Abstract
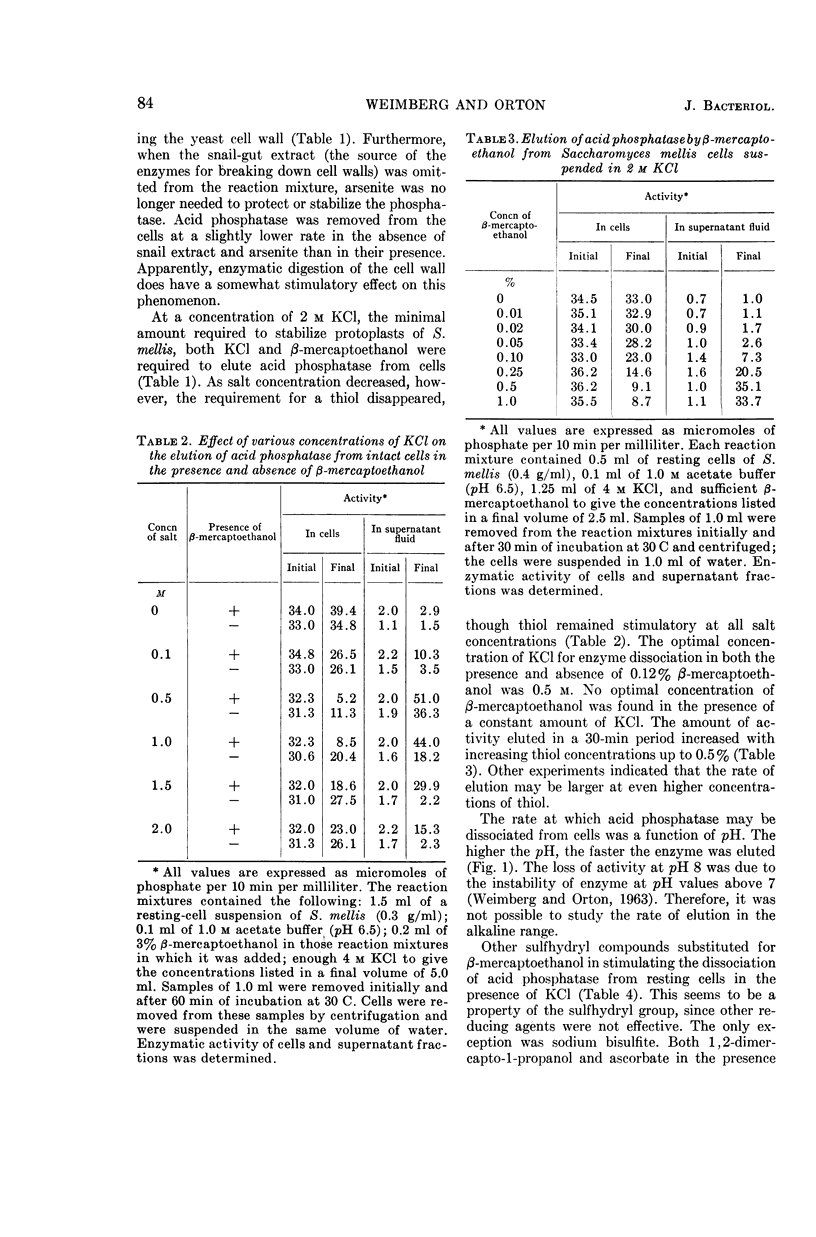
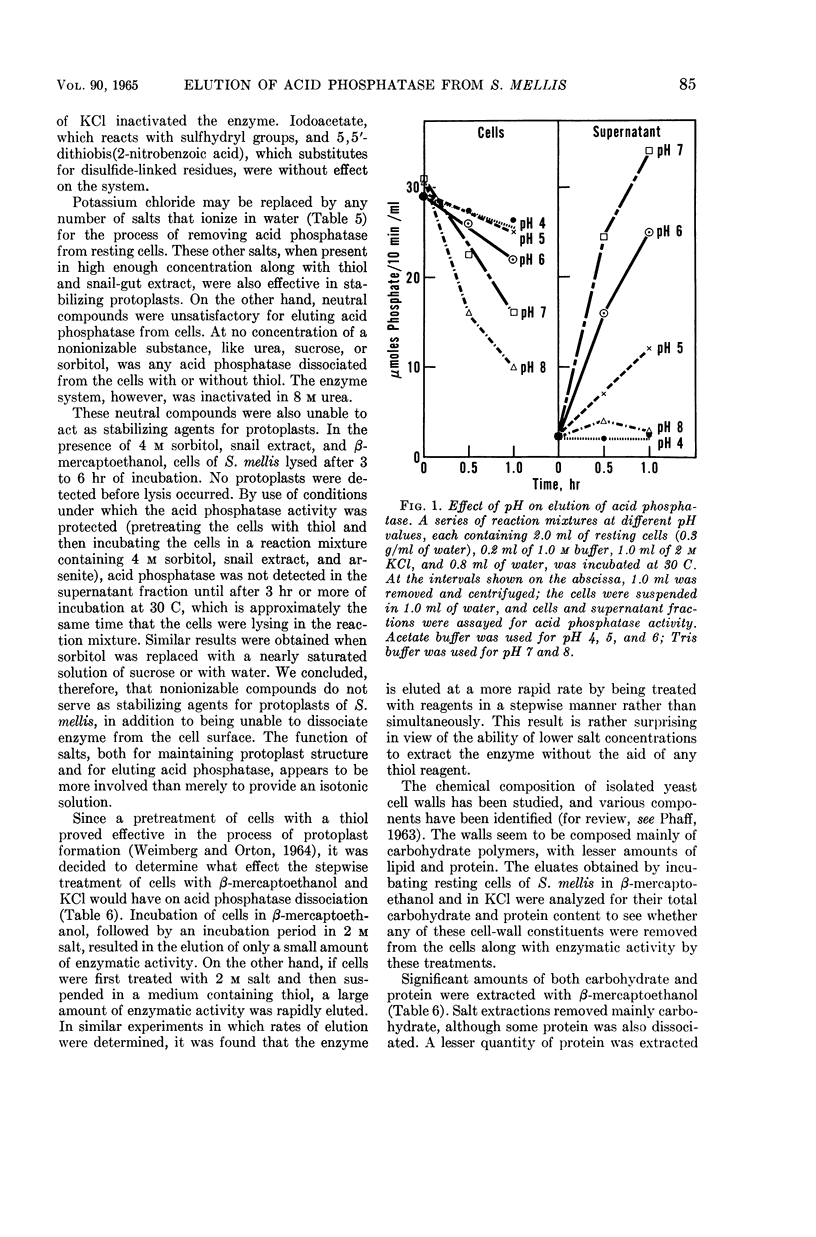
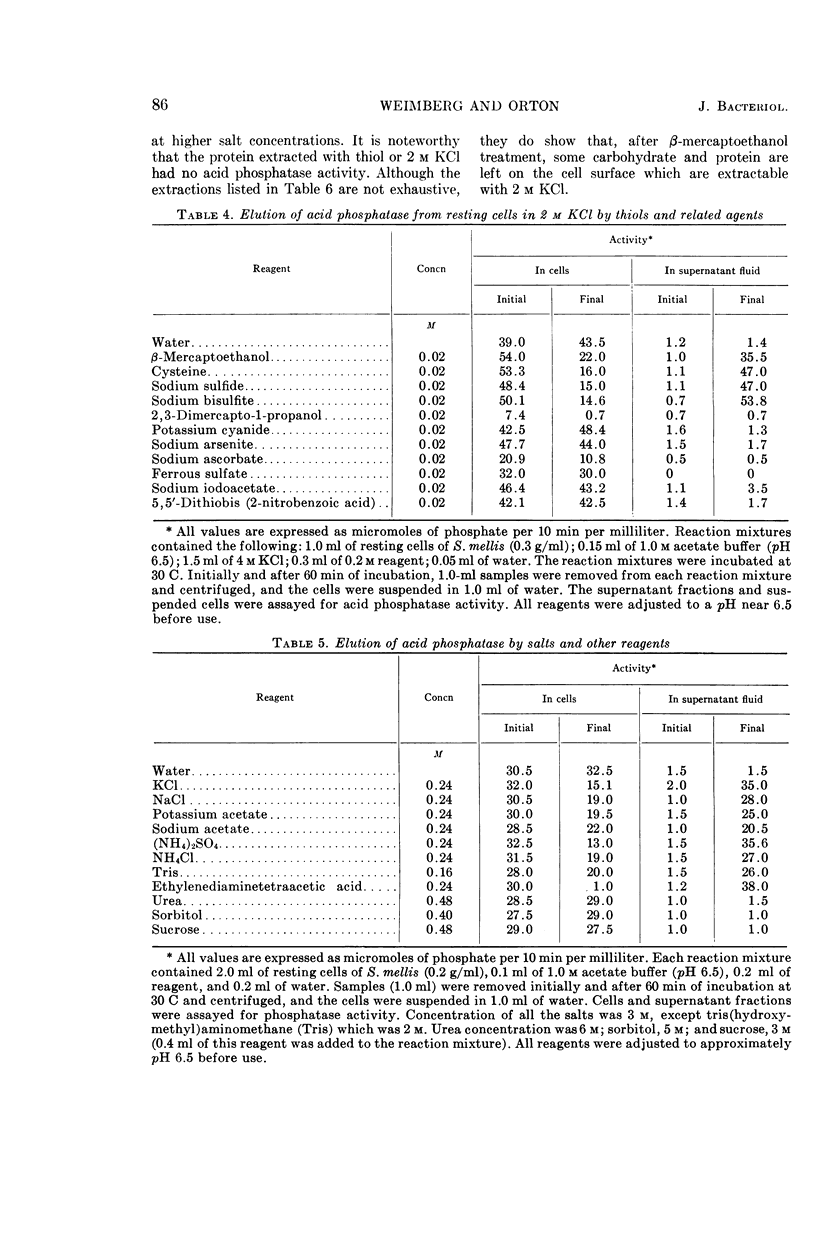
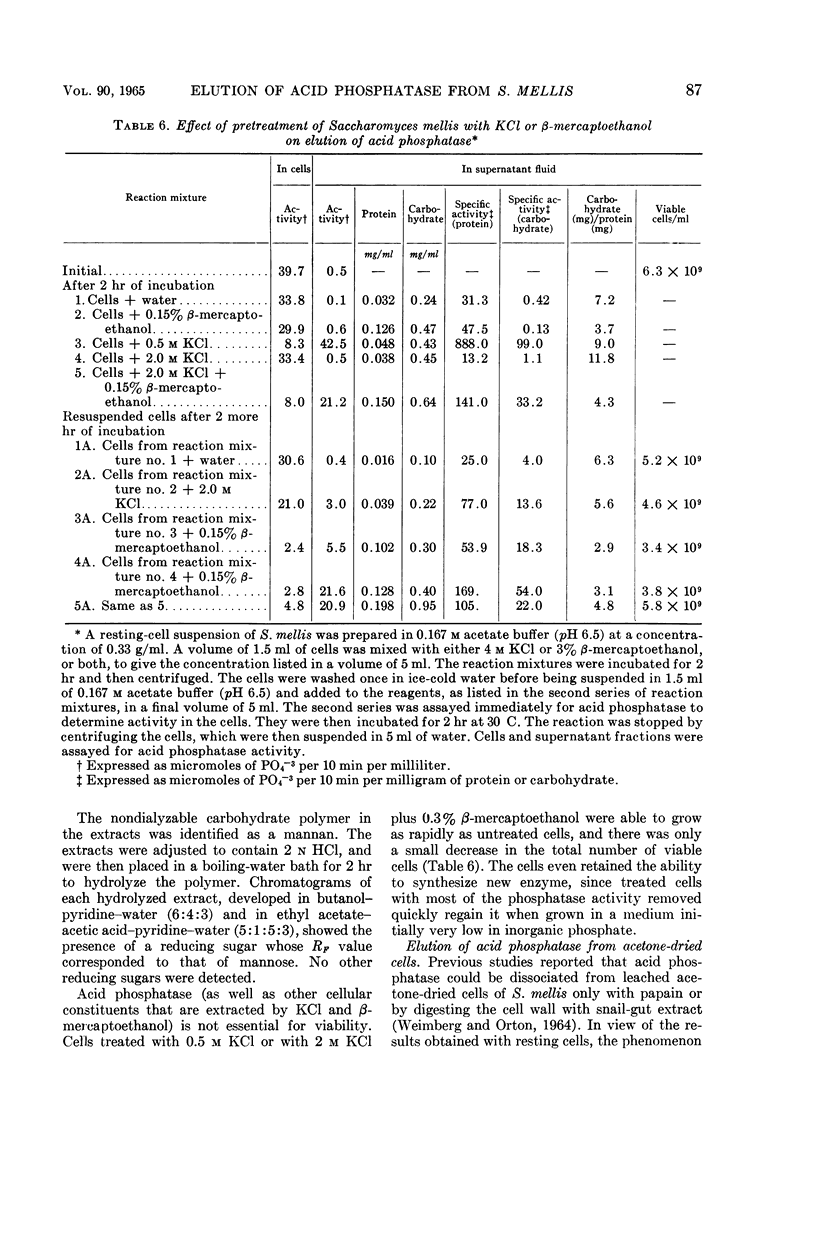
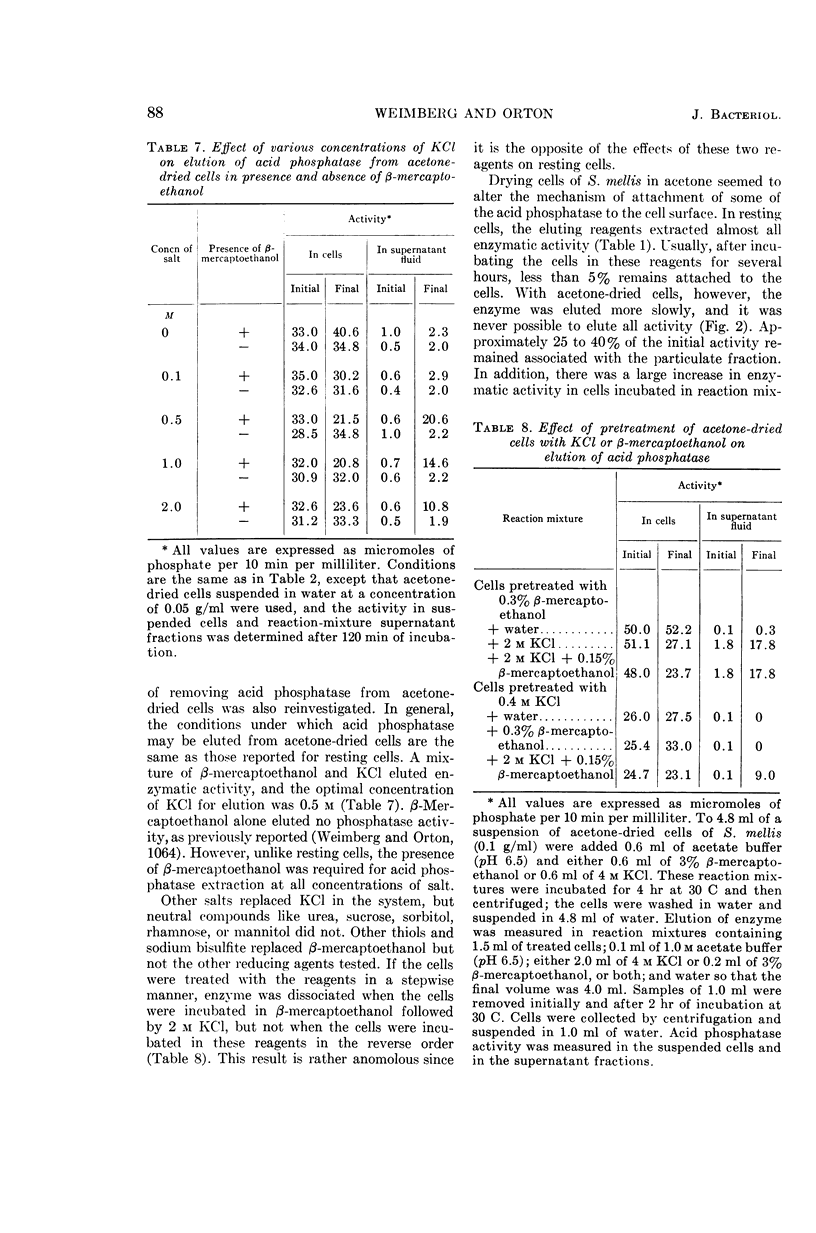
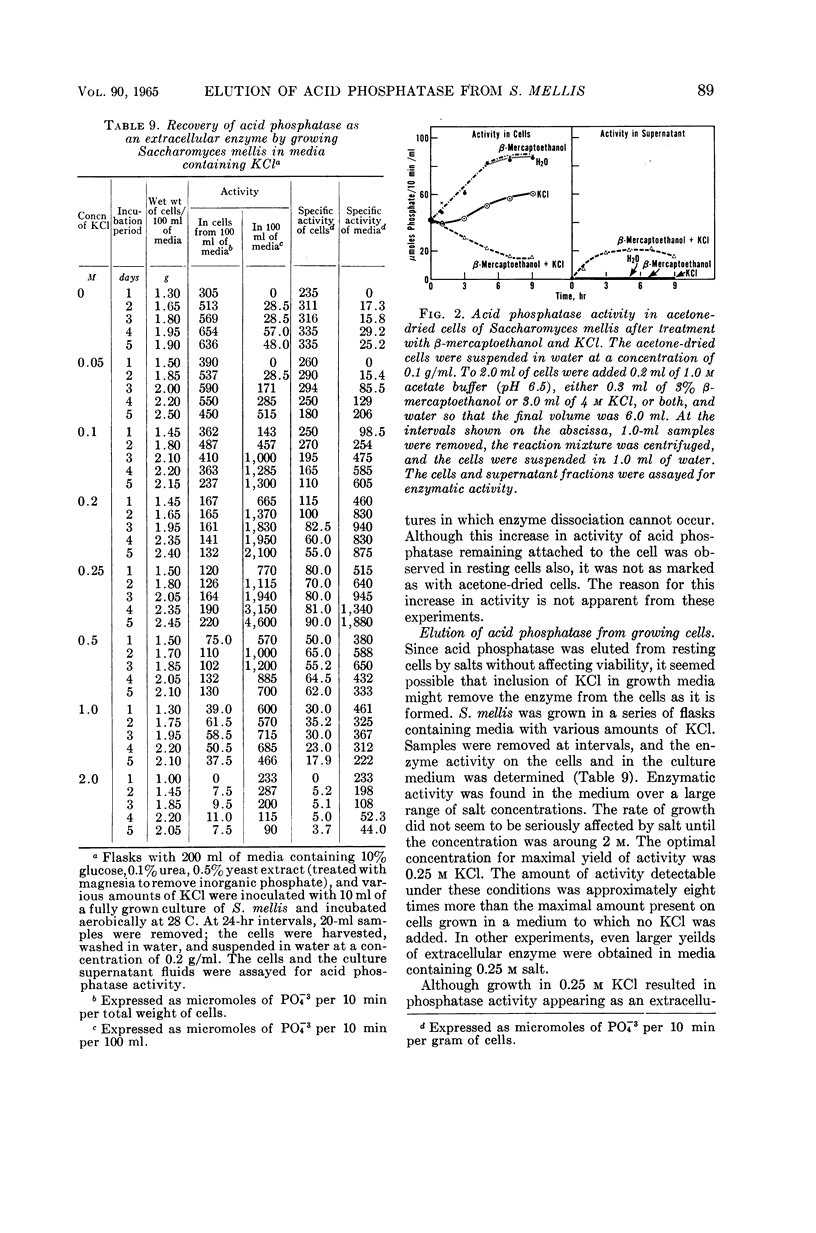
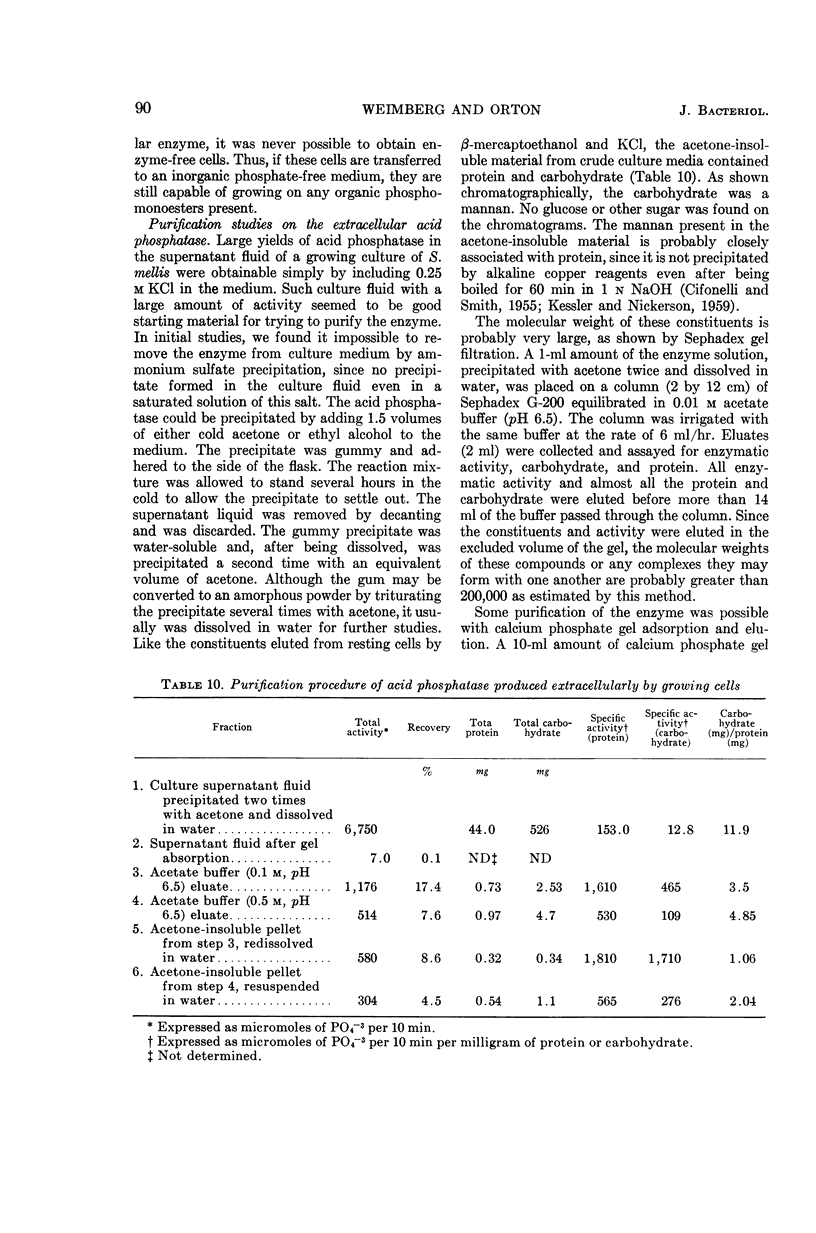
Weimberg, Ralph (Northern Regional Research Laboratory, Peoria, Ill.), and William L. Orton. Elution of acid phosphatase from the cell surface of Saccharomyces mellis by potassium chloride. J. Bacteriol. 90:82–94. 1965.—Acid phosphatase of Saccharomyces mellis may be eluted from intact resting cells by 0.5 m KCl or other salts. However, the enzyme is not eluted at higher salt concentrations of about 2 m unless a thiol, such as β-mercaptoethanol, is included in the reaction mixture. These treatments do not significantly affect viability of the cells. Neutral compounds like sorbitol or sucrose