caloric restriction plus moderate-intensity aerobic exercise, and 3) caloric restriction plus vigorous-intensity exercise. Women from Forsyth County, NC, and the surrounding areas were recruited through local advertisements and were enrolled on the basis of the following criteria: 1) abdominal obesity [BMI (in kg/m2): 25–40; and waist circumference >88 cm], 2) age (50–70 y), 3) postmenopausal status (no menses for >1 y), 4) nonsmoking (for >1 y), 5) not receiving hormone replacement therapy, 6) sedentary (<15 min exercise 2 times/wk in the past 6 mo), and 7) weight stable (<5% weight change) for ≥6 mo before enrollment. After the calorie-restriction interventions, women were asked to return for 6- and 12-mo follow-up visits to monitor changes in body weight, composition, and fat distribution. No contact by study personnel was made during this interim period. The study was approved by the Wake Forest University Institutional Review Board, and all participants signed university approved informed consent documents.
Weight-loss interventions and follow-up
The weight-loss interventions were previously described in detail (15). Briefly, all women in the study underwent caloric restriction for 5 mo. The caloric restriction consisted of a controlled program of underfeeding during which all food was prepared and provided at individual calorie levels by our metabolic kitchen. Individual energy needs were calculated from each participant's measured resting metabolic rate and an activity factor based on self-reported daily activity. The degree of caloric restriction was adjusted so that total caloric deficit (∼400 kcal/d; 2800 kcal/wk; ∼1675 kJ/d; 11,725 kJ/wk) was similar for all groups. All exercise sessions (3 d/wk) took place in our supervised center.
At the end of the 5-mo weight-loss intervention, women were asked to return for anthropometric and body-composition measures 6 and 12 mo after the intervention (11 and 17 mo, respectively). The women were provided with one counseling session with a registered dietitian concerning strategies for maintenance of weight loss at the end of the 5-mo weight-loss intervention. With the exception of scheduling phone calls and the 6- and 12-mo follow-up visits, no other contact by study-related personnel was provided during the 12-mo follow-up period. A total of 95 women completed their assigned 5-mo weight-loss intervention, and 78 and 73 women returned for the 6- and 12-mo follow-up visits, respectively.
Body weight, composition, and fat-distribution measures
Height and weight were measured with shoes and outer garments removed. BMI was calculated as weight (in kg) divided by the square of height (in m). Whole-body fat mass, lean mass, and percentage body fat were measured by dual-energy X-ray absorptiometry (Hologic Delphi A 11.0 QDR; Hologic), and the lean-to-fat mass ratio was calculated. Waist (minimal circumference) and hip (maximal gluteal protuberance) were measured in triplicate, and the waist-to-hip-ratio (WHR) was calculated. All measurements were performed at baseline, immediately after the intervention, and 6 and 12 mo after the intervention.
Statistical analysis
All numerical summaries and statistical comparisons were conducted by using SAS version 9.2 (SAS Institute Inc), and the type I error rate (α) was held constant at 0.05 for all tests. Data are reported as means ± SDs. Absolute changes in body weight, composition, and fat-distribution variables were calculated as the baseline value subtracted from the follow-up values (5, 11, and 17 mo). Differences between body weight, composition, and fat-distribution data over time were determined with paired t tests. Distributions of individual changes in body weight during the follow-up periods were plotted as frequency histograms.
The primary purpose of this study was to assess the relative changes in body composition in response to weight regain after a weight-loss intervention. To this end, the mean change in lean-to-fat mass ratios during the time of the weight-loss intervention and after the intervention were modeled by using a repeated-measures analysis. Age, intervention group, race, and baseline BMI were included as covariates in the model. The model also contained time-period indicator variables for the 5 mo of intervention and the 6- and 12-mo postintervention periods. The change in lean-to-fat mass ratio served as the outcome measure and was defined as the difference between the 5 mo and baseline ratio, and the difference between both postintervention ratios and the 5-mo ratio.
