Abstract
Transcranial direct current stimulation (tDCS) can up- and down-regulate cortical excitability depending on current direction, however our abilities to measure brain-tissue effects of the stimulation and its after-effects have been limited so far. We used regional cerebral blood flow (rCBF), a surrogate measure of brain activity, to examine regional brain-tissue and brain-network effects during and after tDCS. We varied the polarity (anodal and cathodal) as well as the current strength (0.8 to 2.0 mA) of the stimulation. Fourteen healthy subjects were randomized into receiving either anodal or cathodal stimulation (two subjects received both, one week apart) while undergoing Arterial Spin Labeling (ASL) in the MRI scanner with an alternating off-on sampling paradigm. The stimulating, MRI-compatible electrode was placed over the right motor region and the reference electrode over the contralateral supraorbital region. SPM5 was used to process and extract the rCBF data using a 10mm spherical volume-of-interest (VOI) placed in the motor cortex directly underneath the stimulating scalp electrode. Anodal stimulation induced a large increase (17.1%) in rCBF during stimulation, which returned to baseline after the current was turned off, but exhibited an increase in rCBF again in the post-stimulation period. Cathodal stimulation induced a smaller increase (5.6%) during stimulation, a significant decrease compared to baseline (−6.5%) after cessation, and a continued decrease in the post-stimulation period. These changes in rCBF were all significant when compared to the pre-stimulation baseline or to a control region. Furthermore, for anodal stimulation, there was a significant correlation between current strength and the increase in rCBF in the on-period relative to the pre-stimulation baseline. The differential rCBF after-effects of anodal (increase in resting state rCBF) and cathodal (decrease in resting state rCBF) tDCS support findings of behavioral and cognitive after-effects after cathodal and anodal tDCS. We also showed that tDCS not only modulates activity in the brain region directly underlying the stimulating electrode but also in a network of brain regions that are functionally related to the stimulated area. Our results indicate that ASL may be an excellent tool to investigate the effects of tDCS and its stimulation parameters on brain activity.
Introduction
Non-invasive, transcranial electrical brain stimulation, a technique developed many decades ago (Bindman, Lippold et al. 1964), has recently re-emerged as a promising tool to non-invasively modulate brain activity, to causally probe cortical representations of sensorimotor and cognitive functions, and to facilitate treatment of various neurologic and psychiatric disorders (Priori, Berardelli et al. 1998; Nitsche and Paulus 2000; Schlaug and Renga 2008). Transcranial direct current stimulation (tDCS) modulates the excitability of a targeted brain region non-invasively by altering neuronal membrane potentials (Bindman, Lippold et al. 1962; Purpura and McMurtry 1965). Bindman and colleagues (Bindman, Lippold et al. 1964) showed in animals that DC stimulation may increase, decrease, or even silence firing of neurons in the primary motor region (M1). Nitsche and Paulus (Nitsche and Paulus 2000; Nitsche, Liebetanz et al. 2002) showed that cathodal polarization of the motor cortex reduced the size of the transcranial magnetic stimulation (TMS) induced motor evoked potentials (MEP) in humans. In contrast, anodal stimulation increased the size of the MEP (up to 150%), suggesting a differential effect of polarization on cortical excitability. The duration of these electrophysiological effects outlasted the duration of the stimulation by up to 90 minutes after sessions of 1 mA polarization lasting 9–13 minutes (Nitsche, Fricke et al. 2003) (Nitsche and Paulus 2000; Nitsche, Liebetanz et al. 2002) (Nitsche, Nitsche et al. 2003).
Cathodal tDCS has mainly been used to create temporary cortical dysfunctions (“virtual lesions”) to causally probe cortical sensorimotor and cognitive functions affected by the stimulation (Vines, Nair et al. 2006; Vines, Schnider et al. 2006). Following cathodal stimulation, decreases in performance have been found in motor skills after stimulating the motor cortex (Vines, Nair et al. 2006), and auditory-discrimination, short-term auditory memory (Vines, Schnider et al. 2006; Mathys, Loui et al. 2010), and tactile perception after somatosensory cortex stimulation (Rogalewski, Breitenstein et al. 2004). Similarly, following anodal tDCS, improved performances have been observed in implicit motor learning (Nitsche, Schauenburg et al. 2003), sensorimotor skills (Vines, Nair et al. 2006; Vines, Cerruti et al. 2008; Vines, Nair et al. 2008; Reis, Schambra et al. 2009), visuomotor coordination (Antal, Nitsche et al. 2004), visual, auditory, and motor memory functions (Ragert, Vandermeeren et al. 2008; Sparing, Dafotakis et al. 2008; Elmer, Burkard et al. 2009; Galea and Celnik 2009; Chi, Fregni et al. 2010) and probabilistic classification (Kincses, Antal et al. 2004). However, some studies have not found any enhancement effects compared to sham stimulation when anodal tDCS was applied (Rogalewski, Breitenstein et al. 2004; Mathys, Loui et al. 2010).
The prolonged effects of tDCS have been attributed to long-term potentiation (LTP) and longterm depression (LTD) (Hattori, Moriwaki et al. 1990; Moriwaki 1991; Islam, Aftabuddin et al. 1995). Dextromethorphan, an NMDA (N-methyl-D-aspartic acid)-receptor antagonist, suppressed post-tDCS effects of both anodal and cathodal stimulation, strongly suggesting the involvement of NMDA receptors in both types of DC-induced neuroplasticity. In contrast, Carbamazepine, which stabilizes the inactivated state of sodium channels, selectively eliminated anodal effects, suggesting that after-effects of anodal tDCS require a depolarization of membrane potentials (Liebetanz, Nitsche et al. 2002). This study (Liebetanz, Nitsche et al. 2002) provided pharmacological evidence that induction of the after-effects of tDCS requires a combination of glutamatergic and membrane mechanisms, similar to the induction of established types of short- or long-term neuroplasticity.
To date, there has only been indirect evidence for tDCS-induced modulation of cortical excitability, through TMS-induced MEPs, behavioral effects, pharmacological effects, and theoretical modeling data. In using more direct measures of the brain activity, one might be able to better examine quantitative brain-tissue effects during the stimulation, effects due to changes in tDCS parameters, and to test the focality of tDCS or to determine whether focally applied tDCS also leads to changes in an interconnected network of brain regions.
Neuroimaging techniques have the advantage of measuring correlates of neuronal activity not only under or in close proximity to the externally applied electrode, but also in remote brain regions before, during and after stimulation. Some studies have looked at blood flow changes with respect to TMS (Baudewig, Siebner et al. 2001) and our tDCS study can be related to these TMS studies. Using tDCS in conjunction with positron emission tomography (PET), Lang and colleagues (Lang, Siebner et al. 2005) found increased rCBF effects during a motor task after tDCS stimulation. However, they only measured the post-stimulation rCBF differences after real and sham stimulation and did not examine changes in rCBF going from off to on to off conditions.