cannot substitute for ionic compounds in eluting the enzyme from resting cells. Furthermore, the neutral compounds are also inadequate for stabilizing the protoplast structure. It is suggested that the enzyme is held on the cell surface by a combination of electrostatic forces and disulfide bonds. Thiol alone dissociates protein and carbohydrate from the cell surface, but the eluate has no acid phosphatase activity. Salts also remove protein and carbohydrate from the cell surface, but the amount of protein removed is considerably less than that dissociated by thiol. A concentration of 0.5 m KCl elutes more protein than does a 2 m concentration, and enzymatic activity is present only in the 0.5 m KCl eluate. The carbohydrate eluted by either reagent has been identified as a mannan. Conditions for eluting acid phosphatase from acetonedried cells of S. mellis are essentially the same as those for resting cells. Significantly, though, thiol is required at all salt concentrations to dissociate the enzyme. Pretreatment of the cells with thiol, followed by KCl, elutes acid phosphatase, whereas the reverse procedure does not. Acid phosphatase is excreted by growing cells of S. mellis into growth media if the medium contains 0.25 m KCl. The total yield of enzymatic activity may be 8 to 10 times greater than is usually present on derepressed cells grown in a salt-free medium. The enzyme can be precipitated from the culture fluid with acetone. The acetone-precipitated fraction contains mannan and protein in a ratio of 12:1 by weight. Partial purification of the enzyme by calcium phosphate gel and elution resulted in an enzyme fraction in which the specific activity on the basis of protein increased 12-fold, and the carbohydrate-protein ratio was reduced to 1:1.