To have comparable time frames, the 5-mo weight-loss period and the first 6 mo after the intervention were the primary foci of the study. The 12-mo postintervention time point was a secondary interest, and those analyses used 68 subjects with complete baseline and 5-, 11-, and 17-mo data. However, only the 78 subjects with complete baseline and 5- and 11-mo body-composition data were included in the model. With the use of the time-indicator variables in the model, least-squares means were calculated to estimate the change in lean-to-fat mass ratios for the 3 time periods. Statistical comparisons tested whether time-period specific lean-to-fat mass ratios were different from zero and whether body-composition changes during the 6- and 12-mo follow-up periods differed from the changes during the weight-loss phase. Participants were then stratified by weight-regain status, where weight regain was defined as a gain ≥2 kg from the postintervention time point measured at either 6 or 12 mo, and the analyses were rerun. As a secondary analysis on those subjects with weight regain, univariate linear regression models were run to assess the relation between the changes in lean and fat mass during and 12 mo after the intervention.
RESULTS
Main results from the 5-mo weight-loss intervention were previously published (15). Briefly, average weight loss for the 95 women who completed the study was 12.1 ± 4.5 kg (13.4 ± 4.6%) and was not significantly different between intervention groups. Both fat and lean mass decreased significantly with each intervention (P < 0.001), and the absolute decrease in lean mass was similar between groups.
The results provided here are from the subset of participants who completed the follow-up portion of the study (n = 78 and 68 for the 6- and 12-mo follow-up visits, respectively). Mean (±SD) changes in body weight, composition, and fat distribution with weight loss and during follow-up to weight loss are presented in Table 1. All body mass and composition variables were significantly improved immediately after weight loss compared with baseline (all P < 0.05). On average, the weight-loss interventions reduced body weight by 11.55 ± 4.13 kg (12.9 ± 4.2%; P < 0.001), fat mass by 8.19 ± 3.29 kg (20.7 ± 7.9%; P < 0.001), and lean mass by 3.65 ± 1.90 kg (7.0 ± 3.5%; P < 0.001). More fat than lean mass was lost with weight loss, which resulted in body-composition changes favoring a lower percentage of body fat and a higher lean-to-fat mass ratio (P < 0.001). Both waist and hip circumferences were reduced with weight loss (both P < 0.05) and weight loss reduced WHR (P = 0.007), which indicated greater declines in waist than hip girth.
TABLE 1.
Changes (Δ) in body weight, composition, and fat distribution after the 5-mo weight-loss intervention and after 6 and 12 mo of follow-up1
| Variable | Baseline(n = 78) | After weight loss(5 mo; n = 78) | Δ5 mo − baseline | After 6 mo of follow-up(11 mo; n = 78) | Δ11 mo − baseline | After 12 mo of follow-up(17 mo; n = 68) | Δ17 mo − baseline |