Thus far, there has only been one study (Kwon, Ko et al. 2008) that showed brain activity changes in the stimulated region concurrently with tDCS. These authors applied anodal tDCS over the hand region of the precentral gyrus while using blood oxygen level dependent (BOLD) imaging and showed brain activity changes in M1, supplementary motor area (SMA) and the contralateral parietal region. However, signal changes were only seen in the fourth session of stimulation (after 63 seconds of tDCS) and no further data after the fourth session was presented. A recent study also used BOLD imaging in conjunction with tDCS on the primary motor cortex, but they failed to observe changes in the targeted region after anodal and cathodal stimulation of the left precentral gyrus with 1 mA (Antal, Polania et al. 2011). The stimulation was applied in a 20s ON-OFF paradigm with 8 repetitions. The short stimulation sessions and the low dynamic range of BOLD signal changes might have been responsible for this negative study. Effects of electrodes and possibly the tDCS currents themselves on the T2* signal from BOLD scans may also have contributed to the reduced sensitivity of these BOLD studies. A different approach is the use of Magnetic Resonance Spectroscopy (MRS) to examine tDCS effects on neuronal and transmitter-receptor markers. A recent study showed that anodal stimulation lead to locally reduced GABA (gamma Aminobutyric acid) while cathodal stimulation resulted in a decrease in glutamatergic neuronal activity (Stagg, Best et al. 2009).
A relatively new imaging technique, arterial spin labeling (ASL), which uses magnetically labeled arterial blood water as an endogenous tracer (Detre, Leigh et al. 1992; Williams, Detre et al. 1992; Alsop and Detre 1998) offers the possibility to determine baseline perfusion values and to measure regional cerebral blood flow (rCBF) quantitatively in order to assess the immediate and after-effects of tDCS. The excellent temporal stability of ASL experiments (Aguirre, Detre et al. 2002) is particularly useful for examining tDCS effects as compared to BOLD imaging, since tDCS is usually applied for several minutes in order to modulate behavior and cognition (Nitsche and Paulus 2000; Nitsche, Liebetanz et al. 2002). A recent TMS study has used ASL to assess rCBF changes of high and low frequency stimulation (Moisa, Pohmann et al. 2009) and found robust rCBF increases in motor and premotor areas due to stimulation. However, ASL has not been used to assess effects of tDCS.
Given the lack of studies looking at direct brain-tissue effects during and after tDCS, our aims were four-fold. First, we aimed to show that tDCS’s regional effects on the brain will lead to rCBF changes and that these effects can be replicated by turning the current on and off. Second, we aimed to examine a possible differential effects of current polarity (anodal versus cathodal) and current strength on rCBF. Third, we wanted to examine how the documented behavioral effects after cessation of tDCS, which may be more directly related to NMDA receptor activities, would be reflected in post-stimulation rCBF changes. Finally, we intended to take advantage of the whole-brain ASL data to also examine possible effects of tDCS on regions of the brain that were remote from the stimulation site.
Methods and Materials
Subjects
Fourteen healthy, young adults participated in this study (mean age 25.7; SD 5.7; 9 males). Six of the subjects underwent only anodal stimulation and six underwent only cathodal stimulation. Two of the subjects underwent both conditions, separated by at least a week between the sessions, amounting to a total of eight anodal and eight cathodal sessions. The mean ages for the anodal and cathodal groups were 26.3 (SD 6.9) and 24.3 (SD 4.3) years old, respectively. All participants were right handed, as determined by the Edinburgh Handedness Inventory (Oldfield 1971) with laterality quotients ranging from 80–100. All subjects gave their written informed consent following protocol approved by the Committee on Clinical Investigations at our institution.
TDCS setup
The tDCS was delivered by a battery-driven constant-current stimulator (Iomed Phoresor II Auto Model PM850) through a pair of MRI-compatible Ag/AgCl electrodes (Brain Vision LLC, Richardson, TX), which were placed inside saline-soaked circular foam pads with a surface area of 20cm2. The electrodes were modified by adding 16.5 kΩ resistance, in the form of a 15kΩ electrode head and five equidistantly distributed 330Ω resistors in the wires, to help dampen any potential resonant coupling between the radiofrequency transmission of the MRI scanner and the wires (Angelone, Vasios et al. 2006). Such coupling is a potential source of RF heating near the wires that could potentially cause burns to the subject. Lead wires were kept more than 2 cm from the subject with pads and towels to provide further insurance against burns. Subjects were also instructed of the potential for heating and asked to signal immediately if any heating was perceived near the electrodes or wires. The subjects were given anodal and cathodal tDCS. The average current dosages for anodal (1.4 mA; SD 0.26; range 1.0–1.7) and cathodal (1.4 mA; SD 0.38; range 0.8–2.0) stimulations were the same.
The same unihemispheric montage was used for both anodal and cathodal stimulations, but the polarity was switched to expose the underlying brain region to either anodal or cathodal stimulation. We placed the stimulating electrode over the C4 (using the 10–20 EEG system) and the reference electrode over the left supra-orbital region. For details on electrode location and/or stimulation set-up see Okamoto et al (Okamoto, Dan et al. 2004) and our previous publication (Schlaug and Renga 2008).
MR Image Acquisition and Analysis
After the electrodes were positioned and held securely by elastic bandages, subjects were positioned in a 3-Tesla General Electric MR scanner and images were acquired using body coil transmission and a standard 8-channel radiofrequency receive only head coil. Head motion was minimized by using foam padding and forehead restraining straps. The battery-operated direct current stimulator was positioned in the MRI room approximately 2 meters away from the center of the MR scanner. Cables of approximately 3 meters connected the stimulator to the subject’s scalp via the MRI compatible electrodes.
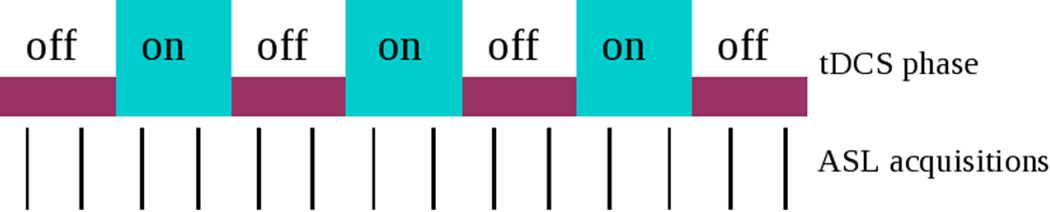
A scout image was first acquired to grossly assess the head positioning in the scanner, followed by a high-resolution strongly T1-weighted Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (voxel size 0.93×0.93×1.5mm). This was then followed by a series of ASL acquisitions during the tDCS on and off phases. Subjects kept their eyes closed during all the ASL acquisitions. ASL images were acquired with background suppression, pulsed continuous labeling, a post-labeling delay of 1.5s and a stack of interleaved spirals fast spin echo acquisitions. Eight spiral interleaves and a 24 cm axial FOV provided 3.7mm in place resolution. The nominal slice thickness was 4 mm but the long echo train acquisition slightly blurs resolution in the slice direction. Each ASL scan required approximately 3.5 minutes and the acquisition of a reference image for rCBF quantification added an additional minute to the acquisition (Dai, Garcia et al. 2008). Two ASL acquisitions were performed during the tDCS ON periods while two ASL images were acquired during the OFF periods. A total of three ON periods were conducted on each subject, beginning with an OFF period to serve as baseline, to examine the reproducibility of tDCS effect. Three ASL images were acquired during the initial OFF period or baseline phase lasting approximately ten minutes. The other periods lasted about seven minutes each for a total experimental duration of approximately 50 minutes (see Figure 1).
Figure 1. Experimental Design.