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWIE D. B., McCLURE F. T. Metabolic pools and the synthesis of macromolecules. Biochim Biophys Acta. 1959 Jan;31(1):236–245. doi: 10.1016/0006-3002(59)90460-3. [DOI] [PubMed] [Google Scholar]
- DUELL E. A., INOUE S., UTTER M. F. ISOLATION AND PROPERTIES OF INTACT MITOCHONDRIA FROM SPHEROPLASTS OF YEAST. J Bacteriol. 1964 Dec;88:1762–1773. doi: 10.1128/jb.88.6.1762-1773.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. Cell-wall mannan-protein of baker's yeast. Science. 1956 Aug 10;124(3215):272–273. doi: 10.1126/science.124.3215.272-a. [DOI] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. Identification of protein disulfide reductase as a cellular division enzyme in yeasts. Science. 1956 Oct 19;124(3225):722–723. doi: 10.1126/science.124.3225.722. [DOI] [PubMed] [Google Scholar]
- Felkner I. C., Wyss O. A substance produced by competent Bacillus cereus 569 cells that affects transformability. Biochem Biophys Res Commun. 1964 May 22;16(1):94–99. doi: 10.1016/0006-291x(64)90217-7. [DOI] [PubMed] [Google Scholar]
- GARCIA MENDOZA C., VILLANUEVA J. R. Production of yeast protoplasts by an enzyme preparation of Streptomyces sp. Nature. 1962 Sep 29;195:1326–1327. doi: 10.1038/1951326a0. [DOI] [PubMed] [Google Scholar]
- HOLTER H., OTTOLENGHI P. Observations on yeast protoplasts. C R Trav Lab Carlsberg. 1960;31:409–422. [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCLELLAN W. L., Jr, LAMPEN J. O. The acid phosphatase of yeast. Localization and secretion by protoplasts. Biochim Biophys Acta. 1963 Feb 12;67:324–326. doi: 10.1016/0006-3002(63)91832-8. [DOI] [PubMed] [Google Scholar]
- MUNDKUR B. ELECTRON MICROSCOPICAL STUDIES OF FROZEN-DRIED YEAST. V. LOCALIZATION OF PROTEIN-BOUND SULPHYDRYL. Exp Cell Res. 1964 Mar;34:155–181. doi: 10.1016/0014-4827(64)90192-2. [DOI] [PubMed] [Google Scholar]
