Abstract
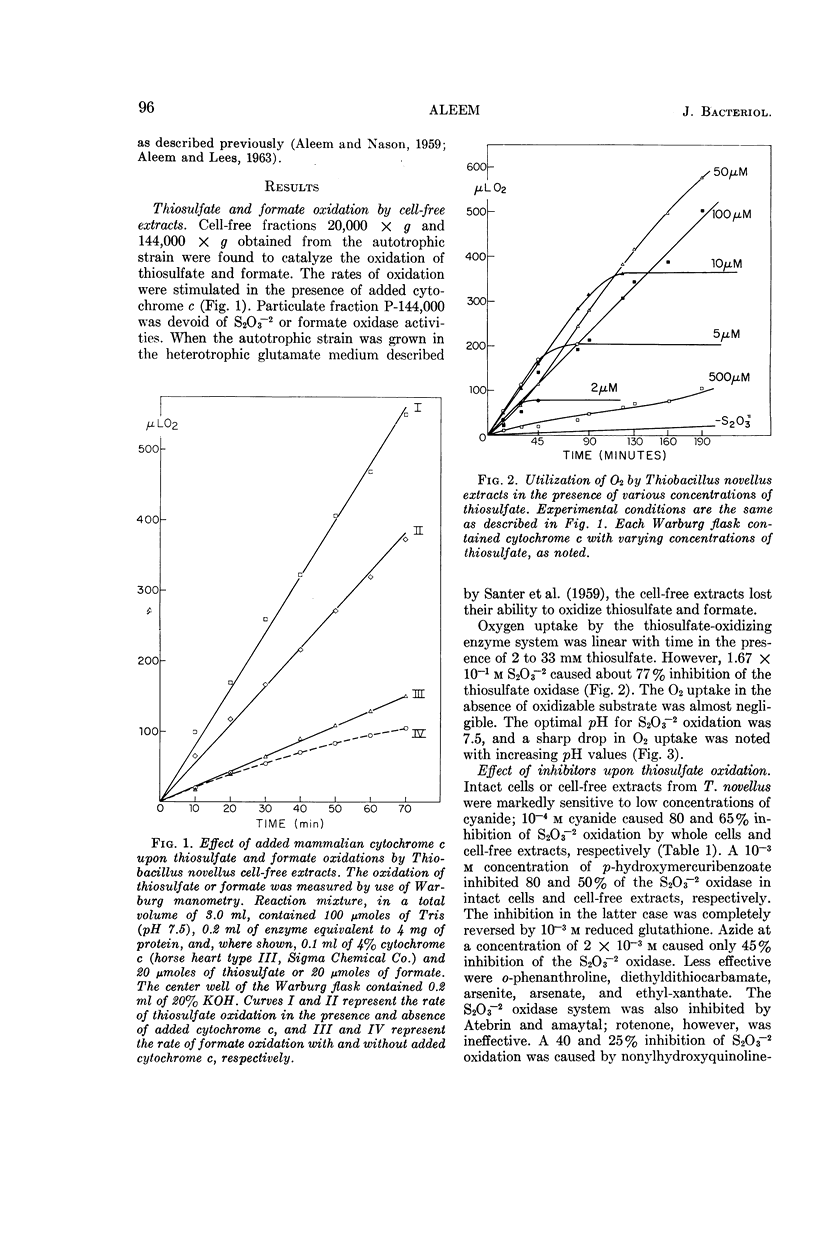
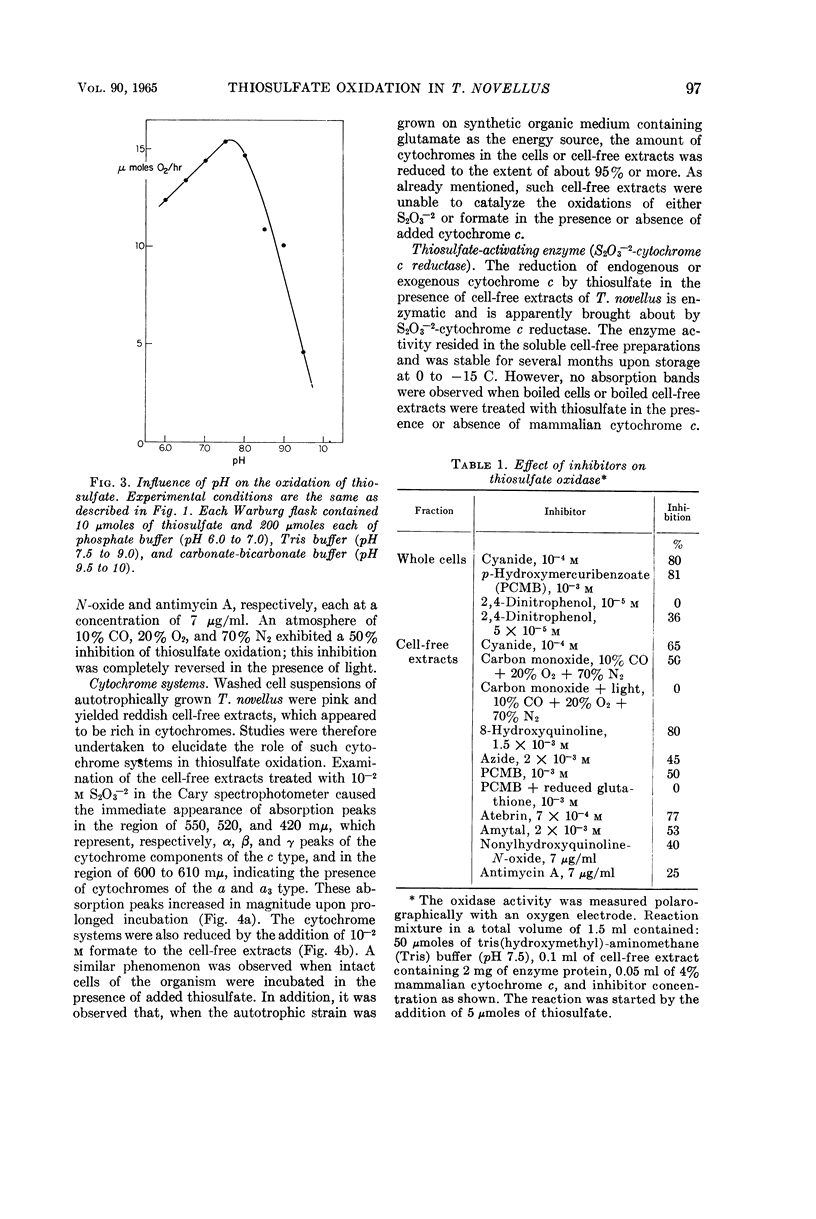
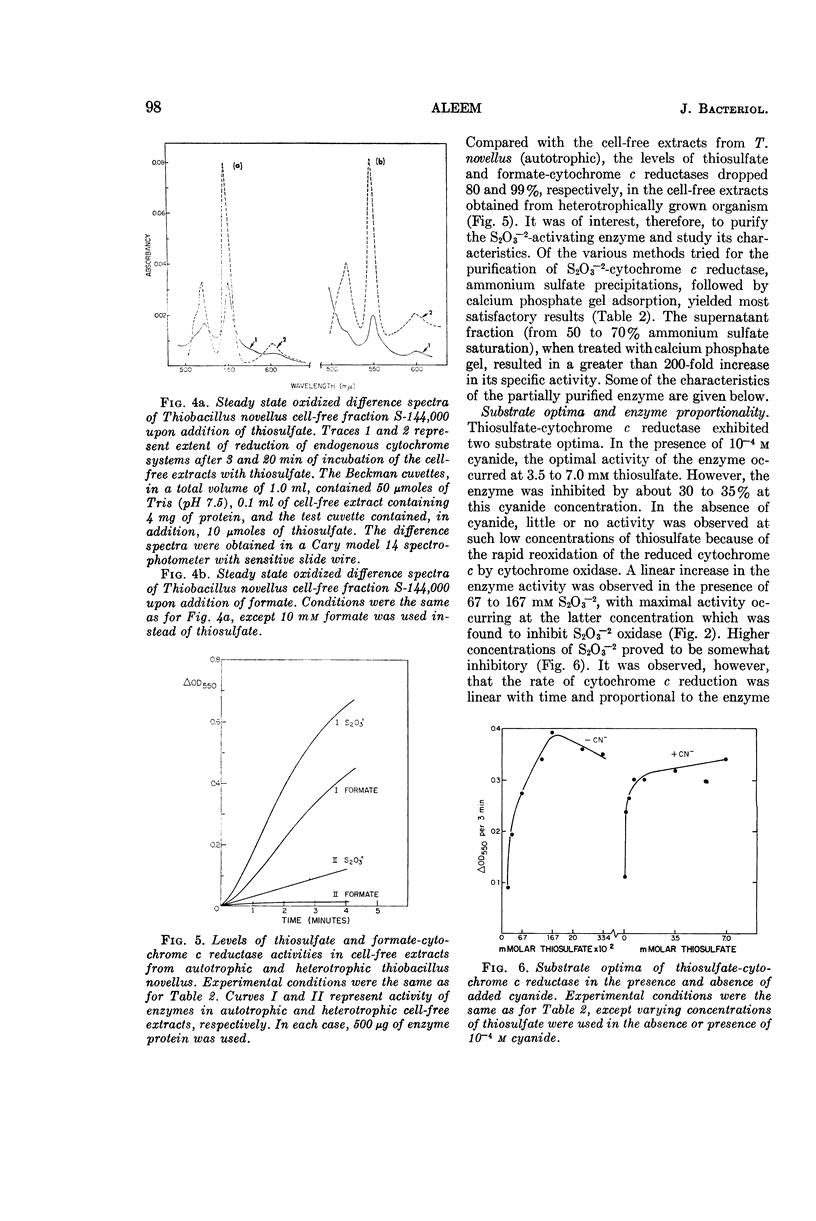
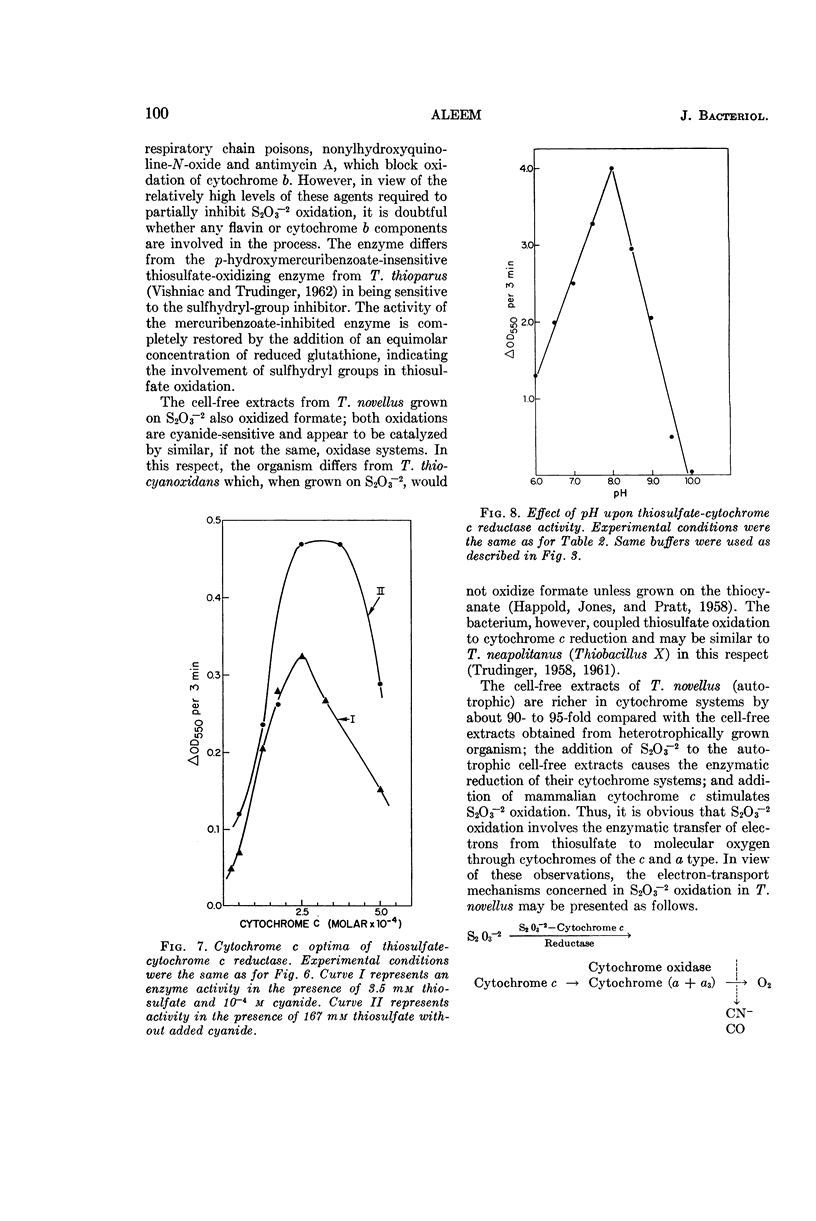
Aleem, M. I. H. (Research Institute for Advanced Studies, Baltimore, Md.). Thiosulfate oxidation and electron transport in Thiobacillus novellus. J. Bacteriol. 90:95–101. 1965.—A cell-free soluble enzyme system capable of oxidizing thiosulfate was obtained from Thiobacillus novellus adapted to grow autotrophically. The enzyme systems of autotrophically grown cells brought about the transfer of electrons from thiosulfate to molecular oxygen via cytochromes of the c and a types; the reactions were catalyzed jointly by thiosulfate oxidase and thiosulfate cytochrome c reductase. The levels of both of these enzymes were markedly reduced in the heterotrophically grown organism. Cell-free extracts