Interleaved tDCS-off and tDCS-on design while acquiring ASL images, where two ASL images were acquired at each on phase and two ASL images were acquired at each off phase, beginning with a baseline consisting of three ASL acquisitions.
Using software routines implemented in SPM5, ASL difference images were corrected for movement, spatially normalized with the PET (for ASL) and T1 (for anatomical) templates, and smoothed with an isotropic Gaussian kernel (8mm full-width at half-maximum). Subject and condition effects were estimated using a general linear model. Global differences in scan intensity were removed by scaling each scan in proportion to its global intensity, and low-frequency drifts were removed using the default temporal high-pass filter. A flexible factorial model was used for the analysis of the ASL data. The off and on phases were modeled as separate conditions.
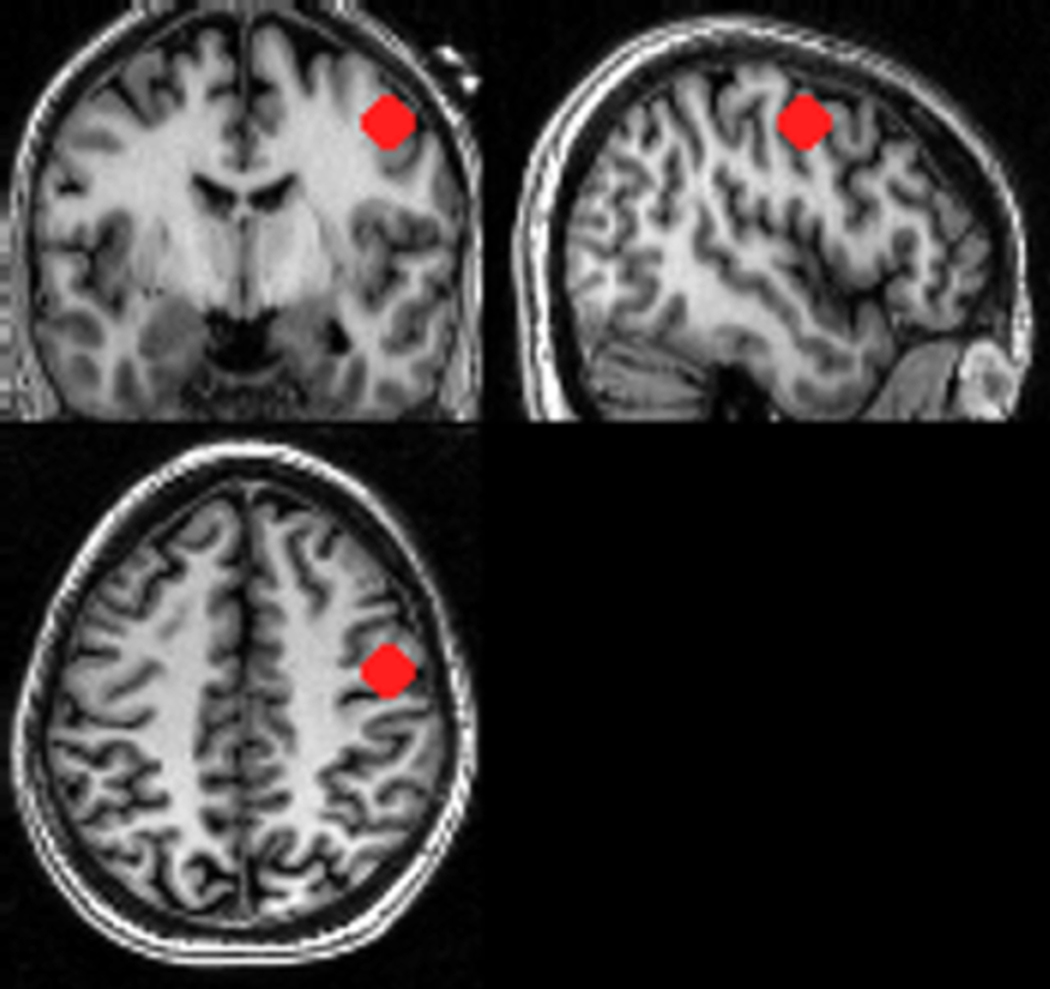
We did two separate analyses using our GLM. The first analysis used ON vs OFF and its reverse contrast for both anodal and cathodal cases to look at global differences between the ON and OFF conditions. A threshold of p<0.001 (T>4.785) was used (uncorrected at the group level). The second analysis used a volume of interest (VOI) placed in the brain region directly underneath the scalp electrode (MRI-compatible markers were used to visualize the electrode position in T1-weighted images) to extract the time course of the rCBF changes across the ON and OFF conditions. The subject-specific normalized anatomical image was used to guide VOI placement. This region was placed to cover the gray matter and underlying white matter of the targeted precentral gyrus, which contains mainly primary motor but also posterior premotor cortex (see Figure 2). The contrasts were then thresholded with a p-value of one, such that none of the voxel activations were suppressed, and the first eigenvariate was extracted from the VOI for each subject. SPM’s first eigenvariate is its estimated weighted mean of the VOI, in this case the time course of rCBF (Friston, Rotshtein et al. 2006). A 10mm spherical VOI was also placed in the left temporo-occipital region (approximately [x=−57, y=−59, z=9] in MNI space) as a control region to ensure that any rCBF changes found in the motor region VOI were not global changes. The extracted time courses of all the ON and OFF periods were then normalized to their means. The multiple ON and OFF periods were used to document the reproducibility and reliability of the signal changes (see Figures 3 and 4). In addition, the eigenvariates were used as vectors for a multiple regression model to detect all regions of the brain that would show similar changes in rCBF. This was done for each individual subject and the corresponding contrast images were then taken to a second level design, where t-tests were done to reveal a network of brain regions that showed a similar time course of rCBF changes to the right precentral gyrus VOI.
Figure 2. VOI Placement.
Positioning of VOI underneath the stimulating electrode (visualized by using an MRI compatible marker) in a subject’s spatially normalized brain. One can clearly see in the three orthogonal projections how well the electrode position of C4 corresponded to the precentral gyrus position
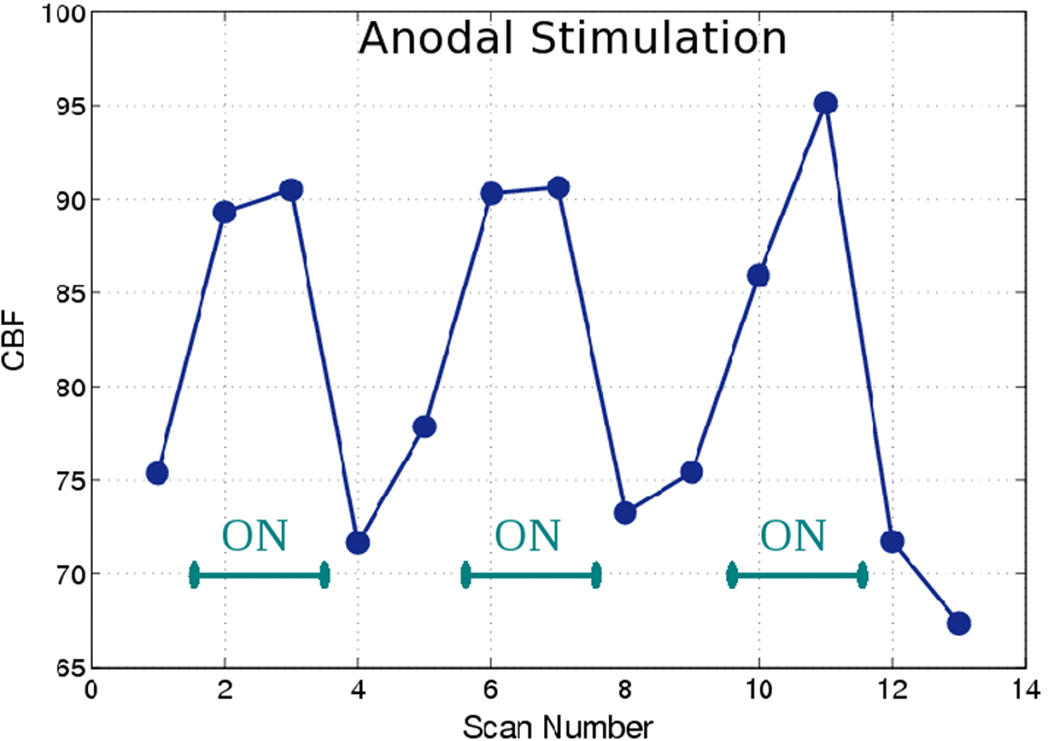
Figure 3. Anodal rCBF changes.
