Abstract
Bovine rotavirus (BRV) is an important cause of diarrhea in newborn calves. Local passive immunity is the most efficient protective strategy to control the disease. IgY technology (the use of chicken egg yolk immunoglobulins) is an economic and practical alternative to prevent BRV diarrhea in dairy calves. The aim of this study was to evaluate the protection and immunomodulation induced by the oral administration of egg yolk enriched in BRV specific IgY to experimentally BRV infected calves. All calves in groups Gp 1, 2 and 3 received control colostrum (CC; BRV virus neutralization Ab titer – VN- =65,536; ELISA BRV IgG1 =16,384) prior to gut closure. After gut closure, calves received milk supplemented with 6% BRV-immune egg yolk [(Gp1) VN=2048; ELISA IgY Ab titer =4096] or non-immune control egg yolk [(Gp2) VN <4; ELISA IgY Ab titer <4) twice a day, for 14 days. Calves receiving CC only or colostrum deprived calves (CD) fed antibody (Ab) free milk served as controls (Gp 3 and 4, respectively). Calves were inoculated with 105.85 focus forming units (FFU) of virulent BRV IND at 2 days of age. Control calves (Gp 3 and 4) and calves fed control IgY (Gp 2) were infected and developed severe diarrhea. Around 80% calves in Gp 1 (IgY 4096) were infected, but they showed 80% (4/5) protection against BRV diarrhea. Bovine RV-specific IgY Ab were detected in the feces of calves in Gp 1, indicating that avian antibodies (Abs) remained intact after passage through the gastrointestinal tract. At post infection day 21, the duodenum was the major site of BRV specific antibody secreting cells (ASC) in all experimental groups. Mucosal ASC responses of all isotypes were significantly higher in the IgY treated groups, independently of the specificity of the treatment, indicating that egg yolk components modulated the immune response against BRV infection at the mucosal level. These results indicate that supplementing newborn calves’ diets for the first 14 days of life with egg yolk enriched in BRV-specific IgY represents a promising strategy to prevent BRV diarrhea. Moreover a strong active ASC immune response is induced in the intestinal mucosa following BRV infection after the administration of egg yolk, regardless the specificity of the treatment.
Keywords: neonatal calf diarrhea, egg yolk immunoglobulins, IgY, bovine rotavirus, mucosal immunity, ELISPOT
1. Introduction
Rotaviruses (RV) are responsible for globally significant gastrointestinal disease, primarily in children <5 years of age and the young of other mammalian and avian species, including calves, pigs and South American camelids (Parreno et al., 2001; Ramig, 2004; Saif LJ, 1994). Diarrhea is the most commonly reported newborn calf disease and the main cause of mortality (Bendali et al., 1999). Bovine rotavirus (BRV) is the most common pathogen involved in neonatal diarrhea in both dairy and beef herds (da Costa Mendes et al., 1993; Garaicoechea et al., 2006). Calves are susceptible to BRV diarrhea up to 8 weeks of age and by the third week of life the susceptibility decreases as the age increases (Bridger, 1994; Dhama et al., 2009). In Argentina, BRV is the main cause of neonatal diarrhea in calves and it was detected in 62.5% (250/400) of calf diarrhea cases in beef and dairy herds during a ten-year-survey from 1994 to 2003 (Garaicoechea et al., 2006). The BRV diarrhea results in greater financial loss to cattle producers than any other infectious disease, particularly due to reduction of weight gain in affected animals and treatment expenses (Aich et al., 2007; Bellinzoni et al., 1990; Bendali et al., 1999). There is no specific treatment for BRV diarrhea. Affected animals recieve therapies to replace lost fluids and electrolytes and antibiotics for secondary bacterial infection. The current strategy for the control of the disease is based on vaccination of the mother to protect the offspring by transference of passive Abs (Bellinzoni et al., 1990; Fernandez et al., 1998; Saif et al., 1987). This strategy reduces severe diarrhea but it does not prevent virus infection or the appearence of clinical signs (Bendali et al., 1999; Parreno et al., 2004; Parreño et al., 2010). A thorough understanding of the mechanisms of intestinal immunity and their correlation with protection of neonates is critical to develop effective vaccines and complementary or alternative passive immunity strategies. Passive immunization by oral administration of specific Abs from different sources such as immune colostrum or chicken egg yolk IgY could represent effective and economic strategies to prevent gastrointestinal infections in food animals.
Rotavirus preferentially infects the mature enterocytes of the upper small intestine, near the tips of the villi. The severity and localization of intestinal infection vary among animal species and between studies; however, the pathological changes are primarily limited to the proximal small intestine (Candy, 2007; Dhama et al., 2009; Estes, 2001; Varshney et al., 1995). Considering the early susceptibility to the infection, the presence of maternal Abs -IgG1 and IgA-, derived from unabsorbed colostrum and milk in the gut lumen plays an important role in the protection against BRV infection and disease (Fernandez et al., 1998). To boost maternal Ab titers, cows are immunized parenterally with BRV vaccines 60 and 30 days before calving (Dhama et al., 2009; Saif and Fernandez, 1996). High titers of passive circulating Abs in newborn calves play a complementary role in protection against BRV induced diarrhea, because IgG1 acquired from colostrum is also transferred from serum to the intestine of neonatal calves (Besser et al., 1988). Virus neutralizing (VN) Ab titers against BRV in newborn calf serum may also be an indicator of protection against BRV diarrhea. However, it has been observed that newborn calves frequently developed BRV diarrhea despite moderately high levels of circulating BRV Abs derived from colostrum and, by the third week of life, most of them have been exposed to BRV in the field (Estes, 2001; Kohara and Tsunemitsu, 2000; Parreno et al., 2004). Although serum Abs are essential in dairy calves, which are usually separated from their dams after colostrum intake and subsequently fed with milk replacers lacking Abs, we previously demonstrated that BRV specific circulating Abs derived from colostrum intake suppress the development of BRV-specific Abs secreting cell (ASC) responses of neonatal calves in a dose dependent manner at both systemic and mucosal levels (Parreno et al., 2004). Following the same line of research, in a second study we also demonstrated that the administration of milk supplemented with BRV immune colostrum for the first 14 days of life induced high protection rates against BRV diarrhea in calves and a positive modulation of the neonatal immune responses toward higher numbers of BRV-specific IgA ASC and greater ASC isotype diversity in the intestinal mucosa. It was also shown that this active immune response protected against BRV challenge after the cessation of the passive Ab supplementation (Parreño et al., 2010).
In this regard, a promising, economically feasible and practical new strategy which has been explored during the past two decades is the supplementation of the milk diet of calves with specific Abs from egg yolk (IgY) (Ikemori et al., 1992; Ikemori et al., 1997; Kuroki et al., 1997; Kuroki et al., 1994; Mine and Kovacs-Nolan, 2002). Bovine RV is highly immunogenic in poultry and IgY technology offers several advantages over the classical ways of Abs production. It is a non-invasive technology, since the Abs can be obtained by collecting eggs, thus animals do not need to be bled. The IgY technology is in alignment with the 3R principles of animal welfare: Reduction, Refinement and Replacement of laboratory animals (Schade, 1996). The only Ab isotype present in chicken egg yolks is IgY. The isolation of IgY Abs is simple and approximately 1500 mg of IgY can be harvested each month from each laying hen (5–25 mg/egg yolk), with between 2 and 10 % being antigen specific IgY, representing a faster and cheaper way of polyclonal Ab production than other sources. Regarding the function, there are four important differences between avian IgY and mammalian IgG: IgY does not bind protein A or G, nor rheumathoid factor; chicken egg yolk immunoglobulins do not interfere with mammalian IgG in serological tests and they do not activate mammalian complement. These differences are advantageous for the application of IgY technology in many areas, from diagnostics to alternatives to antibiotic therapy (Carlander et al., 2000; Schade et al., 1994; Tini et al., 2002).
Passive protection of newborn calves against Group A BRV using specific egg yolk immunoglobulins as a milk supplement within the immediate postnatal period may be a suitable clinical option to control BRV diarrhea. It has been demonstrated previously that treatment of BRV diarrhea using avian egg yolk enriched in BRV specific IgY Abs during the first 2 weeks of life enhanced body weight gain and markedly reduced BRV infection (virus titer in stools as well as the number of calves shedding virus) (Heckert et al., 1999; Kuroki et al., 1997; Touchette et al., 2003). This indicates that egg yolk Abs can be a potential tool against BRV infection when administered daily immediately after birth, when BRV incidence is higher. However, there is no information about how these heterologous Abs and egg yolk itself may modulate the mucosal and systemic immune responses of newborn calves against BRV, which are critical for the development of active immunity to future viral exposures.