- MUNDKUR B. Electron microscopical studies of frozen-dried yeast. I. Localization of polysaccharides. Exp Cell Res. 1960 Jun;20:28–42. doi: 10.1016/0014-4827(60)90219-6. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J., FALCONE G. Enzymatic reduction of disulfide bonds in cell wall protein of baker's yeast. Science. 1956 Aug 17;124(3216):318–319. doi: 10.1126/science.124.3216.318. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. IV. MOLECULAR BASES OF FORM IN YEASTS. Bacteriol Rev. 1963 Sep;27:305–324. doi: 10.1128/br.27.3.305-324.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHCOTE D. H., HORNE R. W. The chemical composition and structure of the yeast cell wall. Biochem J. 1952 May;51(2):232–236. doi: 10.1042/bj0510232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- SVIHLA G., SCHLENK F., DAINKO J. L. Spheroplasts of the yeast Candida utilis. J Bacteriol. 1961 Dec;82:808–814. doi: 10.1128/jb.82.6.808-814.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. EVIDENCE FOR AN EXOCELLULAR SITE FOR THE ACID PHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1964 Dec;88:1743–1754. doi: 10.1128/jb.88.6.1743-1754.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. REPRESSIBLE ACID PHOSPHOMONOESTERASE AND CONSTITUTIVE PYROPHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1963 Oct;86:805–813. doi: 10.1128/jb.86.4.805-813.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. SYNTHESIS AND BREAKDOWN OF THE POLYPHOSPHATE FRACTION AND ACID PHOSPHOMONOESTERASE OF SACCHAROMYCES MELLIS AND THEIR LOCATIONS IN THE CELL. J Bacteriol. 1965 Mar;89:740–747. doi: 10.1128/jb.89.3.740-747.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