| Weight (kg) | 89.76 ± 11.15 | 78.21 ± 10.57 | −11.55 ± 4.13 | 79.93 ± 12.22 | −9.83 ± 7.12 | 81.15 ± 13.49 | −8.12 ± 8.67 |
| BMI (kg/m2) | 33.35 ± 3.79 | 29.06 ± 3.63 | −4.29 ± 1.52 | 29.70 ± 4.29 | −3.65 ± 2.60 | 30.29 ± 4.71 | −3.03 ± 3.17 |
| Fat mass (kg) | 39.55 ± 6.82 | 31.35 ± 6.61 | −8.19 ± 3.29 | 32.32 ± 8.07 | −7.22 ± 5.78 | 33.87 ± 8.98 | −5.65 ± 6.70 |
| Lean mass (kg) | 52.22 ± 5.71 | 48.57 ± 5.63 | −3.65 ± 1.90 | 48.81 ± 5.81 | −3.42 ± 2.12 | 48.45 ± 5.92 | −3.36 ± 2.82 |
| Lean-to-fat mass ratio | 1.34 ± 0.18 | 1.60 ± 0.30 | 0.25 ± 0.17 | 1.59 ± 0.40 | 0.24 ± 0.32 | 1.51 ± 0.40 | 0.18 ± 0.30 |
| Body fat (%) | 42.91 ± 3.28 | 38.94 ± 4.15 | −3.97 ± 2.20 | 39.33 ± 5.01 | −3.58 ± 3.87 | 40.39 ± 5.26 | −2.67 ± 4.16 |
| Waist girth (cm) | 97.93 ± 8.31 | 88.66 ± 8.61 | −9.27 ± 4.42 | 89.97 ± 10.33 | −8.03 ± 5.59 | 89.91 ± 10.97 | −7.45 ± 6.77 |
| Hip girth (cm) | 118.78 ± 9.06 | 109.10 ± 9.07 | −9.68 ± 4.47 | 110.73 ± 10.33 | −8.12 ± 7.05 | 112.19 ± 11.40 | −6.83 ± 8.25 |
| WHR | 0.83 ± 0.07 | 0.81 ± 0.06 | −0.01 ± 0.04 | 0.81 ± 0.07 | −0.01 ± 0.04 | 0.80 ± 0.06 | −0.02 ± 0.04 |
All values are means ± SDs. WHR, waist-hip ratio. n = 77 for waist girth, hip girth, and WHR at 11 mo and subsequent calculations; n = 65 for waist girth, hip girth, and WHR at 17 mo and subsequent calculations. Differences between body weight, composition, and fat-distribution data over time were determined with paired t tests. All body mass and composition variables were significantly improved immediately after weight loss compared with baseline and remained significantly improved during the 12-mo follow up period (all P < 0.05).
Body weight changes during follow-up
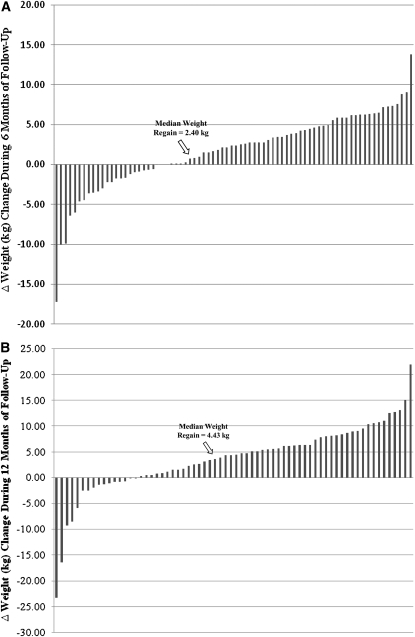
Weight regain did not vary by intervention group (P = 0.11). On average, lost body weight was regained during the 6 mo (+1.72 ± 4.77 kg) and 12 mo (+3.65 ± 6.78 kg) of follow-up, but was still significantly lower than baseline (by 11% and 9% after 6 and 12 mo, respectively; Table 1). However, considerable individual variability in weight regain was observed, as noted in Figure 1. Fifty-three of 78 (68%) women at the 6-mo follow-up and 52 of 68 (76%) women at the 12-mo follow-up had regained some of their weight lost during the intervention. Eleven women (16%) weighed more at the 12-mo follow-up than they did at baseline, and 16 (24%) women continued losing weight after the intervention. Moreover, 75% of those who regained weight gained >2 kg at the 6-mo follow-up, and this number increased to 84% at the 12-mo follow-up. This subgroup of “regainers” (ie, women who gained ≥2 kg during the follow-up period) was subsequently used to examine our question of whether lost lean mass from intentional weight loss was recovered during weight regain (see below).
FIGURE 1.
Distribution of individual changes in body weight over 6 (A) and 12 (B) mo of follow up to the 5-mo weight-loss intervention (n = 78 and 68, respectively). The average (±SD) weight changes were +1.72 ± 4.77 and +3.65 ± 6.78 kg during the first 6 and 12 mo of follow-up, respectively.