The objective of this study was to evaluate if the administration of milk enriched with BRV specific avian IgY Abs during the first 14 days of life is a suitable preventive strategy to protect calves against BRV diarrhea for the entire period of susceptibility to the disease, without inhibiting the development of active immune responses. In this study, newborn calves received 1L of pooled colostrum from non-immunized dams (control colostrum, CC) previous to gut closure within the first 6 hours of life. To study the protective and modulatory effects of chicken egg yolk Abs, calves were fed after gut closure (24 h of life) for 14 days with commercial Ab-free milk supplemented with crude egg yolk from hens hyperimmunized with BRV. A better understanding of the modulatory effects of local passive heterologous Abs on induction of active systemic and mucosal immune responses against BRV infection will improve the application of this strategy to prevent BRV diarrhea in dairy calves and other animals, including human infants.
2. Materials and Methods
2.1. Experimental design
2.1.1. Colostrum feeding and calf inoculation
Twenty-eight newborn Holstein male calves procured from the same dairy farm were removed from their mothers at birth prior to suckling and moved into isolation facilities within the first 4 hours of life. The animals were housed in individual isolation rooms under a strict management protocol as previously described (Parreno et al., 2004). The protocol for animal management and euthanasia met the requirements of The Institutional Animal Care Committee (IACC) of the Veterinary College (University of Buenos Aires) and the Office of Laboratory Animal Welfare (OLAW, NIH). Calves in Groups (Gp) 1, 2 and 3 received 1 L of control colostrum (CC) within the first 6 hours of life (previous to gut closure). This colostrum had a virus neutralization Ab (VN) titer to BRV of 65,536 and a BRV IgG1 Ab titer of 16,384 (determined by ELISA) (Table 1). All the animals in these groups reached a circulating BRV IgG1 Ab titer of ~1024 in serum. Calves in Gp 4 (colostrum deprived –CD-) received 1 L of Ab free milk at this time point. Thereafter, for the first 24 h of life, Ab free milk was given to all the animals (no more than 3 L during this period of time). Animals assigned to passive treament groups (Gp 1 and 2) started the treatment after this period of time, to assure that the administration of heterologous Abs started after gut closure (Stott and Fellah, 1983). Calves were randomly assigned to one of the following feeding groups: Gp 1: CC + milk supplemented with BRV-specific egg yolk with a final BRV IgY Ab titer of 4096 (n=5); Gp 2: CC + milk supplemented with normal egg yolk with a final BRV IgY Ab titer of <4 (n=6). IgY Ab titers in milk were determined by ELISA. Two groups of calves were negative controls; one received one dose of CC (Gp 3; n=9) and the others were colostrum deprived (CD) calves (Gp 4; n=8) and thereafter were fed milk without BRV Abs (Gonzalez et al., 2010). Calves in Gp 1 and 2 were fed twice a day with 2 L of each supplemented milk. From day 15 onwards, all calves in these groups were fed with milk without supplemental Abs. The milk used in the experiment was commercial sterilized bovine milk for human consumption (Mastellone Hnos., Bs. As., Argentina) (Table 1). All animals were orally inoculated with 105.85 Focus Forming Units (FFU) of virulent INDIANA (IND P[5]G6) BRV between the third and fourth feeding [approximately 36 hours after colostrum intake; 0 post-inoculation day (PID)]. This viral dose was previously confirmed to cause diarrhea and virus shedding in 100% of inoculated CD calves (Parreno et al., 2004). All the calves were euthanized at 21 PID to study the primary Ab responses to BRV infection.
Table 1.
Virus neutralization (VN) and ELISA isotype antibody titers to BRV IND in the supplemented milks, colostrum pools and egg yolk pools used to feed the experimental calves.
| Sample | volume of the corresponding pool added to 2 L of Ab free milk | Ab titer against BRV IND P[5]G6 | |
|---|---|---|---|
| ELISA | VNa | ||
| Control Colostrum (CC) | - | 16,384 (IgG1) | 65,536 |
| CONTROL IgY POOL | - | 256 (IgY) | 64 |
| IND BRV IgY POOL | - | 65,536 (IgY) | 16,384 |
| Gp 1: IND BRV IgY supplemented milk | 125 ml b (6.25 %) | 4096 (IgY) | 2048 |
| Gp 2: CONTROL IgY supplemented milk | 125 ml b (6.25 %) | <4 (IgY) | <4 |
Determined by fluorescence focus forming unit reduction assay.
Volume (percentage) of egg yolk pools (control/IND BRV) added to 2 liters of commercial milk to obtain the indicated final Ab titer against IND BRV.
2.1.2. Clinical observations and sample collection
Calves were examined daily for the development of diarrhea and virus shedding after BRV inoculation. The presence of elevated rectal temperatures was recorded. To estimate the severity of the diarrhea, fecal consistency was scored as follows: 0: normal; 1: pasty; 2: semi-liquid; 3: liquid. A score equal or greater than 2 was considered diarrhea. The scoring was done blindly by qualified technicians. Prior and after BRV inoculation, fecal samples were collected daily to assess virus shedding. Serum samples were collected before the colostrum feeding (within 4 hours after birth), at inoculation, and then weekly (7, 14, 21 PID). Serum Abs to BRV were detected by ELISA and VN assays. The presence of coproantibodies was assessed by ELISA. Peripheral blood lymphocytes (PBL) were sampled at 0 PID and then weekly, to assess ASC responses by ELISPOT assay. Body weight was measured at 0 PID and then weekly. At 21 PID, the animals were euthanized and the number of BRV ASC were also quantified by ELISPOT in the following mucosal-associated lymphoid tissues (MALT): duodenum, jejunum and ileum lamina propria (LP), jejunum and ileum Peyer’s patches (PP), jejunum and cecal mesenteric lymph nodes (MLN); and in systemic lymphoid tissues (spleen and PBL). Large (LIC) and small (SIC) intestinal contents from all the calves were collected at necropsy for Ab detection by ELISA (Parreno et al., 2004).
2.1.3. Virus
Virulent IND BRV (P[5]G6) inoculum used for calf experimental infection consisted on the intestinal contents of a CD calf that had been orally inoculated with a fecal suspension containing virulent BRV, as described previously (Fernandez et al., 1998; Parreno et al., 2004). The cell-culture-adapted IND BRV was propagated in monkey kidney cells (MA-104) for its use in ELISPOT, ELISA and VN assays (Parreno et al., 2004; Parreño et al., 2010).
2.1.4. Control colostrum pool
The control colostrum pool used in this study for initial colostrum intake of experimental calves in Gp 1, 2 and 3, was derived from non-vaccinated BRV seropositive dairy cows, and was prepared in our laboratory as described previously and stored at −20ºC until use. It had an IgG1 Ab titer against BRV of 16,384 determined by ELISA and a VN Ab titer of 65,536 (Parreño et al., 2010).
2.1.5. Egg yolk pools
The egg yolk pools used in this study were obtained from immunized and non-immunized hens. Lohmann Brown Classic laying hens were housed in cages specially designed and built for this purpose in groups of two animals per cage, following animal welfare recommendations (Schade and Hlinak, 1996; Schade, 1996). Room temperature, relative humidity and light/dark cycles were controled. Hens were fed laying hen diet and water ad libitum, and eggs were collected daily starting at the first immunization. Live attenuated IND BRV (P[5]G6) containing 1010 FFU/ml was mixed in a ratio of 1:1 in Freund’s complete adjuvant (FCA) or Freund’s incomplete adjuvant (FIA; both from Sigma-Aldrich). Birds were immunized intramuscularly in the breasts with 500 μl of immunogen. The FCA was used only for the first immunization, while FIA was used for the subsequent booster injections. Egg yolks from immunized hens were diluted in distilled water at a ratio of 1:3, and pools were prepared weekly and stored at −20°C. BRV IgY Ab titer was determined by IND BRV ELISA and VN assays, and pools with high BRV IgY Ab titers were maintained at −20°C (BRV IgY POOL). Egg yolks from non-immunized hens were processed in a similar manner (CONTROL IgY POOL) (Table 1).