Changes in rCBF over time in a typical subject fitted with the anodal montage, showing immediate, reproducible, and significant increases in rCBF during stimulation in our VOIs, with subsequent decreases to pre-stimulus levels and a tendency to rise back up again. The on-phases are between the green dotted lines.
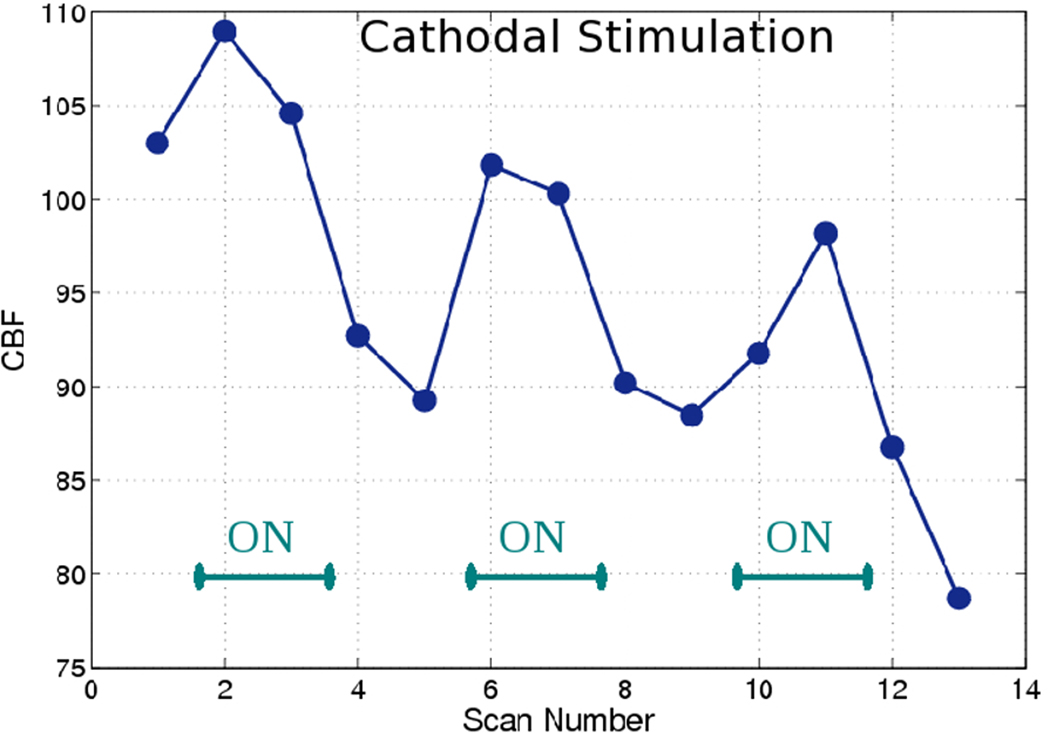
Figure 4. Cathodal rCBF changes.
Changes in rCBF over time in a typical subject fitted with the cathodal montage, showing reproducible but modest (compared to anodal stimulation – see Figure 3) increases in rCBF during stimulation with subsequent decreases to below pre-stimulus levels and a tendency for continued decrease in rCBF. The ON phases are clearly marked.
We only used the first OFF-ON-OFF sequence for quantitative analysis of tDCS induced rCBF effects (see Figure 5) in order to avoid any residual effects of the first stimulation on the subsequent tDCS stimulation phases (see also (Fricke, Seeber et al. 2011) for a discussion on homeostatic effects). The baseline was taken as the average of all the pre-stimulation time points excluding the first one to minimize magnetization equilibrium and stress effects of the subject. The maximum value is used for the ON phase to capture the peak rCBF change of the stimulation. The post-stimulation OFF phase had two imaging time points before stimulation was started again (see Figure 1) and thus were split into two time points (first OFF and second OFF) to detect possible trends in rCBF in the post-stimulation period. The relative changes in rCBF were calculated between the time points and compared between anodal and cathodal stimulation as well as with our control region.
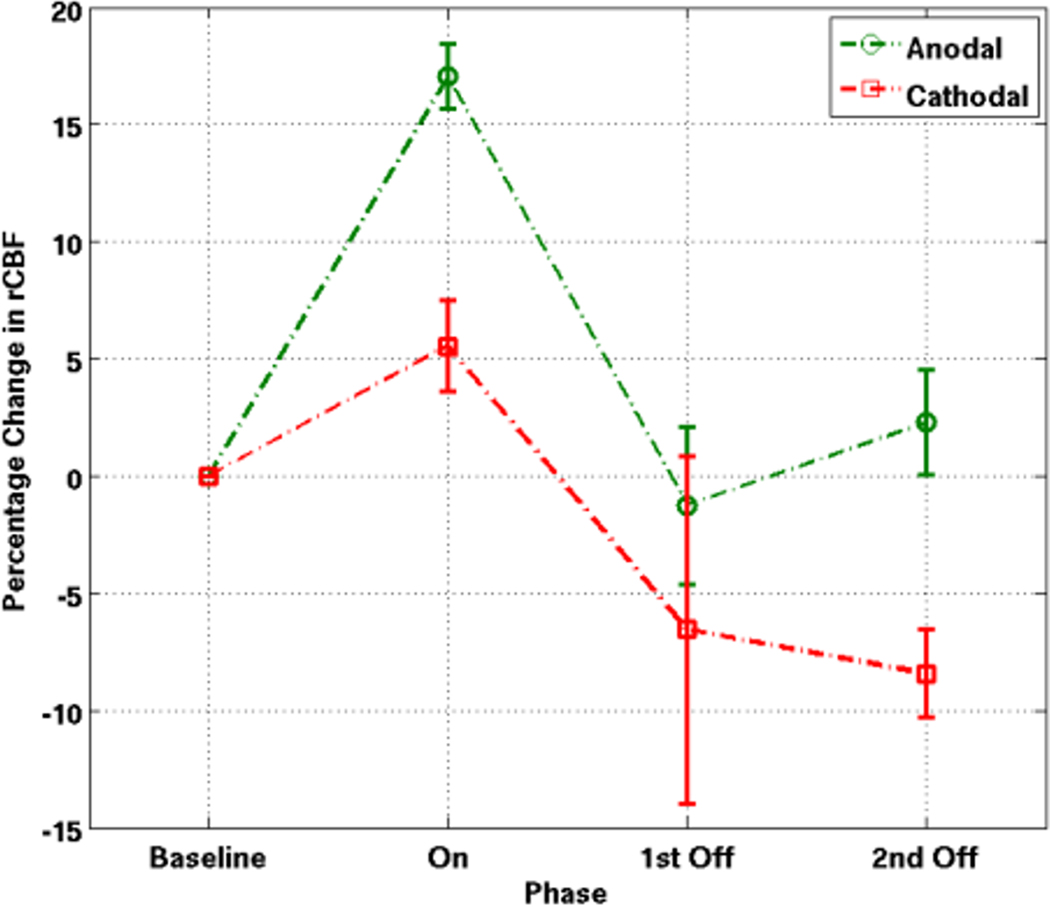
Figure 5. Average rCBF changes during and after the anodal and cathodal stimulation.