Changes in body composition during follow-up
Overall, lean mass did not significantly change during the follow-up period and, at 12-mo of follow-up, was still lower than baseline (P < 0.001). On the other hand, average fat mass was higher at both 6 (P = 0.02) and 12 (P < 0.001) mo than at the postintervention time point; however, fat mass was also still lower than baseline (−18.4 ± 13.8% and −14.4 ± 15.5% at 6 and 12 mo, respectively). Accordingly, by 12 mo, the lean-to-fat mass ratio had decreased significantly (P = 0.01) from the postintervention time point, but was still significantly greater than that at baseline (P < 0.001).
To determine whether body composition changed similarly during weight loss and during follow-up to weight loss (eg, whether the amount of lean mass changed with respect to fat mass), we conducted a repeated-measures analysis modeling the ratio of lean mass to fat mass over time. Adjustment was made for age, group, race, and baseline BMI. The results showed a noticeable decreasing trend in this ratio overall (Table 1), which indicated greater fat mass than lean mass accretion as time goes beyond the weight-loss intervention period. Moreover, the direction of this relation differs by weight-regain status (Table 2). In women who did not gain ≥2 kg (n = 24) in the year after the intervention, a positive increase for lean-to-fat mass ratio (least-squares mean > 0) was observed, whereas a decrease (least-squares mean < 0) was observed for women who gained ≥2 kg body weight (n = 54). This suggests that fat mass was increasing to a greater degree than was lean mass in women who regained weight during the postintervention period.
TABLE 2.
Adjusted changes in lean-to-fat mass ratio estimates during and 6 and 12 mo after the 5-mo weight-loss intervention1
|
P value |
||||
| Adjusted change in lean-to-fat mass ratio | LSM | SE | Baseline to 5 mo | H0: LSM = 0 |
| All | ||||
| Baseline to 5 mo | 0.25 | 0.03 | <0.001 | |
| 5–11 mo | −0.01 | 0.03 | <0.001 | 0.682 |
| 5–17 mo | −0.09 | 0.03 | <0.001 | 0.001 |
| Non–2-kg gainers (n = 24) | ||||
| Baseline to 5 mo | 0.31 | 0.06 | <0.001 | |
| 5–11 mo | 0.19 | 0.06 | 0.025 | 0.003 |
| 5–17 mo | 0.11 | 0.06 | 0.005 | 0.070 |
| ≥2-kg gainers (n = 54) | ||||
| Baseline to 5 mo | 0.23 | 0.02 | <0.001 | |
| 5–11 mo | −0.09 | 0.02 | <0.001 | <0.001 |
| 5–17 mo | −0.19 | 0.02 | <0.001 | <0.001 |
Values were adjusted for age, group, race, and BMI. In women who did not gain ≥2 kg body weight (n = 24), a greater positive direction for lean-to-fat mass ratio [least-squares mean (LSM) >0] was observed, whereas the less desirable direction (LSM <0) was observed for women who gained ≥2 kg body weight. The LSM was for adjusted change in the lean-to-fat mass ratio. H0, assumes the null hypothesis of no change in lean to fat ratio over time.
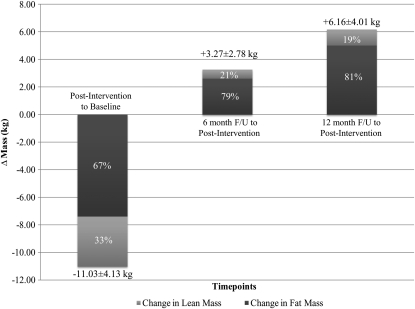
The composition of the weight lost during the 5-mo weight-loss interventions and the composition of the weight regained during follow-up to weight loss in the women who regained ≥2 kg body weight are shown in Figure 2. Of the weight lost during the intervention, 67% was fat and 33% was lean tissue. Of the weight regained during the first 6-mo of follow-up to weight loss, 79% was fat and 21% was lean tissue. During the entire 12 mo of follow-up, 81% of weight regained was fat and 19% was lean tissue. On average, by 12 mo after the intervention, 26% of fat mass lost was regained, whereas only 6% of lean mass lost was regained.