2.1.6. Pilot Dose-Response Study and preparation of egg yolk supplemented milk
To establish the final BRV IgY Ab titer needed in the milk diet of neonatal calves to protect them against diarrhea, supplemented milks were prepared by the addition of crude liquid egg yolk from previously frozen pools. Two immune milks containing final IgY Ab titers to BRV of 1024 and 4096 (determined by ELISA) were initially tested in a pilot study. Calves fed milk with a final IgY Ab titer of 1024 were not protected against diarrhea and virus shedding (data not shown), but the supplementation of milk with a final IgY Ab titer of 4096 prevented BRV induced diarrhea in calves. Hence, for the present study, and specifically to address the potential interference effect of the local passive heterologous Ab treatments, we tested a supplemented milk that induced a high protection against diarrhea but not against virus shedding (BRV IgY final Ab titer of 4096) and a milk supplemented with control egg yolk (BRV IgY final Ab titer <4). We compared these with colostrum deprived calves (CD) or calves receiving control colostrum (CC), and fed milk without Abs.
Titers of VN and isotype specific Abs to IND BRV (determined by ELISA) in bovine colostrum, egg yolk pools and the egg yolk supplemented milks used to feed the calves are summarized in Table 1. The main isotype present in colostrum was IgG1, while IgY was the primary Ab isotype present in the egg yolk pools and supplemented milks. Milk used to feed calves assigned to Gp 1 and Gp 2, was prepared by adding 125 ml of egg yolk pools (BRV IgY POOL and CONTROL IgY POOL, respectively) to 2 L of commercial sterilized milk. The volume of egg yolk added to the milks represented 6.25% of the total feeding volume (Table 1). The addition of BRV IgY Pool to the milk of Gp 1 conferred it a final IgY Ab titer to BRV of 4096 and a VN titer of 2048, while a final IgY Ab titer to BRV of <4 and VN titer of <4 for Gp 2 milk were achieved.
2.1.7. Rotavirus antigen detection
Rotavirus shedding was detected in fecal samples using an antigen capture ELISA as described previously (Cornaglia E.M., 1989).
2.1.8. Cell culture immunofluorescence assay (CCIF)
Virus infectious titer was assessed by a cell culture immunofluorescence (CCIF) assay (Parreño et al., 2010). Fluorescent foci were visualized using a FITC-labeled hyperimmune bovine antiserum to IND BRV, and fluorescent foci-forming cells were counted using a fluorescence microscope. Titers were expressed as the number of fluorescent focus forming units per ml (FFU/ml).
2.1.9. Bovine isotype-specific antibody ELISA
The IgM, IgA and IgG1 Ab titers to IND BRV were quatitated in colostrum pools, calf sera, feces, LIC and SIC. Specific IgG1 Abs were detected by an indirect ELISA using the reagents and protocol described previously (Fernandez et al., 1998; Parreno et al., 2004). Specific IgM and IgA Abs were measured by capture ELISAs standardized in our laboratory, using anti-bovine IgM MAb (kindly supplied by Dr L.J. Saif, The Ohio State University, USA) and affinity purified sheep anti-bovine IgA polyclonal antibody (Bethyl Laboratories INC., Montgomery, Texas, U.S.A.) (Parreno et al., 2004).
2.1.10. BRV IgY specific antibody ELISA
The IgY Ab titers to IND BRV were determined in crude egg yolk pools, calf and hen sera, calf feces and supplemented milks by an indirect ELISA. Briefly, 96 well ELISA plates (Maxisorp, NUNC) were coated with a BRV hyperimmune antiserum prepared in guinea pigs. After a blocking step with albumin from chicken egg white (Sigma-Aldrich, Germany), the supernatant of cell culture lysate BRV-infected or mock-infected MA-104 were added, followed by serial four-fold dilutions of the samples. The reaction was developed using a peroxidase labeled polyclonal goat anti-chicken IgY Ab (Sigma-Aldrich, Germany), as conjugate and hydrogen peroxide and ABTS as susbtrate/cromogen system.
2.1.11. Fluorescent focus reduction virus neutralization (FFN) test
Virus neutralizing (VN) Ab titers to IND BRV in colostrum pools, egg yolk pools, supplemented milks and calf sera were determined by a fluorescent focus neutralization (FFN) test as previously described (To et al., 1998). The VN titer was expressed as the reciprocal of the highest sample dilution that resulted in >80% reduction in the number of fluorescent foci.
2.1.12. Isolation of mononuclear cells (MNC)
Approximately 15 cm of tissue samples of duodenum, jejunum, and ileum lamina propria were collected. Discrete Peyer’s patches (PPs) (n=4) were identified at different points along the mucosal surface of the jejunum and collected individually. A segment of intestine corresponding to a 10 cm portion of the distal ileum continuous Peyer’s patch was also obtained. The mesenteric lymph nodes (MLN) corresponding to the jejunum and ileocecal regions were collected and processed separately. Intestinal tissues were initially digested with dithiotreithol (GIBCO, BRL, Grand Island, NY, USA) and collagenase type II (Sigma Chemical Co., St Louis, MO, USA). MNC suspensions were obtained by centrifugation through 30%, 43% and 70% consecutive Percoll density gradients (Amersham Pharmacia Biotech, Uppsala, Sweden) as previously described for pig tissues (Yuan et al., 1996). Mononuclear cells from blood and spleen were extracted to evaluate ASC responses in systemic lymphoid tissues. The tissue was passed through stainless steel 80-mesh screens of a cell collector (Cellector; E-C Apparatus Corp., Petersburg, FL, USA). Mononuclear cells from blood were isolated by Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation following the recommendations of the manufacturer. Puri ed MNC from all tissues were resuspended at a nal concentration of 5 × 106 MNC/ml in RPMI-1640 (GIBCO, BRL, Grand Island, NY, USA) supplemented with 10% FCS, 20 mM HEPES, 2 mM Glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential aminoacids, 100 IU/ml penicillin, 67 mg/ml streptomycin and 50 mg/ml gentamycin (E-RPMI). The cell viability of each MNC suspension was assessed by Trypan blue exclusion (in all cases it was >95%).
2.1.13. ELISPOT assay for BRV-specific ASC
An ELISPOT assay for quantification of anti-BRV IgM, IgA, IgG1 and IgG2 ASC was conducted to evaluate effector B-cell responses from all calves at 21 PID, as previously described (Parreno et al., 2004). Briefly, IND BRV infected MA-104 cells in 96-well plates were fixed with 70% acetone, air dried, and stored at −20ºC until use. Mononuclear cells from different tissues were counted using Trypan blue staining and three dilutions of single-cell suspensions per tissue were plated (5 × 105; 5 × 104 and 5 × 103 cells/well). Plates were incubated for 14 hours at 37ºC in 5% CO2. The plates were incubated with a set of commercial peroxidase-labeled goat polyclonal Abs to bovine IgM (1:10,000); IgG1 (1:8000); IgG2 (1:3000) and IgA (1:1000) (Bethyl Laboratories INC., Montgomery, Texas, USA) for 1 h at 37°C and the spots were developed with a tetramethylbenzidine peroxidase substrate system (TMB, KPL, Gaithersburg, MD, USA).
2.2. Statistical analysis
Fisher’s exact test was used to compare proportions of calves with diarrhea and virus shedding among groups. The Kruskall-Wallis rank sum (non-parametric) test was used to compare days of onset and duration of diarrhea and virus shedding, cumulative diarrhea scores, cumulative titers of virus shed and days with high rectal temperature, among groups. Neutralizing and isotype-specific Ab titers were log10-transformed prior to statistical analysis. Differences in Ab titers among groups were evaluated by general analysis of variance (ANOVA) followed by DGC multiple comparison test on repeated measures throughout time. Negative samples at a dilution of 1:4 were assigned an arbitrary Ab titer of 2 for the calculation of geometric mean titers (GMTs). At 21 PID, the ASC numbers were compared among groups using the Kruskall-Wallis rank sum test. Statistical significance was assessed at p<0.05 for all comparisons. Differences among body weight gained were analyzed by Student t test. All the statistical analyses were conducted using STATISTIX 8.0 (Analytical Software, Tallahasee, USA) and MedCalc® version 11.1.1.0 statistical software.
3. RESULTS
3.1. The BRV immune egg yolk confers significant protection rates against BRV diarrhea
Results of the various parameters associated with BRV infection and disease are summarized in Table 2. After inoculation with BRV, 80% (4/5) of calves fed milk supplemented with BRV immune egg yolk for 14 days (BRV IgY 4096; Gp 1) were protected against diarrhea. One animal was completely protected against the infection and disease, three animals shed virus asymptomatically and only one calf developed mild diarrhea 4 days after inoculation and for 2 days only (Table 2 and Figure 2). The onset of the clinical signs in this calf was slightly delayed, while the duration and severity of the illness was significantly diminished compared with the groups of animals that did not receive any passive Ab treatment (Gp 3 and Gp 4) (Table 2). In addition, this calf showed a significant reduction in the number of days with high temperature associated with diarrhea (0.2 days) in comparison to groups Gp 3 and Gp 4 (7.4 and 4.4, respectively) (Table 2). On the other hand, 100% of calves receiving milk supplemented with IgY derived from non-immunized hens (Gp 2) developed diarrhea (Table 2). However, the severity of the disease, in terms of diarrhea score and days with elevated temperatures, was significantly reduced and of shorter duration compared with the calves receiving control colostrum (Gp 3) or CD calves (Gp 4), fed with Ab free milk (Table 2). Compared with Gp 1 (IgY 4096), the severity and mean duration of diarrhea and the number of days with fever in Gp 2 (control IgY) were higher, but not significantly so (Table 2).