Average changes in rCBF (normalized to zero) for the first OFF-ON-OFF of anodal and cathodal stimulation across all subjects. The description 1st off and 2nd OFF refers to the two acquisitions after the end of the stimulation and reflects the trend in rCBF after the stimulation has been turned off.
In addition to looking at the blood flow changes of the first peak, we also examined the effects of current strength on rCBF changes extracted from our VOI in the precentral gyrus. We applied linear correlations to look at rCBF changes between each time point of interest (baseline, ON, first OFF, and second OFF) with the different current strengths applied.
Results
1. Safety and assessment of image distortion
Our results showed that transcranial direct current stimulation can be safely administered in the MR environment. None of our subjects reported any adverse effects and we were able to obtain brain signal changes that correlated with the alternating OFF and ON periods of the direct current stimulation (see Figures 3 and 4). The brain images did not show any distortion, signal loss, or signs of elevated flip angles near the electrodes.
2. Regional CBF changes comparing on- and off-periods, anodal and cathodal stimulation
Anodal stimulation led to a large increase in rCBF in the brain tissue underlying the stimulating electrode (mean of +17.1%; SD 1.4). This response was reliable and reproducible both within and between subjects (see Figures 3 and 4 for typical anodal and cathodal responses). Upon termination of the tDCS stimulus (after about 8 min), the rCBF returned to slightly below the pre-stimulus level (−18.3% compared to the peak rCBF; SD 3.4) with a slight increase in the OFF period (+3.5%; SD 2.4) from the first OFF time point to the second OFF time point (see first OFF-ON-OFF average rCBF changes in Figure 5). These changes in rCBF were all significant (p<0.05) when compared to the rCBF changes in our control region. The changes in the control region were +5.7% (SD 3.8) for the OFF-ON transition and −5.4% (SD 6.8) for the ON-OFF transition and −1.7% (SD 7.5) for the percentage difference between first OFF and second OFF time points. The magnitudes of change from OFF to ON and ON to OFF for the anodal condition did not differ significantly.
Cathodal stimulation led to a modest increase in rCBF (mean of +5.6%; SD 2.0) when the stimulation was turned on and a more than two-fold decrease in rCBF from the peak of the ON period (−12.1%; SD 7.4) when the stimulation was turned off. In contrast to the anodal condition, there was a significant difference between the magnitudes of change from OFF to ON and ON to OFF for the cathodal condition (p<0.05). There was also a further decrease in rCBF between the first OFF and second OFF time points (−1.9%; SD 1.8). These changes in rCBF were significant at all three phases (p<0.05) when compared to the rCBF changes in our control region. The changes in the control region were −3.9% (SD 10.4) for the OFF-ON transition and −3.4% (SD 9.5) for the ON-OFF transition and +4.3% (SD 4.9) for the percentage difference between the two imaging timepoints in the OFF period.