FIGURE 2.
Composition of weight lost during the 5-mo intervention and composition of weight regained over the 6- and 12-mo follow-up (F/U) period in women who regained ≥2 kg body mass. n = 54 at 5 mo after the intervention and at the 6-mo follow-up; n = 44 at the 12-mo follow-up.
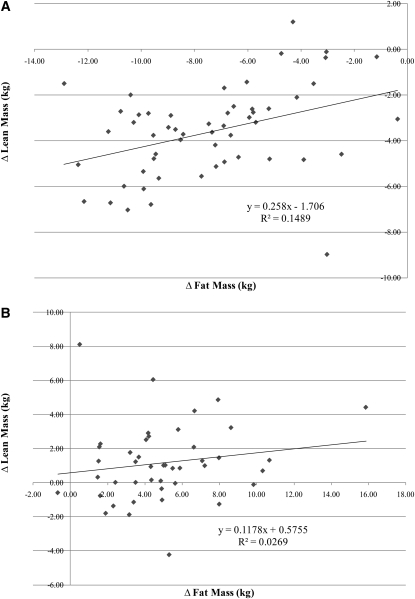
We also examined the relation between changes in lean mass and changes in fat mass during both weight loss and weight regain (n = 54; Figure 3). These changes were more strongly correlated during weight loss than during weight regain (r = 0.39 and r = 0.16, respectively). Furthermore, these data showed that for every 1 kg fat lost during the weight-loss intervention, 0.26 kg lean tissue was lost and that, for every 1 kg fat regained over the following year, only 0.12 kg lean tissue was regained (Figure 3). However, these results are only suggestive because the 2 slopes were not significantly different (P = 0.28).
FIGURE 3.
Relation of changes in lean and fat mass during the 5-mo weight-loss intervention (A) and during the 12-mo follow-up period to the weight-loss intervention (B) in women who regained ≥2 kg body mass. n = 54 at 5 mo after the intervention; n = 44 at the 12-mo follow-up.
Changes in body-fat distributions during follow-up
WHR was unchanged from after the intervention during the first 6 mo of follow-up (P = 0.91); however, WHR did decrease significantly by 12 mo (P = 0.03). The mean follow-up values were still significantly lower than the value at baseline (−1.4 ± 4.9% and −2.1 ± 5.2% at 6 and 12 mo, respectively). Waist and hip girth measures increased during the postintervention period, but these values remained significantly less than what was observed at baseline (all P < 0.001).
DISCUSSION
This study examined whether, and how, body-composition changes with weight regain in postmenopausal women. The novel findings were that, after significant (13%) weight reduction induced by adherence to a 5-mo hypocaloric diet, women who regained ≥2 kg lost weight had a reduced lean-to-fat mass ratio. This suggests that, for these women, fat mass was regained to a greater degree than was lean mass. Importantly, considerable variability was observed with weight regain status. Although more than two-thirds of women regained >2 kg during the 12-mo follow-up period, 84% weighed less at the 1-y follow-up than they did at baseline. In addition, although the average fat mass was higher at both 6 and 12 mo than at the postintervention time point, absolute fat mass was still lower han that at baseline.
Our results for the prevalence of weight regain are more encouraging than are other findings in the literature. In a 1999 study by McGuire et al (7), successful weight-loss maintenance [defined as achieving an intentional weight loss of ≥10% of initial body weight and maintaining this weight loss for 1 y (16)] was shown to occur in ≈20% of overweight/obese persons. On the basis of this definition, 46% (31/68) of the women in our study would be considered successes. Because female sex is a known predictor of weight-loss maintenance (17), this may explain our increased number of successes. Additionally, the prevalence reported by McGuire et al was from a community-based sample, whereas our participants were highly motivated research participants. Considering the amount of weight regained, our study found an average sustained weight loss of 3.65 kg 1 y after the intervention. These results concur with those from a recent meta-analysis of weight-loss outcomes in weight-loss clinical trials with a minimum of 1 y of follow-up, which showed that a weight loss of ∼3–4 kg is maintained after the intervention (18).