Table 2.
Diarrhea and fecal virus shedding in calves after oral inoculation with BRV IND (P[5]G6).
| Treatment Group |
n | Inoculum infectious titer (FFU/ml) | Diarrheab,e | Virus sheddinga | Days with fever asoc to BRV (>39ºC) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Affected Calvesd | Mean Onset (PID) | Mean Duration (days) | Mean Cumulative Scorec | % Affected Calvesd | Mean Onset (PID) | Mean Duration (days) | Mean Peak shed virus (CCIF) | Mean RV titer (CCIF) | ||||
| Gp 1: CC + IND BRV IgY supplemented milk | 5 | 10 4.76 | 20% A | 4.0 | 0.4 B | 2.00 B | 80% | 2.00 | 5.2 | 5.27 | 4.83 AB | 0.2 C |
| Gp 2: CC + CONTROL IgY supplemented milk | 6 | 10 4.79 | 100% B | 3.3 | 5.2 AB | 12.89 AB | 100% | 1.75 | 6.7 | 6.47 | 6.27 A | 1.3 BC |
| Gp 3: CC + Ab free milk | 9 | 10 4.55 | 100% B | 2.2 | 7.4 A | 20.22 A | 100% | 2.11 | 8.0 | 5.36 | 3.98 B | 7.4 A |
| Gp 4: CD + Ab free milk | 8 | 10 4.12 | 100% B | 1.1 | 9.4 A | 26.88 A | 100% | 1.50 | 5.5 | 5.71 | 4.45 AB | 4.4 AB |
Determined by ELISA and CCIF.
Diarrhea duration was defined as the number of days with fecal score ≥2. Stool consistency was scored daily (0=normal; 1=pasty; 2=semi-liquid; 3=liquid).
Mean cumulative scores from 0 to 21 PID calculated as a measure of severity of diarrhea (sum daily fecal score)/n.
Proportions in the same column with different superscript upper case letters differs significantly (Fisher’s exact test, p<0.005).
Means in the same column with different superscript upper case letters differ significantly (Kruskall-Wallis rank sum test; p<0.05).
CC= control colostrum / CD = colostrum deprived. Calves were fed with 2 liters of milk, twice a day, for 14 days, with or without the addition of the corresponding volume of IND BRV IgY pool or control IgY pool, according to the group.
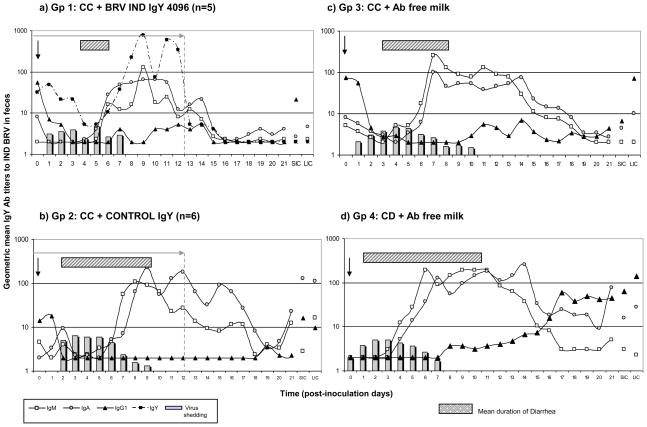
Figure 2.
Geometric mean isotype-specific Ab titers (GMT) to IND BRV detected daily in feces of a) Gp 1: calves fed control colostrum (CC) followed by BRV egg yolk supplemented milk with a final BRV IgY Ab titer of 4096; b) Gp 2: calves receiving CC followed by CONTROL egg yolk supplemented milk with a final BRV IgY Ab titer <4; c) Gp 3: calves receiving CC and d) Gp 4: colostrum deprived calves, both fed Ab-free milk. All animals were orally inoculated at 2 days of age/36 hours after colostrum intake [0 days post inoculation (0 PID)], and euthanized at 21 PID. SIC, small intestinal contents; and LIC, large intestinal contents, were collected at 21 PID. Columns represent the mean titer of virus shed per day per group (from CCIF assay). Bars represent the mean days of diarrhea duration for each group. Statistical analysis of the Ab titers in feces analyzed every 7 days is detailed in Supplementary material, Table 2 (ANOVA followed by DGC multiple comparison test through time, p<0.05).
Regarding BRV infection, 80% (4/5) of Gp 1 calves (IgY 4096) shed virus after oral inoculation, with a mean duration and titers similar to the control groups (Gp 2, 3 and 4) (Table 2). Specifically, one animal did not shed detectable virus, three calves shed virus asymptomatically and only one calf developed diarrhea and shed virus for a more prolongued period (7 days) (Table 2 and Figure 2). The BRV-specific IgY was detected in the feces of all Gp 1 calves from the beggining of the treatment until the onset of virus shedding, and thereafter until the end of the treatment. In particular, in one calf that did not shed virus, a reduction of BRV IgY Ab titers in feces was evident during the initial days after inoculation (data not shown), suggesting that this animal was infected but the virus shed was not detected because of the IgY Abs present in the gut.
All the calves fed milk supplemented with non-specific IgY (Gp 2) shed virus with a pattern similar to those of the control groups, Gp 3 (CC) and Gp 4 (CD). However, the mean titer of virus shed by Gp 2 calves was significantly higher that the other groups. Compared to Gp 1 (IgY 4096), the mean BRV titer in feces of control IgY treated calves was higher, but not statistically significant.
The pattern of virus shedding in CC and CD calves (Gp 3 and 4, respectively) was characterized by a rapid onset. Calves from CC + Ab free milk diet (Gp 3) showed different patterns between animals, some calves shed virus for almost 20 days and others only for 3 days post inoculation, with an average for the group of 10 PID (Table 2). However, CD animals (Gp 4) showed a more uniform virus shedding pattern and a faster resolution (average of 5.5 PID) (Table 2 and Fig. 2).
Concerning body weight gains, calves fed milk supplemented with egg yolk from hyperimmunized hens (Gp 1) had significantly increased body weights at 21 PID compared with control IgY treated calves (Gp 2) (Student T test, p=0.0182 ; Gp 1: 7.06 kg vs. Gp 2: 3.72 kg) (Supplementary material, Table 3 and Fig. 1).
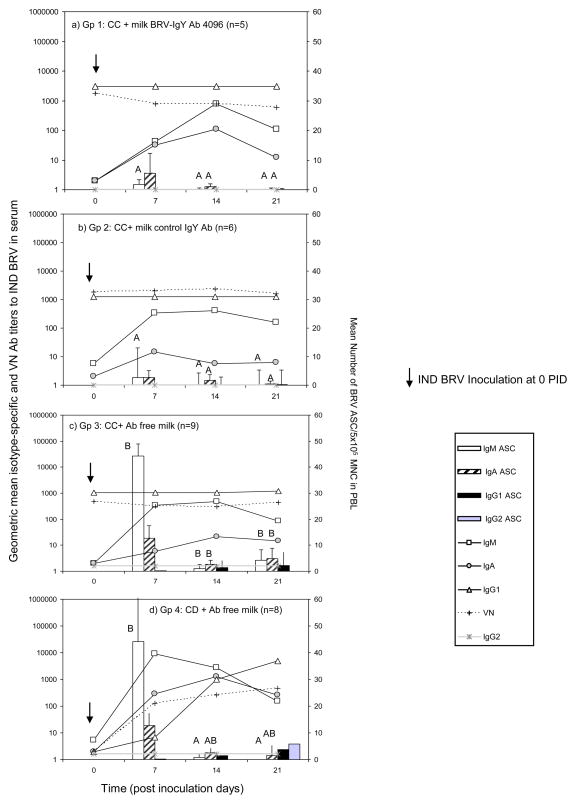
Figure 1.