Comparing the changes between the anodal and cathodal conditions, we found significant differences in the relative rCBF increases (p<0.001) when the stimulation was turned on, when the stimulation was switched off (p<0.05), and between the first OFF and second OFF time points (p<0.001).
3. Regional CBF changes and their relation to current strength
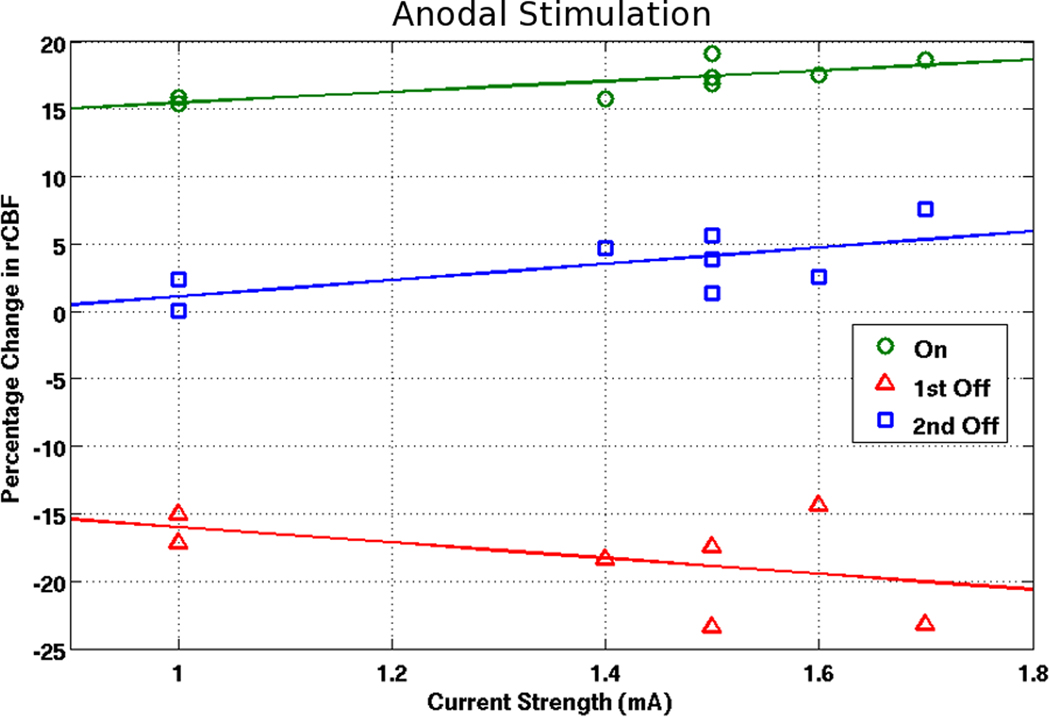
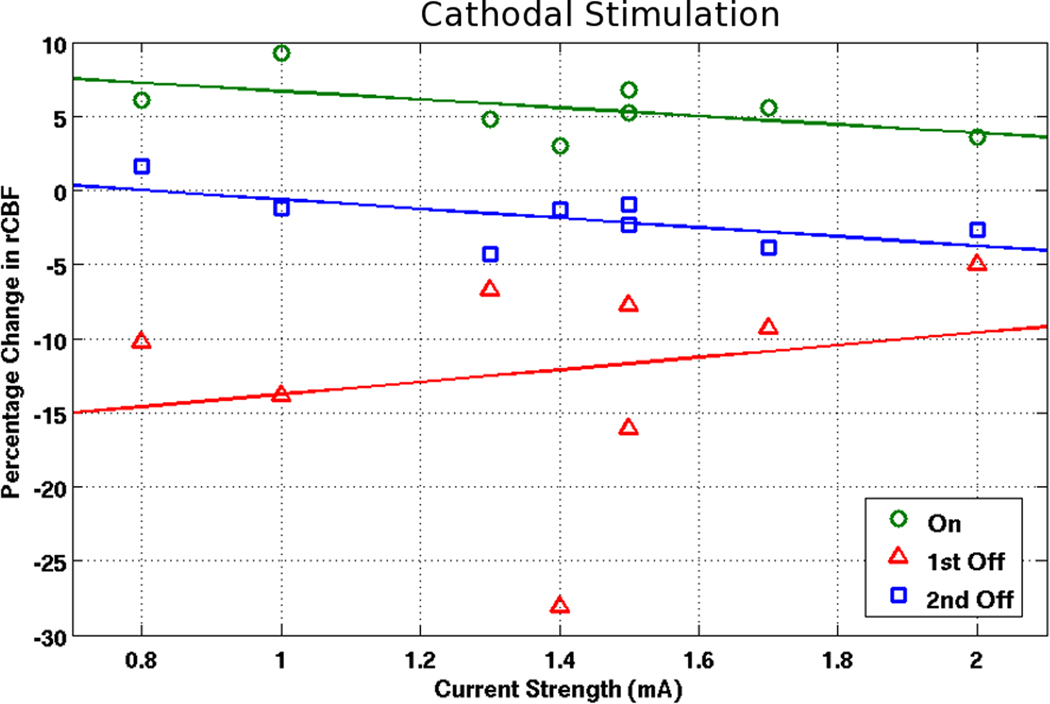
For anodal cases (see Figure 6), there was a significant correlation (r=0.77; p<0.05) for larger increases with higher currents when the stimulation was turned on. For cathodal cases (see Figure 7), there was an inverse relationship (not significant) for smaller increases with higher currents (r=−0.55; p=0.16). Furthermore, there was a positive trend (r=0.65; p=0.08) for the change in rCBF between the first and second timepoint in the OFF phase for the anodal condition and a negative trend (r=−0.64; p=0.09) for the cathodal condition. No particular trends were found for the decreases in rCBF upon cessation of stimulation in relation to current applied.
Figure 6. Correlating rCBF changes with current strength (anodal condition).
Correlating current strength with rCBF changes in the ON, first OFF, and second OFF time point for anodal montages.
Figure 7. Correlating rCBF changes with current strength (cathodal condition).
Correlating current strength with CBF changes in the ON, first OFF, and second OFF time point for cathodal montages.
4. Local and remote rCBF changes
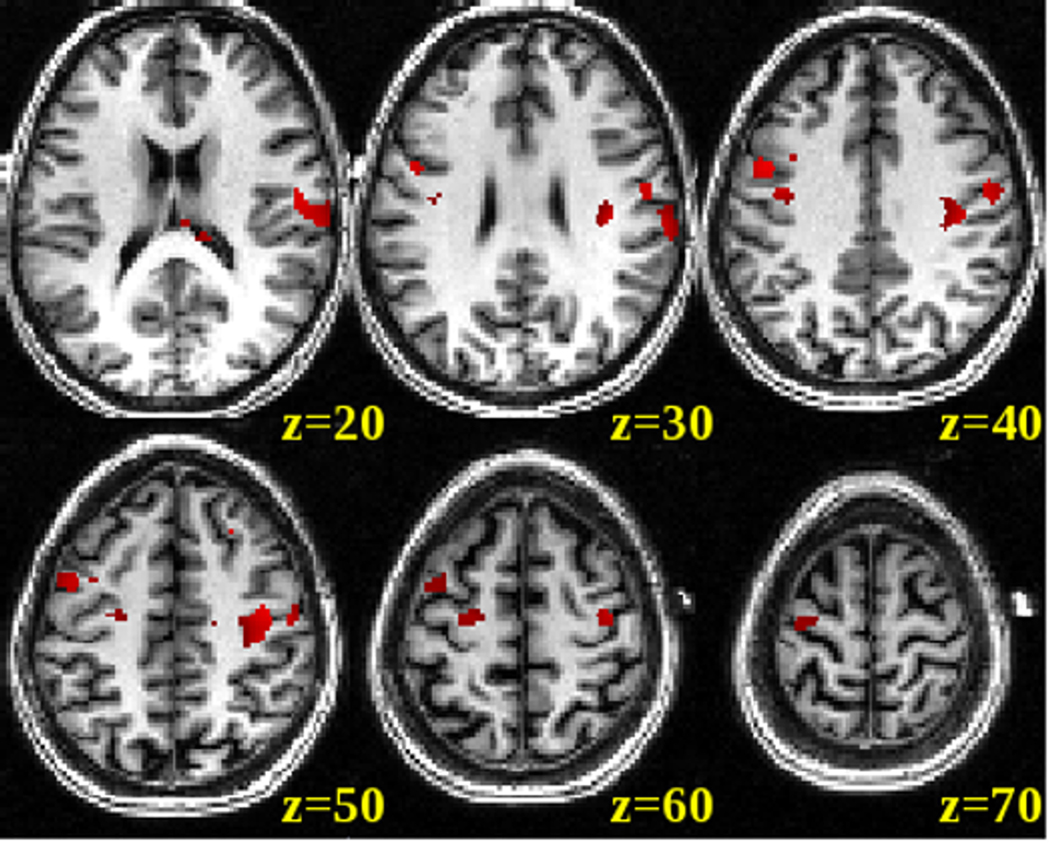
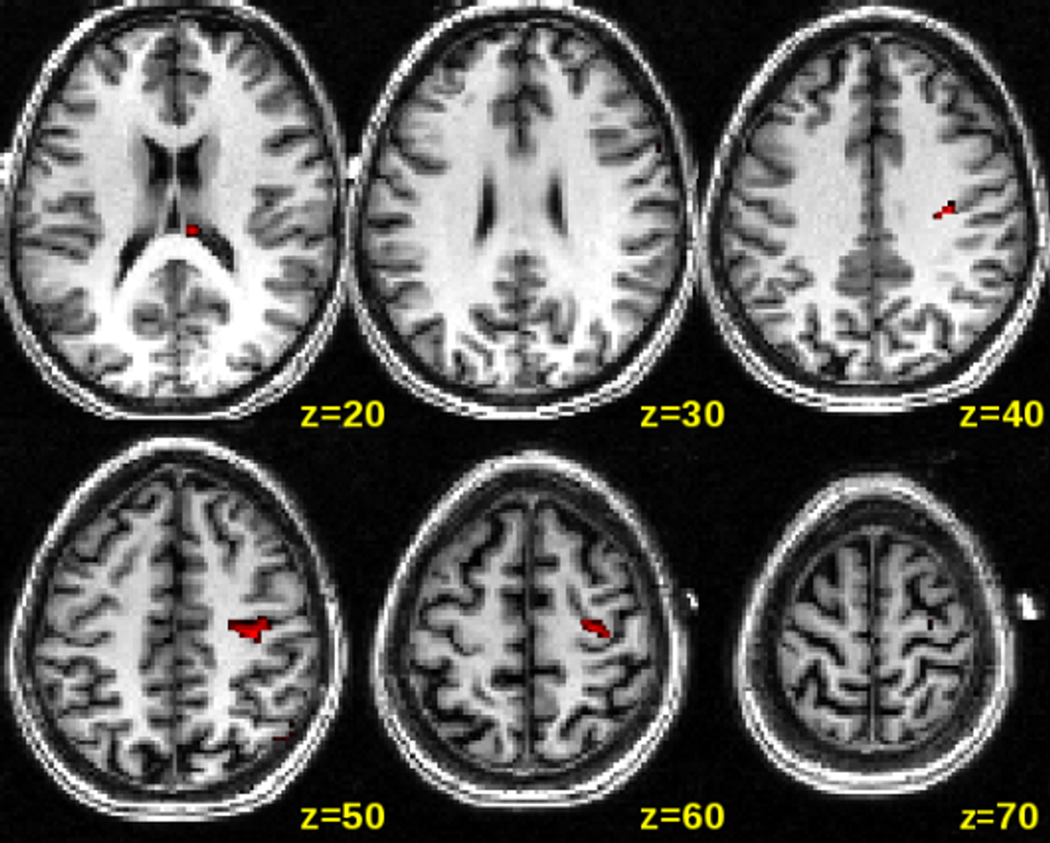
Using the time course extracted from the VOI in a regression analysis across the entire brain space, we found a network of brain areas that showed similar rCBF changes to the targeted brain region (p<0.001). This network of brain regions included the targeted stimulation site (right precentral gyrus), ipsilateral inferior motor and premotor regions, but also somewhat less strong contralateral motor and premotor regions (see Figure 8). It is interesting to note that the ipsilateral regions are similar to the clusters we found in the direct voxel-by-voxel ON vs OFF contrast for the anodal condition (see Figure 9). The voxel-by-voxel ON vs OFF contrast for the cathodal condition did not yield any significant voxels at the p<0.001 threshold. The reverse contrasts of OFF vs ON for both anodal and cathodal conditions did not yield any significant voxels at the p<0.001 threshold.