Diet-induced weight loss results in a decrease in both fat and lean mass. Previous studies have reported that ∼75% of weight loss is attributable to fat mass loss and 25% to lean mass loss (19–21). Our results indicate a slightly higher contribution of lean mass loss to total weight loss (31% of lost weight was lean mass and 69% was fat mass), but they generally agree with the findings of previous studies. Little is known about the composition of weight change across weight cycling, especially in older adults. A recent observational study showed that for each 1 kg weight lost, there was an average of 0.06 kg lean mass lost over a 2-y period in older (70–79 y) women. Moreover, for each 1 kg weight gained during a 2-y weight-gain period, there was an average of 0.32 kg lean mass gained in women (22). Our study found that, for every 1 kg weight lost during the weight-loss intervention, 0.32 kg lean tissue was lost and for every 1 kg weight regained over the following year, only 0.08 kg lean tissue was regained. Thus, in this randomized controlled trial, proportionally more lean mass relative to total body mass was lost during the weight-loss period than was gained during the weight-regain period.
The health ramifications of our findings remain to be seen. On the one hand, lean mass lost during weight loss was not fully recovered during weight regain, which rendered the women more “sarcopenic obese” than they were before the intervention. Whereas it is intuitive that having high levels of fat mass together with low muscle mass may lead to more functional limitations (23) and metabolic disorders (24), some studies suggest that muscle quality, rather than muscle quantity, may be the more important predictor of health risk (25–27). In the Health, Aging, and Body Composition Study cohort, for example, muscle strength but not mass was independently associated with an increased risk of mobility loss (26) and mortality (27). Unfortunately, data on muscle strength or quality were not evaluated in our current study and will be the focus of future investigations. Moreover, because aging is accompanied with a progressive increase in fat mass (28) and loss in muscle mass (13), without a nonweight loss control group, we do not know whether regainers are more “sarcopenic obese” than they would have become over the course of 17 mo without the weight-loss intervention. On the other hand, however, the moderate absolute reduction in weight (−9%) and fat mass (−14%) maintained at the 12-mo follow-up is associated with cardioprotective benefit (29, 30). Intentional weight loss of this magnitude can improve or prevent many of the obesity-related risk factors for coronary heart disease (31, 32) and diabetes (33) and is associated with prolonged survival (34, 35). We previously showed that, in a 6-mo weight-loss intervention on muscle strength and quality in older obese adults, greater fat loss was associated with greater gains in muscle strength and quality, despite the loss of lean body mass (36). Thus, the benefits of weight and fat mass loss may trump any potential negative complications of lost lean mass.
As mentioned previously, weight regain was not ubiquitous and for the “nongainers” the lean-to-fat mass ratio 12-mo after the intervention was similar to that immediately after weight loss. Although our study did not attempt to capture the phenotypic differences between gainers and nongainers, several articles have been published on successful strategies to maintain weight loss. In animals, regular exercise after weight loss attenuates the metabolic drive to regain weight (37) and, in humans, successful weight maintenance is associated with greater physical activity (38). These findings were likely due, in part, to attenuated suppression of resting energy expenditure, which results from caloric restriction and is a risk factor for weight regain (39, 40). Dietary composition and approach during weight loss may also influence weight-loss maintenance success. Hypocaloric diets high in protein (41, 42) and diets low in saturated but high in monounsaturated fat (43) may reduce weight regain. In another study, the large initial weight loss achieved by individuals following a very-low-energy diet approach to weight loss was not maintained over time, whereas individuals who had used a self-guided approach maintained their initial weight loss with greater success (44). Finally, in a comprehensive study that examined the behavioral and psychological predictors of weight regain after significant weight loss, Wing et al (45) found that decreases in physical activity and increases in depressive symptoms, disinhibition, and hunger were related to weight regain, whereas increased frequency of self-weighing and dietary restraint were found to be protective. Application of these findings to aid in the development of successful weight-loss-maintenance strategies needs to be the focus of the next generation of weight-loss clinical trials. Further research is also needed to determine the predictors of where excess energy is stored (as fat or lean tissue) when lost weight is regained. Previous work has shown that weight regain is negatively correlated with baseline fat mass (46, 47), also known as the fat-free mass sparing effect, but whether baseline body composition predicts regain of more fat or lean tissue is not known.