Left axis: Geometric mean isotype-specific and neutralizing Ab titers (GMT) against IND BRV in serum collected weekly (lines); right axis: Number of BRV-specific ASC/5×105 MNC in PBL (bars; error bars: SD) from: a) Gp 1: calves fed with control colostrum (CC) followed by BRV egg yolk supplemented milk with a final BRV IgY Ab titer of 4096; b) Gp 2: calves receiving CC followed by CONTROL egg yolk supplemented milk with a final BRV IgY Ab titer <4; c) Gp 3: calves receiving CC and d) Gp 4: colostrum deprived calves, both fed Ab-free milk. All calves were orally inoculated with IND BRV at 0 PID. Bars corresponding to ASC of the same isotype with different upper case superscript letters indicate significant differences among groups for the same isotype, (Kruskal-Wallis non-parametric sum rank test, p<0.05). Statistical analysis of the Ab titers in serum are detailed in Supplementary material, Table 1 (ANOVA followed by DGC multiple comparison test through time, p<0.05).
3.2. Passive Ab treatments did not interfere with the isotype-specific and VN Abs to IND BRV in serum and the active ASC responses in PBL
Isotype-specific and VN Ab responses to IND BRV in serum and the number of anti-BRV ASC in PBL are depicted in Fig. 1 (statistical analysis detailed in Supplementary material, Table 1). After colostrum administration, all calves in Gp 1, 2 and 3, showed high IgG1 Abs and VN Ab titers to BRV in the sera (Fig. 1a, b and c). Calves in Gp 1 showed significantly higher IgG1 Ab titers than Gp 3 at 0 PID, however this difference disappeared at 7 PID. Bovine RV IgG1 and VN titers remained high and constant within each group (Supplementary material, Table 1). The detection of high IgG1 Ab titers was indicative of colostrum intake.
Colostrum-deprived calves (Gp 4) showed a strong immune response against BRV, developing IgG1 and VN Abs to BRV in serum, at titers similar to those detected in the other experimental groups at 21 PID. There was an increasing number of IgG1 ASC for Gp 4 along the experience (Fig 1.d).
After BRV inoculation, IgM and IgA Abs were detected in the serum from all the animals in association with the appearance of IgM and IgA ASC in blood, indicating that all calves were primed by BRV inoculation, with or without supplemental IgY Abs in milk. At PID 7, CD calves (Gp 4) showed significantly higher IgM and higher IgA Ab titers than the calves receiving colostrum (Gp 1, 2 and 3) (supplementary material, Table 1). For the ASC response in blood, the numbers of IgM ASC were significantly higher in calves without milk supplementation (Gp 3 and Gp 4) than in calves fed milk supplemented with egg yolk (Gp 1 and Gp 2) at this same time point. A similar profile but of lower magnitude was observed for IgA ASC in blood and IgA Abs in serum with a peak Ab titer detected at 14 PID (Fig. 1).
No IgG2 Ab responses were detected in serum, while few IgG2 ASC were detected in blood of CD calves (Gp 4) at 21 PID (Fig. 1).
3.3. Isotype-specific Ab responses to IND BRV in feces and intestinal contents were not inhibited by passive Ab treatments
Isotype-specific Ab responses to IND BRV in feces (evaluated daily), and in LIC and SIC (determined on 21 PID) are shown in Figure 2 (Statistical analysis detailed in Supplementary material, Table 2). A strong IgG1 Ab response to BRV inoculation was detected in CD group (Gp 4), and in lower magnitude in CC group (Gp 3) (Fig. 2). At 21 PID, CD calves (Gp 4) showed significantly higher IgG1 Abs in feces than all other experimental groups (Fig.2 and Supplementary material Table 2).
BRV-specific IgM and IgA responses were detected at 7 PID and titers were high until at least 14 PID. The appearance of these Abs was associated with virus clearance. Calves receiving passive IgY treatment cleared virus shedding faster than calves fed Ab free milk, once an active immune response was detected (Gp 1: 1 day; Gp 2: 2 days; Gp 3: 4 days; Gp 4: 3 days). No significant differences were detected among groups for these Ab isotypes, suggesting that milk supplementation did not affect the active immune response to BRV, although BRV IgY Abs were present in the gut of Gp 1 calves ( Fig. 2a).
The Ab responses in SIC and LIC were examined for the 3 isotypes. At 21 PID, calves from Gp 2 (CONTROL IgY) had highest BRV IgM and IgA Ab titers, animals from Gp 3 (CC) and 4 (CD) had an intermediate profile, while animals from Gp 1 (BRV IgY 4096) had the lower Ab titers (Supplementary material, Table 2). At 21 PID, calves from all groups had IgG1 Abs in intestinal contents with the highest titers in CD calves. However, all the differences observed were not statistically significant.
3.4. Passive Ab treatments caused a reduction in the active ASC responses to BRV in systemic tissues and MLN at 21 PID
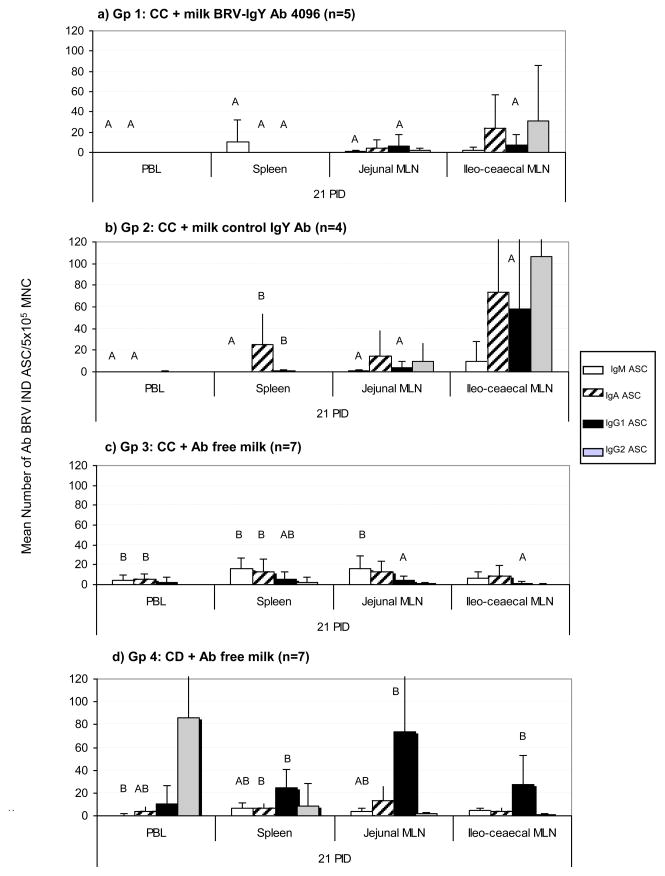
At 21 PID, calves were euthanized and tissues were collected. Mononuclear cells from systemic and GALT-associated tissues were obtained and tested to determine the isotype, number and distribution of ASC specific against BRV. The magnitude and profile of BRV ASC responses, evaluated by ELISPOT in PBL, spleen and MLN are depicted in Fig. 3.
Figure 3.
Mean numbers of BRV ASC per 5 × 105/MNC obtained from systemic lymphoid tissues (PBL and Spleen) and MLN draining the small and large intestine, at 21 PID. a) Gp 1: calves fed with control colostrum (CC) followed by BRV egg yolk supplemented milk with a final BRV IgY Ab titer of 4096; b) Gp 2: calves receiving CC followed by CONTROL egg yolk supplemented milk with a final BRV IgY Ab titer <4; c) Gp 3: calves receiving CC and d) Gp 4: colostrum deprived calves, both fed Ab-free milk. For each tissue, when comparing mean ASC numbers of the same isotype, among treatment groups: bars with different letter indicate a significant difference, (Kruskal-Wallis rank sum test, p<0.05). n= number of calves in each group. Error bars indicate SD.
Relatively small numbers of BRV ASC of all the isotypes were found in PBL at 21 PID (Fig. 1 and Supplementary Material Table 3). However, calves from Gp 3 (CC + Ab free milk) had a significantly higher numbers of IgM and IgA ASC than calves from Gp 1 and 2 (BRV IgY 4096 and Control IgY milk treatment, respectively).
Spleens of calves from BRV IgY 4096 (Gp 1) had fewer BRV ASC of all isotypes than the control groups (Gp 2, 3 and 4), at 21 PID. As expected, calves from CC and CD groups (Gp 3 and 4, respectively) had BRV ASC of all isotypes. In particular, IgG1 was the predominant isotype for Gp 4, while IgA and IgM ASC were more abundant in Gp 3 calves (Fig. 3).