Figure 8. CBF changes in a network of brain regions for the anodal condition.
Averaged distribution of CBF response across the entire brain space correlated with the timecourse obtained from the VOI under the electrode for the anodal condition. Significant correlations (p<0.001, uncorrected at the group level) were overlaid onto a single spatially standardized brain.
Figure 9. Voxel-wise whole brain analysis of ON vs OFF for the anodal condition.
Significant voxels (p<0.001, uncorrected at the group level) were overlaid onto a single spatially standardized brain. Besides the strong activation of the precentral gyrus, there were also very small clusters of voxels in the premotor, and parietal cortex on the ipsilateral hemisphere.
Discussion
Our results show that non-invasive brain stimulation with tDCS and simultaneous noninvasive blood flow imaging in the MRI environment is technically feasible and safe. TDCS can modulate rCBF quickly and reproducibly. Both modes of stimulation led to an increase in rCBF during the stimulation phase, although the magnitude of change was about three times higher for anodal stimulation than for cathodal stimulation. One can speculate that the difference in blood flow increases during the stimulation phase between anodal and cathodal stimulation may be due to the smaller number of inhibitory synapses (although some would argue higher efficiency) as compared to excitatory synapses; this might account for the smaller increases in blood flow during the cathodal stimulation (Koos and Tepper 1999; Megias, Emri et al. 2001). Thus, activation of both excitatory and inhibitory networks leads to an increase in rCBF, but due to fewer synapses, and hence reduced demand for energy, activation of an inhibitory network might lead to a smaller local increase in rCBF. Alternatively, the difference may be due to modulations in glutamatergic activity, which is sufficient to induce LTD (Stagg, Best et al. 2009) or a direct effect of tDCS on blood vessels (Durand, Fromy et al. 2002).
The magnitudes of change in rCBF for the anodal and cathodal stimulations were comparable to the range of changes seen in TMS studies using either PET or ASL (Moisa, Pohmann et al. 2009). The 17.1% increase during anodal stimulation is comparable with local blood flow increases seen in suprathreshold high frequency (10Hz) TMS for the motor cortex. Similarly, the much lower increases in rCBF with cathodal stimulation is comparable to blood flow increases seen with low frequency (<2 Hz) TMS (Fox, Ingham et al. 1997; Chouinard, Van Der Werf et al. 2003; Fox, Narayana et al. 2006; Moisa, Pohmann et al. 2009). Subthreshold rTMS, where the stimulation does not elicit muscle twitches in the contralateral hand, showed mixed results, with some studies finding no significant rCBF changes (Bestmann, Baudewig et al. 2004) and others finding a small increase in the range of 5 to 10 percent (Fox, Narayana et al. 2006). Higher frequency repetitive TMS is generally considered to cause an increase in excitability (like anodal stimulation) while lower frequency or subthreshold TMS is thought to increase local inhibition (similar to cathodal stimulation).
An interesting observation was made during the tDCS OFF period in our data. For anodal stimulation, rCBF increased in the second OFF period, possibly reflecting an increase in excitability that outlasted the anodal stimulation period. Contrary to that, after the cathodal stimulation, there was a continued decrease in rCBF, possibly reflecting a decrease in excitability or a persistent local inhibition that outlasted the cathodal stimulation period. This suggests that rCBF may also be a surrogate marker of the after-effects of tDCS that have been shown in behavioral and neurophysiological experiments to persist for up to 90 minutes after sessions of 1 mA polarization lasting 9–13 minutes (Nitsche 2002; Nitsche, Fricke et al. 2003) (Nitsche, Nitsche et al. 2003).
We found a linear increase in rCBF with increasing anodal current strength. This is similar to the results of a recent TMS-CASL study (Moisa, Pohmann et al. 2009), but is different from a study by Paus and colleagues (Paus, Jech et al. 1998) in which a negative correlation between rCBF and pulse trains (10 Hz rTMS spaced at 2s intervals) applied to M1 was observed. The discrepancy in these two TMS studies might have been due to using supra-versus sub-motor threshold stimulation. However, the variation in rCBF as a function of current strength is small within the tested range of 1.0–1.7 mA when compared to the much larger rCBF changes observed when the polarity of stimulation was reversed, highlighting the importance of current polarity over current dosage.
Functionally related regions on both hemispheres showed rCBF changes that mirrored the time course of rCBF changes in the region directly under the electrode. This network of brain regions showed some similarity to a network of activated motor-related regions also described in TMS studies (Fox, Ingham et al. 1997; Paus, Jech et al. 1998; Brandt, Brocke et al. 2001; Bestmann, Baudewig et al. 2003; Chouinard, Van Der Werf et al. 2003; Bestmann, Baudewig et al. 2004; Fox, Narayana et al. 2006). Importantly, we observed a positive correlation between the stimulated (right) and the contralateral (left) motor area, which has not been seen in any TMS-PET study, elucidating possible coupling of neuroactivity between the motor regions, although in general motor regions on the stimulated hemisphere were more strongly activated than motor regions on the contralateral hemisphere.