In conclusion, our study showed that, although not all postmenopausal women who lose weight will regain it within 1 y, fat mass is regained to a greater degree than is lean mass in those who do. The strengths of this study were the long-term follow-up and the sophisticated body-composition techniques used. Unfortunately, without a “normal aging” control group we were unable to determine whether the regainers were more “sarcopenic obese” than they would have been without the weight-loss intervention. Additionally, we did not analyze muscle strength and quality, which are more important than muscle mass in predicting clinical outcomes. Last, the results from this study are limited to sedentary, abdominally obese, postmenopausal women, and the findings may differ in men or in younger populations. Future studies of weight cycling are needed to determine its effects on muscle strength, quality, and function and body composition in older adults after all weight lost is regained.
Acknowledgments
The authors’ responsibilities were as follows—BJN: designed the research; MFL and XW: conducted the research; CCD: analyzed the data; KMB, CCD, DPB, and BJN: wrote the manuscript; and KMB: had primary responsibility for the final content. All authors read and approved the final manuscript. The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. None of the authors had any conflicts of interest to report.
REFERENCES
- 1.Zamboni M, Mazzali G, Zoico E, Harris TB, Meigs JB, Di Francesco V, Fantin F, Bissoli L, Bosello O. Health consequences of obesity in the elderly: a review of four unresolved questions. Int J Obes (Lond) 2005;29:1011–29 [DOI] [PubMed] [Google Scholar]
- 2.Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc 2009;109:1886–95 [DOI] [PubMed] [Google Scholar]
- 3.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging 2008;12:487–91 [DOI] [PubMed] [Google Scholar]
- 4.Chaston TB, Dixon JB, O'Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–50 [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–96 [DOI] [PubMed] [Google Scholar]
- 6.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr 1993;123:465–8 [DOI] [PubMed] [Google Scholar]
- 7.McGuire MT, Wing RR, Hill JO. The prevalence of weight loss maintenance among American adults. Int J Obes Relat Metab Disord 1999;23:1314–9 [DOI] [PubMed] [Google Scholar]
- 8.Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Consensus Development Conference, 30 March to 1 April 1992. Ann Intern Med 1993;119:764–70 [PubMed] [Google Scholar]
- 9.Barte JC, ter Bogt NC, Bogers RP, Teixeira PJ, Blissmer B, Mori TA, Bemelmans WJ. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 2010;11:899–906 [DOI] [PubMed] [Google Scholar]
- 10.Westover SA, Lanyon RI. The maintenance of weight loss after behavioral treatment. A review. Behav Modif 1990;14:123–37 [DOI] [PubMed] [Google Scholar]
- 11.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–67 [DOI] [PubMed] [Google Scholar]
- 12.Hensrud DD, Weinsier RL, Darnell BE, Hunter GR. A prospective study of weight maintenance in obese subjects reduced to normal body weight without weight-loss training. Am J Clin Nutr 1994;60:688–94 [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8 [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, Harris TB, Newman AB. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2010;65:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicklas BJ, Wang X, You T, Lyles MF, Demons J, Easter L, Berry MJ, Lenchik L, Carr JJ. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr 2009;89:1043–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr 2001;21:323–41 [DOI] [PubMed] [Google Scholar]
- 17.de Zwaan M, Hilbert A, Herpertz S, Zipfel S, Beutel M, Gefeller O, Muehlhans B. Weight loss maintenance in a population-based sample of German adults. Obesity (Silver Spring) 2008;16:2535–40 [DOI] [PubMed] [Google Scholar]
- 18.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755–67 [DOI] [PubMed] [Google Scholar]