Collectively, these results suggest a trend towards a reduction in the number of BRV ASC in systemic (PBL and spleen) as the specificity and duration of the treatment increase, from colostrum deprived calves (CD, Gp 4) to calves fed 1 L of control colostrum (CC, Gp 3), followed by calves receiving colostrum plus milk supplemented with egg yolk (Gp 1). Eventhough a possible explanation for this phenomenon could have been that increasing viral antigen in the intestine may result in greater systemic responses, no correlation was observed between virus shedding and the systemic responses (data not shown).
In egg yolk supplemented groups, ileocecal MLN developed a strong ASC response to BRV infection. The most abundant ASC isotypes were IgA and IgG2, followed by IgG1. Interestingly, a completely inverted profile was observed in CD calves (Gp 4), where in jejunal MLN, the predominant isotype of BRV ASC was IgG1, followed by IgA and IgM, while IgG2 ASC were almost not detectable at 21 PID (Figure 3).
3.5. Passive Ab treatments positively modulated the active immune response toward higher numbers of ASC in intestinal lymphoid tissues at 21 PID
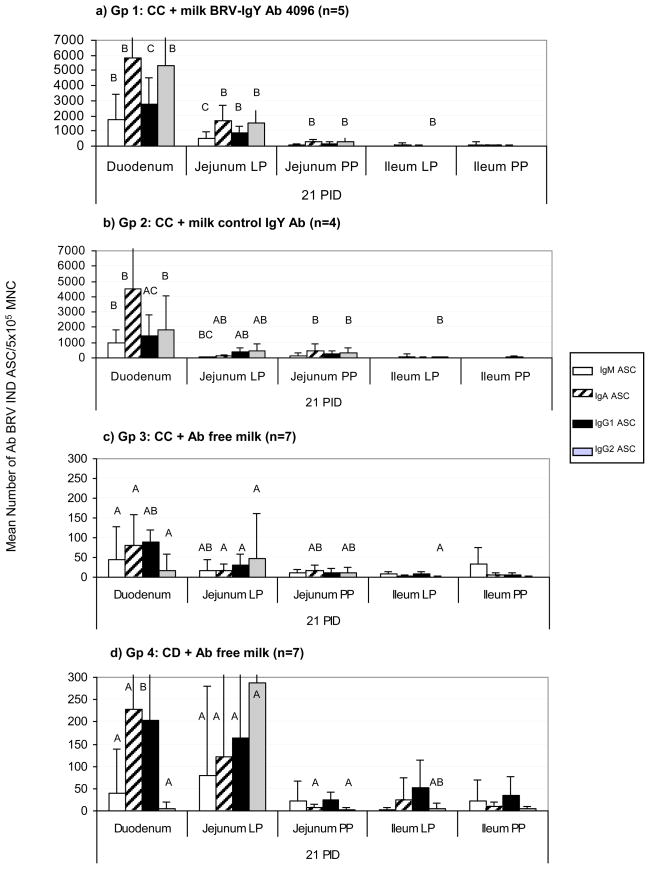
The ASC responses to BRV in the intestinal mucosa are depicted in Figure 4. Rotavirus isotype-specific ASC were detected in all the lymphoid tissues tested. At 21 PID, most ASC were present in the proximal intestinal lamina propria (duodenum, jejunum) and, in lower magnitude, in PPs, independently of the treatment. This finding is in agreement with the site of BRV infection and replication in the small intestine and represents the main GALT effector and inductive sites, respectively.
Figure 4.
Mean numbers of BRV ASC per 5 × 105/MNC obtained from the intestinal mucosa associated lymphoid tissues (GALT): Duodenum, Jejunum LP and PPs, Ileum LP and PPs, at 21 PID. a) Gp 1: calves fed with control colostrum (CC) followed by BRV egg yolk supplemented milk with a final BRV IgY Ab titer of 4096; b) Gp 2: calves receiving CC followed by CONTROL egg yolk supplemented milk with a final BRV IgY Ab titer <4;c) Gp 3: calves receiving CC and d) Gp 4: colostrum deprived calves, both fed Ab-free milk. For each tissue, when comparing mean ASC numbers of the same isotype, among treatment groups: bars with different letters indicate a significant difference, (Kruskal-Wallis rank sum test, p<0.05). n= number of calves in each group. Error bars indicate SD. Please note diff axis.
The ASC responses to BRV in the duodenum and jejunum lamina propria were significantly higher in the egg yolk treated groups (Gp 1 and Gp 2) than in the Ab free diet groups (Gp 3 and 4) for all the isotypes tested, independently of the specificity of the IgY (control or BRV-specific). In addition, the profile of the ASC responses in the duodenum of the calves fed milk supplemented with egg yolk was characterized by high numbers of IgA followed by IgG2 ASC, while the profile observed in non-treated calves (Gp 3 and Gp 4) was characterized by the dominance of IgA followed by IgG1 ASC. The BRV IgY 4096 group (Gp 1) showed a strong BRV ASC response restricted to duodenum and jejunum lamina propria and a weak response in the ileum compared with control groups (Gp 3 and 4). Calves receiving control egg yolk (Gp 2) showed a strong immune response in duodenum as observed for Gp 1, and an intermediate behavior between Gp 1 and control groups (Gp 3 and 4) in the jejunum lamina propria. The other regions of the GALT did not show important differences regarding the number, isotype nor profile of the ASC responses to BRV infection.
4. Discussion
We evaluated milk supplemented with IgY Abs from crude egg yolk for its ability to prevent BRV diarrhea in calves. Our goal was to provide passive Abs in the gut that prevent the disease, but still allow a subclinical infection to prime the neonatal mucosal immune response to BRV.
The group of calves fed milk supplemented with crude egg yolk at a final ELISA IgY Ab titer to BRV of 4096 showed 80% protection rate against BRV diarrhea. The results indicate that the treatment caused a significant reduction in the percentage of affected animals, the duration and severity of illness and BRV-associated high temperatures wich also coincided with the presence of BRV IgY Abs in feces. The detection of BRV IgY Abs in feces of Gp 1 treated animals clearly demonstrates that IgY Abs passed through the intestinal tract and remained biologically active. The IgY Abs in feces showed a similar kinetic pattern for all the calves, whereas a decrease in BRV IgY Ab titer was associated with virus shedding. This binding of BRV IgY Abs with infectious virions may explain the absence of diarrhea after this treatment, although the virus replicated in the gut of the newborn calves. In contrast, the supplementation of milk with non-specific crude egg yolk (Gp 2, CONTROL IgY milk), failed to protect calves against diarrhea. The onset, duration and severity of the illness were not reduced in Gp 2 compared to CC or CD calves (Gp 3 and 4, respectively). Eventhough the differences were not statistically significant, the mean BRV titer in feces of control IgY treated calves was higher than in Gp 1 calves (IgY 4096).
Although crude egg yolk was given as a passive treatment starting immediately after colostrum intake, an important condition of the experimental design was to administer the first dose right after gut closure (24 h post delivery, (Stott and Fellah, 1983)), to avoid egg yolk IgY Ab uptake into serum. Consequently, no IgY Abs were detected in serum samples from any of the animals from Gp 1 or 2 (BRV IgY 4096 and CONTROL IgY, respectively); thus, no systemic effects were expected or observed after oral administration of egg yolk.
In addition to being economical, eggs possess many characteristics of a high quality animal feed ingredient. Typical nutrient analysis of liquid eggs shows 55 % protein, 40 % fat and 4 % ash, and also they are a valuable source of iron, phosphorous, trace minerals and vitamins. For this reason, egg yolk and whole eggs have been studied extensively as an alternative protein source for animal feeding, with promising results. Most of the authors conclude that when egg is included at levels up to 10 % of the diet (around 30 % of the total protein), growth performance of the calves is improved or similar to that observed when they receive only Ab free milk diets (Touchette et al., 2003). In our work, we supplemented the milk diet of newborn calves with 6.25 % of crude egg yolk (Gp 1 and Gp 2). Calves fed milk supplemented with egg yolk enriched in IgY Abs to BRV (Gp 1), showed significantly increased mean body weights compared with calves fed milk supplemented with control egg yolk (Gp 2) (p=0.0182) post-BRV challenge. Similar results have been reported by Kuroki et al. in calves under field conditions (Kuroki et al., 1997). These results indicate that the effect of crude egg yolk itself on body weight gain is improved by the presence of BRV specific IgY Abs, which induced high protection rates against BRV diarrhea.
Salmonella is also an important pathogen responsible for neonatal diarrheas in calves (Bellinzoni et al., 1990). Eventhough it is not the main bacterial agent responsible for diarrhea outbreaks in herds from Argentina, this potential biohazard was considered for this study. Laying hens affected to this work were vaccinated against Salmonella to prevent the contamination of the eggs that may result in the subsequent bacterial infection of the calves under treatment.