from the autotrophically grown T. novellus catalyzed formate oxidation and enzymatically reduced cytochrome c with formate. Both formate oxidation and cytochrome c reduction activities were abolished under heterotrophic conditions. The thiosulfate-activating enzyme S2O3−2-cytochrome c reductase, as well as thiosulfate oxidase, was localized chiefly in the soluble cell-free fractions, and the former enzyme was purified more than 200-fold by ammonium sulfate fractionation and calcium phosphate gel adsorption procedures. Optimal activity of the purified enzyme occurred at pH 8.0 in the presence of 1.67 × 10−1m S2O3−2 and 2.5 × 10−4m cytochrome c. The thiosulfate oxidase operated optimally at pH 7.5 and thiosulfate concentrations of 1.33 × 10−3 to 3.33 × 10−2m in the presence of added cytochrome c at a concentration of 5 × 10−4m. Both enzymes were markedly sensitive to cyanide and to a lesser extent to some metal-binding agents. Although a 10−3m concentration of p-hydroxymercuribenzoate had no effect on S2O3−2-cytochrome c reductase, it caused a 50% inhibition of S2O3−2 oxidase, which was completely reversed in the presence of 10−3m reduced glutathione. Carbon monoxide also inhibited S2O3−2 oxidase; the inhibition was completely reversed by light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEEM M. I., LEES H. Autotrophic enzyme systems. I. Electron transport systems concerned with hydroxylamine oxidation in Nitrosomonas. Can J Biochem Physiol. 1963 Mar;41:763–778. [PubMed] [Google Scholar]
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPPOLD F. C., JONES G. L., PRATT D. B. Utilization of thiocyanate by Thiobacillus thioparus and T. thiocyanoxidans. Nature. 1958 Jul 26;182(4630):266–267. doi: 10.1038/182266a0. [DOI] [PubMed] [Google Scholar]
- SANTER M., BOYER J., SANTER U. Thiobacillus novellus. I. Growth on organic and inorganic media. J Bacteriol. 1959 Aug;78:197–202. doi: 10.1128/jb.78.2.197-202.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Cytochromes and thiosulphate oxidation in an aerobic Thiobacillus. Biochim Biophys Acta. 1958 Oct;30(1):211–212. doi: 10.1016/0006-3002(58)90274-9. [DOI] [PubMed] [Google Scholar]
- TRUDINGER P. A. Thiosulphate oxidation and cytochromes in Thiobacillus X. 2. Thiosulphate-oxidizing enzyme. Biochem J. 1961 Apr;78:680–686. doi: 10.1042/bj0780680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., TRUDINGER P. A. Symposium on autotrophy. V. Carbon dioxide fixation and substrate oxidation in the chemosynthetic sulfur and hydrogen bacteria. Bacteriol Rev. 1962 Jun;26:168–175. [PMC free article] [PubMed] [Google Scholar]