Our study does have a number of limitations. For example, by only looking at blood flow changes which are indirect markers of neuronal activity, we aimed to further elucidate the mechanisms how tDCS affects the brain, but we cannot directly determine what really happens at the neuronal or synaptic level. A second limitation is that we cannot separate the correlation of distal brain regions with the stimulated motor cortex as a stimulated effect from merely an effect of functional connectivity. In order to rule this out, it would have been ideal to do functional connectivity analyses on resting-state data, but we did not acquire these kinds of data. Furthermore, it is important to emphasize the potential for safety and technical problems with wires and electrodes within the MRI scanner. These safety issues and design considerations are very similar to those for electroencephalography (EEG) within the MRI. Undesired coupling of the wires to the transmit coil could produce currents capable of burning the subject and distorting flip angles and receive sensitivity near the wire. In general, these safety concerns cause potential problems for MRI based functional measures in the study of tDCS and other electrical therapies, but solutions and approaches to MRI in the presence of wires and electrodes suggest MRI may remain a valuable tool for characterizing electrical therapies.
In summary, we showed that tDCS can be safely administered in the MR environment and the induced rCBF changes in the stimulated region are reproducible. We found that tDCS modulates rCBF differentially depending on the polarity and, to a lesser degree, the strength of the stimulation. Furthermore, differential rCBF after-effects of anodal (increase in resting state rCBF) and cathodal (decrease in resting state rCBF) tDCS support findings of behavioral and cognitive after-effects after cathodal and anodal tDCS (Vines, Nair et al. 2006; Vines, Schnider et al. 2006; Vines, Nair et al. 2008). We also showed that tDCS not only modulates activity in a network directly under the stimulating electrode but also in a network of brain regions that are functionally related to the stimulated area. Our results demonstrated the efficacy of using ASL to examine and explore differential effects of tDCS and its stimulation parameters.
Research Highlights.
TDCS can be applied safely in the MR environment and rCBF changes are reproducible.
TDCS modulates rCBF differentially depending on polarity.
Differential rCBF effects outlast anodal and cathodal stimulation.
TDCS leads to regional and remote CBF changes in a network of brain regions.
Acknowledgements
This study was supported by a grant from NIH/NINDS (NS045049), NIH/NIDCD (R01 DC009823-01) and from CIMIT (W81XWH-09-2-0001). The authors express their gratitude to Drs. Dinesh Nair, Vijay Renga, Bradley Vines, Robert Lindenberg, and Christoph Mathys who helped with data acquisition during various phases of this research. XZ and GS have no conflicts of interest related to this manuscript, including employment, consultancies, honoraria, ownership or options, expert testimony, grants or patents receiving or pending, or royalties. DCA discloses inventorship on patents related to the ASL technique used in this study for which he has received and may in the future receive royalties, and the receipt of research support from GE Healthcare Technologies, an MRI scanner vendor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aguirre GK, Detre JA, et al. Experimental design and the relative sensitivity of BOLD and perfusion fMRI. Neuroimage. 2002;15(3):488–500. doi: 10.1006/nimg.2001.0990. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- Angelone LM, Vasios CE, et al. On the effect of resistive EEG electrodes and leads during 7 T MRI: simulation and temperature measurement studies. Magn Reson Imaging. 2006;24(6):801–812. doi: 10.1016/j.mri.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Antal AM, Nitsche A, et al. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16(4):521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- Antal A, Polania R, et al. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2010.11.085. in press. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Siebner HR, et al. Functional MRI of cortical activations induced by transcranial magnetic stimulation (TMS) Neuroreport. 2001;12(16):3543–3548. doi: 10.1097/00001756-200111160-00034. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, et al. On the synchronization of transcranial magnetic stimulation and functional echo-planar imaging. J Magn Reson Imaging. 2003;17(3):309–316. doi: 10.1002/jmri.10260. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, et al. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19(7):1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, et al. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, et al. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. J Physiol. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SA, Brocke J, et al. In vivo assessment of human visual system connectivity with transcranial electrical stimulation during functional magnetic resonance imaging. Neuroimage. 2001;14(2):366–375. doi: 10.1006/nimg.2001.0847. [DOI] [PubMed] [Google Scholar]
- Chi RP, Fregni F, et al. Visual memory improved by non-invasive brain stimulation. Brain Res. 2010;1353:168–175. doi: 10.1016/j.brainres.2010.07.062. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, et al. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90(2):1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detre JA, Leigh JS, et al. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Durand S, Fromy B, et al. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. J Physiol. 2002;540(Pt 1):261–269. doi: 10.1113/jphysiol.2001.013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer S, Burkard M, et al. Direct current induced short-term modulation of the left dorsolateral prefrontal cortex while learning auditory presented nouns. Behav Brain Funct. 2009;5:29. doi: 10.1186/1744-9081-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P, Ingham R, et al. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8(12):2787–2791. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, et al. Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Hum Brain Mapp. 2006;27(6):478–487. doi: 10.1002/hbm.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke K, Seeber AA, et al. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105(3):1141–1149. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, et al. A critique of functional localisers. Neuroimage. 2006;30(4):1077–1087. doi: 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102(1):294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Moriwaki A, et al. Biphasic effects of polarizing current on adenosine-sensitive generation of cyclic AMP in rat cerebral cortex. Neurosci Lett. 1990;116(3):320–324. doi: 10.1016/0304-3940(90)90094-p. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, et al. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684(2):206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Kincses TZ, Antal A, et al. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42(1):113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2(5):467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, et al. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett. 2008;435(1):56–59. doi: 10.1016/j.neulet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, et al. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125(Pt 10):2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Mathys C, Loui P, et al. Non-invasive brain stimulation applied to Heschl's gyrus modulates pitch discrimination. Frontiers in Psychology. 2010;1 doi: 10.3389/fpsyg.2010.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megias M, Emri Z, et al. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102(3):527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- Moisa M, Pohmann R, et al. Interleaved TMS/CASL: Comparison of different rTMS protocols. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Moriwaki A. Polarizing currents increase noradrenaline-elicited accumulation of cyclic AMP in rat cerebral cortex. Brain Res. 1991;544(2):248–252. doi: 10.1016/0006-8993(91)90061-y. [DOI] [PubMed] [Google Scholar]
- Nitsche MA. Transcranial direct current stimulation: a new treatment for depression? Bipolar Disord. 2002;4(Suppl 1):98–99. doi: 10.1034/j.1399-5618.4.s1.43.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553(Pt 1):293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, et al. [Modulation of cortical excitability by transcranial direct current stimulation] Nervenarzt. 2002;73(4):332–335. doi: 10.1007/s00115-002-1272-9. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, et al. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114(4):600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15(4):619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, et al. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79(2):1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular Activities and Evoked Potential Changes during Polarization of Motor Cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, et al. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol. 2008;119(4):805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalewski A, Breitenstein C, et al. Transcranial direct current stimulation disrupts tactile perception. Eur J Neurosci. 2004;20(1):313–316. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Rev Med Devices. 2008;5(6):759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R, Dafotakis M, et al. Enhancing language performance with non-invasive brain stimulation--a transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46(1):261–268. doi: 10.1016/j.neuropsychologia.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, et al. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29(16):5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, et al. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Nair D, et al. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008;28(8):1667–1673. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair DG, et al. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17(6):671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- Vines BW, Schnider NM, et al. Testing for causality with transcranial direct current stimulation: pitch memory and the left supramarginal gyrus. Neuroreport. 2006;17(10):1047–1050. doi: 10.1097/01.wnr.0000223396.05070.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water [published erratum appears in Proc Natl Acad Sci U S A 1992 May 1;89(9):4220] Proc Natl Acad Sci U S A. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]