In this study, the development and modulation of active serum and fecal Ab responses to BRV infection were evaluated. The BRV-specific ASC responses in systemic and mucosal-associated lymphoid tissues were also determined in conventional calves fed milk supplemented with BRV immune egg yolk.
Although some differences in Ab titers and kinetics were found between the experimental groups, it is clear that all the animals developed a specific immune response to BRV. Antibody supplemented diets (Gp 1 and 2) caused a short delay in the development of active immune responses in serum that may be related to the milder clinical signs, as previously shown here. In serum and feces from all the experimental groups, IgM was the first isotype to appear, followed by IgA and later by IgG1.
In Gp 1 calves, the administration of milk supplemented with chicken egg yolk induced low systemic (PBL and spleen) ASC responses to BRV, in agreement with the response to a subclinical infection. Similar results have been reported in calves fed milk supplemented with BRV-immune colostrum (Parreño et al., 2010). Collectively, ASC responses in systemic tissues (PBL and spleen) suggest a trend towards a reduction in the number of BRV ASC as the specificity and duration of the treatment increase, from colostrum deprived calves (CD, Gp 4) to calves fed 1 L of control colostrum (CC, Gp 3) followed by calves receiving colostrum plus milk supplemented with egg yolk (Gp 1).
Passive treatment based on the administration of milk supplemented with BRV immune colostrum to newborn calves for the first 14 days of life induces a high protection rate against diarrhea and a positive modulation of the neonatal immune responses towards higher numbers of IgA ASC and greater isotype diversity in the intestinal mucosa (Parreño et al., 2010). It is unclear whether this immunomodulation is due to the Abs themselves or to the presence of other bioactive factors, when colostrum is the source of BRV IgG1 Abs. When the milk diet was supplemented with crude chicken egg yolk, a significant diversification and enhancement of the local immune responses against BRV was observed. Duodenum, jejunum lamina propria and ileocecal MLN were the primary tissues involved and IgA was the dominant ASC isotype, followed by IgG2 instead of IgG1. Interestingly, this effect was independent of the specificity of the treatment and was significantly more pronounced than the one observed previously in calves receiving milk supplemented with the same titer of homologous bovine IgG1 Abs from colostrum (Parreño et al., 2010). These results are in agreement with the presence of immunomodulating molecules in egg yolk, that promote B cell clonal expansion and IgA switching related to anti-inflammatory responses to dietary antigens (Nelson, 2007).
It is unclear which component of crude egg yolk is responsible for the observed increase numbers of ASC to BRV in calves from Groups 1 and 2. There are many possible explanations for the immunomodulatory properties of crude egg yolk. Bioactive molecules (hormones and cytokines) are present in egg yolk. Xu et al. described the immunomodulatory properties of Tranfer factor (TF) found in the egg yolk of immunized hens. The TF increases the thymus-spleen index and stimulates proliferation of lymphocytes in mice. This factor seems to have not only specific immune activity against Hepatitis B virus but also non-specific immune activity, making it a potential immunoregulator in egg yolk for enhancing treatments for this viral infection (Xu et al., 2006). Nelson et al. also found that egg yolk protein digests from non-immunized hens have immunomodulatory activities in the gut in vivo. They may act directly to block pathogen entry or indirectly by the activation of the immune system to prevent the infection. These authors further demonstrated that an egg yolk low lipid peptide digest induced IgA ASC in the gut which prevent or limit adherence and colonization by enteropathogens. Also the egg yolk compound orchestrated Th1/Th2 responses, inducing the secretion of IL-4 and IL-10, potent activators of B cells and anti-inflamatory agents in the gut (Nelson, 2007). It is also well known that egg lysophosphatidylcholines (LPCs) possess adjuvant properties, promoting dendritic cell maturation and a Th1-oriented response in vitro and inducing a Th2 Ab response against specific viral antigens, when administered systemically in mice (Bach et al., ; Perrin-Cocon et al., 2006).
Despite these stimulatory activators of egg yolk, it is interesting that amplified immune responses ocurred only in the effector sites within the first portion of the gut, the duodenum and jejunal lamina propria, and the mesenteric lymph nodes draining the large intestine (ileocecal MLN), while no differences were detected in the jejunal PPs and the ileum.
Collectively, our results indicate that the practice of feeding crude egg yolk supplemented milk at a final IgY Ab titer to BRV of 4096 should be highly effective to control BRV diarrhea in endemic dairy farms where neonatal calf diarrhea represents an important economic problem (Svensson et al., 2003). The administration of milk supplemented with BRV immune egg yolk for the first 14 days of life induced high protection rates against BRV diarrhea in calves during the period of peak susceptibility to the infection. Secondly, positive modulation of the neonatal immune responses, including higher numbers of ASC and greater isotype diversity was observed in the intestinal mucosa. The immunomodulatory effects of egg yolk on the mucosal immune system characterized here may represent a novel advantage of IgY technology that will allow its use not only as a preventive strategy but also as a specific treatment of BRV diarrhea.
Supplementary Material
Table 1. Serum Ab titers against BRV measured weekly for all the experimental groups of calves.
Table 2. Ab titers against BRV in feces, measured weekly. Statistical analysis.
Table 3. Body weight measured at 0 PID and at 21 PID. Statistical analysis.
Figure 1. Box and whisker graph of the body weight gain from 0 to 21 PID of calves from Gp 1 and Gp 2.
Acknowledgments
We gratefully acknowledge the technical assistance of Diego Franco, Nancy Suárez Pérez, Alan Kahl, Gustavo Asenzo, José Vallejos, Osvaldo Zábal, Verónica Rocha and Matilde Villalba. We would like to thank to Dr. Horacio Terzolo from INTA Balcarce for his advice in IgY technology. We also thank the assistance of “Las Marías” dairy farm, which provided all the calves. This study was supported by a Basic Biomedical (FIRCA-BB) (R03), Fogarty Foundation, National Institute of Health (NIH), FIRCA Grant N° 60014561, USA; and INTA-BID PICT Nº 25375, Agencia de Promoción Científica, y Técnica, SECyT, Argentina. Lic. Celina G. Vega salary was provided by SECyT and CONICET fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aich P, Wilson HL, Kaushik RS, Potter AA, Babiuk LA, Griebel P. Comparative analysis of innate immune responses following infection of newborn calves with bovine rotavirus and bovine coronavirus. J Gen Virol. 2007;88:2749–2761. doi: 10.1099/vir.0.82861-0. [DOI] [PubMed] [Google Scholar]
- Bach G, Perrin-Cocon L, Gerossier E, Guironnet-Paquet A, Lotteau V, Inchauspe G, Fournillier A. Single lysophosphatidylcholine components exhibit adjuvant activities in vitro and in vivo. Clin Vaccine Immunol. 17:429–438. doi: 10.1128/CVI.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni RC, Blackhall J, Terzolo HR, Moreira AR, Auza N, Mattion N, Micheo GL, La Torre JL, Scodeller EA. Microbiology of diarrhoea in young beef and dairy calves in Argentina. Rev Argent Microbiol. 1990;22:130–136. [PubMed] [Google Scholar]
- Bendali F, Sanaa M, Bichet H, Schelcher F. Risk factors associated with diarrhoea in newborn calves. Vet Res. 1999;30:509–522. [PubMed] [Google Scholar]
- Besser TE, McGuire TC, Gay CC, Pritchett LC. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J Virol. 1988;62:2234–2237. doi: 10.1128/jvi.62.7.2234-2237.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger JC. A definition of bovine rotavirus virulence. J Gen Virol. 1994;75 ( Pt 10):2807–2812. doi: 10.1099/0022-1317-75-10-2807. [DOI] [PubMed] [Google Scholar]