- 19.Ballor DL, Katch VL, Becque MD, Marks CR. Resistance weight training during caloric restriction enhances lean body weight maintenance. Am J Clin Nutr 1988;47:19–25 [DOI] [PubMed] [Google Scholar]
- 20.Dengel DR, Hagberg JM, Coon PJ, Drinkwater DT, Goldberg AP. Effects of weight loss by diet alone or combined with aerobic exercise on body composition in older obese men. Metabolism 1994;43:867–71 [DOI] [PubMed] [Google Scholar]
- 21.Gallagher D, Kovera AJ, Clay-Williams G, Agin D, Leone P, Albu J, Matthews DE, Heymsfield SB. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab 2000;279:E124–31 [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, Harris TB, Newman AB. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2010;65:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004;12:1995–2004 [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, Kim KW, Lim JY, Park KS, Jang HC. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, Harris TB. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci 2000;904:456–61 [PubMed] [Google Scholar]
- 26.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–33 [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–7 [DOI] [PubMed] [Google Scholar]
- 28.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001;2:141–7 [DOI] [PubMed] [Google Scholar]
- 29.Van Gaal LF, Wauters MA, De Leeuw IH. The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord 1997;21(suppl 1):S5–9 [PubMed] [Google Scholar]
- 30.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 1998;6(suppl 2):51S–209S [PubMed] [Google Scholar]
- 31.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2004;110:2952–67 [DOI] [PubMed] [Google Scholar]
- 32.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93 [DOI] [PubMed] [Google Scholar]
- 33.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med 1990;7:228–33 [DOI] [PubMed] [Google Scholar]
- 35.Shea MK, Houston DK, Nicklas BJ, Messier SP, Davis CC, Miller ME, Harris TB, Kitzman DW, Kennedy K, Kritchevsky SB. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci 2010;65:519–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci 2007;62:866–71 [DOI] [PubMed] [Google Scholar]
- 37.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, Giles ED, Brown IE, Hill JO. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol 2009;297:R793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67–85 [DOI] [PubMed] [Google Scholar]
- 39.Astrup A, Gøtzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr 1999;69:1117–22 [DOI] [PubMed] [Google Scholar]
- 40.Menozzi R, Bondi M, Baldini A, Venneri MG, Velardo A, Del Rio G. Resting metabolic rate, fat-free mass and catecholamine excretion during weight loss in female obese patients. Br J Nutr 2000;84:515–20 [PubMed] [Google Scholar]
- 41.Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Additional protein intake limits weight regain after weight loss in humans. Br J Nutr 2005;93:281–9 [DOI] [PubMed] [Google Scholar]
- 42.Westerterp-Plantenga MS, Lejeune MP, Nijs I. van Ooijen M, Kovacs EM. High protein intake sustains weight maintenance after body weight loss in humans. Int J Obes Relat Metab Disord 2004;28:57–64 [DOI] [PubMed] [Google Scholar]
- 43.Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: a 6-mo randomized, controlled trial. Am J Clin Nutr 2008;88:1232–41 [DOI] [PubMed] [Google Scholar]
- 44.Marinilli Pinto A, Gorin AA, Raynor HA, Tate DF, Fava JL, Wing RR. Successful weight-loss maintenance in relation to method of weight loss. Obesity (Silver Spring) 2008;16:2456–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wing RR, Papandonatos G, Fava JL, Gorin AA, Phelan S, McCaffery J, Tate DF. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol 2008;76:1015–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogels N, Diepvens K, Westerterp-Plantenga MS. Predictors of long-term weight maintenance. Obes Res 2005;13:2162–8 [DOI] [PubMed] [Google Scholar]
- 47.Dulloo AG, Jacquet J. The control of partitioning between protein and fat during human starvation: its internal determinants and biological significance. Br J Nutr 1999;82:339–56 [DOI] [PubMed] [Google Scholar]