- Candy DC. Rotavirus infection: a systemic illness? PLoS Med. 2007;4:e117. doi: 10.1371/journal.pmed.0040117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlander D, Kollberg H, Wejaker PE, Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res. 2000;21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornaglia EM, BM, Fitjman N, Schudel AA. Enzyme linked immunosorbent assay, immunofluorescent test and electrophoresis analysis of rotaviral RNA in the diagnosis and characterization of the bovine rotavirus. Revista Latinoamericana de Microbiologia. 1989:59–62. [Google Scholar]
- da Costa Mendes VM, de Beer M, Peenze I, Steele AD. Molecular epidemiology and subgroup analysis of bovine group A rotaviruses associated with diarrhea in South African calves. J Clin Microbiol. 1993;31:3333–3335. doi: 10.1128/jcm.31.12.3333-3335.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Chauhan RS, Mahendran M, Malik SV. Rotavirus diarrhea in bovines and other domestic animals. Vet Res Commun. 2009;33:1–23. doi: 10.1007/s11259-008-9070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. Rotavirus and their replication (Cap 54a) In: Fields BN, DMK, Howley PM, editors. Fields Virology. Lippincott - Raven; Philadelphia: 2001. [Google Scholar]
- Fernandez FM, Conner ME, Hodgins DC, Parwani AV, Nielsen PR, Crawford SE, Estes MK, Saif LJ. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from cows immunized with recombinant SA11 rotavirus core-like particle (CLP) or virus-like particle (VLP) vaccines. Vaccine. 1998;16:507–516. doi: 10.1016/S0264-410X(97)80004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaicoechea L, Bok K, Jones LR, Combessies G, Odeon A, Fernandez F, Parreno V. Molecular characterization of bovine rotavirus circulating in beef and dairy herds in Argentina during a 10-year period (1994–2003) Vet Microbiol. 2006;118:1–11. doi: 10.1016/j.vetmic.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Gonzalez DD, Mozgovoj MV, Bellido D, Rodriguez DV, Fernandez FM, Wigdorovitz A, Parreno VG, Dus Santos MJ. Evaluation of a bovine rotavirus VP6 vaccine efficacy in the calf model of infection and disease. Vet Immunol Immunopathol. 2010;137:155–160. doi: 10.1016/j.vetimm.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Heckert HP, Bardella I, Hofmann W, Oltmer S. Effect of antibody-containing egg powder on development of active immunity in calves. Dtsch Tierarztl Wochenschr. 1999;106:10–14. [PubMed] [Google Scholar]
- Ikemori Y, Kuroki M, Peralta RC, Yokoyama H, Kodama Y. Protection of neonatal calves against fatal enteric colibacillosis by administration of egg yolk powder from hens immunized with K99-piliated enterotoxigenic Escherichia coli. Am J Vet Res. 1992;53:2005–2008. [PubMed] [Google Scholar]
- Ikemori Y, Ohta M, Umeda K, Icatlo FC, Jr, Kuroki M, Yokoyama H, Kodama Y. Passive protection of neonatal calves against bovine coronavirus-induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet Microbiol. 1997;58:105–111. doi: 10.1016/S0378-1135(97)00144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara J, Tsunemitsu H. Correlation between maternal serum antibodies and protection against bovine rotavirus diarrhea in calves. J Vet Med Sci. 2000;62:219–221. doi: 10.1292/jvms.62.219. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Ohta M, Ikemori Y, Icatlo FC, Jr, Kobayashi C, Yokoyama H, Kodama Y. Field evaluation of chicken egg yolk immunoglobulins specific for bovine rotavirus in neonatal calves. Arch Virol. 1997;142:843–851. doi: 10.1007/s007050050123. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Ohta M, Ikemori Y, Peralta RC, Yokoyama H, Kodama Y. Passive protection against bovine rotavirus in calves by specific immunoglobulins from chicken egg yolk. Arch Virol. 1994;138:143–148. doi: 10.1007/BF01310045. [DOI] [PubMed] [Google Scholar]
- Mine Y, Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- Nelson K, Mine Duarte, Matar Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food and Agricultural Immunology. 2007;18:15. [Google Scholar]
- Parreno V, Bejar C, Vagnozzi A, Barrandeguy M, Costantini V, Craig MI, Yuan L, Hodgins D, Saif L, Fernandez F. Modulation by colostrum-acquired maternal antibodies of systemic and mucosal antibody responses to rotavirus in calves experimentally challenged with bovine rotavirus. Vet Immunol Immunopathol. 2004;100:7–24. doi: 10.1016/j.vetimm.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreno V, Constantini V, Cheetham S, Blanco Viera J, Saif LJ, Fernandez F, Leoni L, Schudel A. First isolation of rotavirus associated with neonatal diarrhoea in guanacos (Lama guanicoe) in the Argentinean Patagonia region. J Vet Med B Infect Dis Vet Public Health. 2001;48:713–720. doi: 10.1111/j.1439-0450.2001.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreño V, Marcoppido G, Vega C, Garaicoechea L, Rodriguez D, Saif La, Fernández F. Milk supplemented with immune colostrum: Protection against rotavirus diarrhea and modulatory effect on the systemic and mucosal antibody responses in calves experimentally challenged with bovine rotavirus. Veterinary Immunology and Immunopathology. 2010 doi: 10.1016/j.vetimm.2010.01.003. Article in press. [DOI] [PubMed] [Google Scholar]
- Perrin-Cocon L, Agaugue S, Coutant F, Saint-Mezard P, Guironnet-Paquet A, Nicolas JF, Andre P, Lotteau V. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine. 2006;24:1254–1263. doi: 10.1016/j.vaccine.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol. 2004;78:10213–10220. doi: 10.1128/JVI.78.19.10213-10220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif LJ, Fernandez FM. Group A rotavirus veterinary vaccines. J Infect Dis. 1996;174(Suppl 1):S98–106. doi: 10.1093/infdis/174.Supplement_1.S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif LJ, RB, Parwani A. Animal rotaviruses. In: AZ K, editor. Virus infections of the gastrointestinal tract. Marcel-Dekker; New York: 1994. pp. 279–367. [Google Scholar]
- Saif LJ, Weilnau P, Miller K, Stitzlein L. Isotypes of intestinal and systemic antibodies in colostrum-fed and colostrum-deprived calves challenged with rotavirus. Adv Exp Med Biol. 1987;216B:1815–1823. [PubMed] [Google Scholar]
- Schade R, Burger W, Schoneberg T, Schniering A, Schwarzkopf C, Hlinak A, Kobilke H. Avian egg yolk antibodies. The egg laying capacity of hens following immunisation with antigens of different kind and origin and the efficiency of egg yolk antibodies in comparison to mammalian antibodies. ALTEX. 1994;11:75–84. [PubMed] [Google Scholar]
- Schade R, Hlinak A. Egg Yolk Antibodies, State of the Art and Future Prospects. ALTEX. 1996;13:5–9. [PubMed] [Google Scholar]
- Schade RCS, Hendriksen C, Erhard M, Hugl H, Koch G, Larsson A, Pollmann W, van Regenmortel M, Rijke E, Spielmann H, Steinbusch H, Straughan D. The Production of Avian (Egg Yolk) Antibodies: IgY. ATLA. 1996;24:925–934. [Google Scholar]
- Stott GH, Fellah A. Colostral immunoglobulin absorption linearly related to concentration for calves. J Dairy Sci. 1983;66:1319–1328. doi: 10.3168/jds.S0022-0302(83)81941-9. [DOI] [PubMed] [Google Scholar]
- Svensson C, Lundborg K, Emanuelson U, Olsson SO. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev Vet Med. 2003;58:179–197. doi: 10.1016/s0167-5877(03)00046-1. [DOI] [PubMed] [Google Scholar]
- Tini M, Jewell UR, Camenisch G, Chilov D, Gassmann M. Generation and application of chicken egg-yolk antibodies. Comp Biochem Physiol A Mol Integr Physiol. 2002;131:569–574. doi: 10.1016/s1095-6433(01)00508-6. [DOI] [PubMed] [Google Scholar]
- To TL, Ward LA, Yuan L, Saif LJ. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79 ( Pt 11):2661–2672. doi: 10.1099/0022-1317-79-11-2661. [DOI] [PubMed] [Google Scholar]
- Touchette KJ, O'Brien ML, Coalson JA. Liquid egg as an alternative protein source in calf milk replacers. J Dairy Sci. 2003;86:2622–2628. doi: 10.3168/jds.S0022-0302(03)73857-0. [DOI] [PubMed] [Google Scholar]
- Varshney KC, Bridger JC, Parsons KR, Cook R, Teucher J, Hall GA. The lesions of rotavirus infection in 1- and 10-day-old gnotobiotic calves. Vet Pathol. 1995;32:619–627. doi: 10.1177/030098589503200602. [DOI] [PubMed] [Google Scholar]
- Xu YP, Zou WM, Zhan XJ, Yang SH, Xie DZ, Peng SL. Preparation and determination of immunological activities of anti-HBV egg yolk extraction. Cell Mol Immunol. 2006;3:67–71. [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Serum Ab titers against BRV measured weekly for all the experimental groups of calves.
Table 2. Ab titers against BRV in feces, measured weekly. Statistical analysis.
Table 3. Body weight measured at 0 PID and at 21 PID. Statistical analysis.
Figure 1. Box and whisker graph of the body weight gain from 0 to 21 PID of calves from Gp 1 and Gp 2.