Abstract
Objective
Traumatic brain injury (TBI) induces significant neurological damage, including deficits in learning and memory which contribute to a poor clinical prognosis. Treatment options to limit cognitive decline and promote neurological recovery are lacking, in part, due to a poor understanding of the secondary/delayed processes which contribute to brain injury. In the present study, we characterized the temporal and spatial changes in the expression of PSD-95, a key scaffolding protein implicated in excitatory synaptic signaling, following controlled cortical impact in mice. Neurological injury, as assessed by the open field activity test and the novel object recognition test, were compared with changes in PSD-95 expression.
Methods
Adult male CD-1 mice were subjected to controlled cortical impact to simulate a moderate traumatic brain injury in humans. The spatial and temporal expression of PSD-95 was analyzed in the cerebral cortex and hippocampus at various time points following injury. Neurological assessments were performed to compare changes in PSD-95 with cognitive deficits.
Results
A significant decrease in PSD-95 expression was observed in the ipsilateral hippocampus beginning at day 7 post-injury. The loss of PSD-95 corresponded with a concomitant reduction in immunoreactivity for NeuN, a neuronal-specific marker. Aside from the contused cortex, significant loss of PSD-95 immunoreactivity was not observed in the cerebral cortex. The delayed loss of hippocampal PSD-95 directly correlated with the onset of behavioral deficits, suggesting a possible causative role for PSD-95 in behavioral abnormalities following a head trauma.
Conclusion
Delayed loss of hippocampal synapses was observed following head trauma in mice. These data may suggest a cellular mechanism to explain the delayed learning and memory deficits in humans and provide a potential framework for further testing to implicate PSD-95 as a clinically-relevant therapeutic target.
Keywords: Traumatic Brain Injury, Glutamate, Synaptic Plasticity, Hippocampus
Traumatic brain injury (TBI) is a leading cause of death and disability. Over 3 million Americans exhibit long-term disabilities due to TBI 3,15, placing an economic and emotional burden on society. Clinically, TBI survivors frequently exhibit delayed deficits in learning and memory, including impairments in concentration and attention 2,15,20,25,36,37,39. This complicates patient rehabilitation and contributes to a poor long-term prognosis 13,31,38. Similarly, delayed reductions in learning and memory capacity are observed in pre-clinical animal models, suggesting the cellular mechanisms of neurological injury following TBI may be evolutionarily conserved 3,14,27.
Preventative measures such as helmet and seatbelt laws, stricter enforcement of drunk driving laws, improved safety devices, and educational programs have been effective in reducing the number and severity of brain injuries. However, in spite of advances in critical care medicine and the development of the “trauma center”, medical management of TBI remains limited. Treatment options to maintain cognitive function after TBI are lacking, at least in part, due to poorly understood mechanisms. Neurological injury following a head trauma is complex and likely involves multiple mechanisms and signaling cascades beyond the initial traumatic event. Cellular necrosis within the cerebral cortex occurs immediately after trauma and contributes to increased intracranial pressure and acute patient mortality 16,28. However, delayed cellular loss within the hippocampus correlated with cognitive deficits and long-term prognosis following experimental 12,19 or clinical head injury 15,20,26,36,37,39. The temporal delay between the acute trauma and delayed hippocampal injury represents a clinically-feasible therapeutic window for a treatment strategy to limit secondary damage and promote post-traumatic hippocampal plasticity
Long-term potentiation (LTP), a process which promotes synaptic neurotransmission, may contribute to the processes of learning and memory. Rat brain slices prepared at one week after lateral fluid percussion injury were unable to induce and maintain LTP and exhibited attenuated N-methyl-D-aspartate (NMDA)-induced potentials and glutamate-induced excitatory currents 35. Post-synaptic density protein-95 (PSD-95), a scaffolding protein that is abundantly expressed within excitatory synapses, is implicated in the maturation of pre-synaptic and post-synaptic components in excitatory synapses, promotion of dendritic spine formation, and enhancement of glutamatergic neurotransmission 5,6,9,17,18,23,24,30,33. Notably, PSD-95 controls activity-dependent AMPA receptor incorporation at synapses during LTP in vitro and during experience-driven synaptic strengthening in vivo 8. Together, these data suggest the possibility that the loss of hippocampal PSD-95 expression may underlie the development of delayed cognitive impairments following TBI.
Herein, we report the temporal and spatial pattern of PSD-95 expression within the brain following controlled cortical impact in mice. These changes correlated with delayed hippocampal damage and behavioral deficits. Together, these data provide a framework and rationale for the therapeutic targeting of hippocampal synapses to improve long-term neurological outcome following TBI.
MATERIALS AND METHODS
Controlled Cortical Impact
Animal studies were reviewed and approved by the Committee on Animal Use for Research and Education at the Medical College of Georgia, in compliance with NIH guidelines. Male CD-1 mice (8–10 weeks old; Charles River, Wilmington, MA) were anesthetized with 8 mg/kg xylazine/60 mg/kg ketamine and then placed in a stereotaxic frame (Amscien Instruments, Richmond, VA) and a 3.5 mm craniotomy was made in the right parietal bone midway between bregma and lambda with the medial edge 1 mm lateral to the midline. Mice were impacted at ~4.5 m/s with a 20 ms dwell time and 1 mm depression using a 3 mm diameter convex tip to induce a moderate TBI, as described by our group 22. The incision was then surgically stapled, and mice were placed at 37 C until recovery. Sham-operated controls underwent identical surgical procedures but were not impacted. Throughout all procedures, body temperature was maintained at 37°C using a small animal temperature controller (David Kopf Instruments, Tujunga, CA).
Cresyl violet staining
Gross injury was assessed in coronal sections (12 μM) that were incubated with 0.1% cresyl violet in 100% ethanol (pH 4.0) for 5 minutes. Sections were washed in distilled water followed by successive ethanol washes (70%, 95%, 100%). Sections were then briefly washed three times in xylene and cover-slipped. Digital images of ipsilateral cortices were captured for all treatment groups on a Zeiss Axiophot microscope using a 2.5X objective.
Western blotting
Western blotting was performed as detailed by our group 7,22,32. Blots were incubated overnight at 4°C with an anti-PSD-95 primary antibody (1:1000; Cell Signaling Technology, Beverly, MA) or with an anti-β-actin primary antibody (1:3000; Santa Cruz Biotechnology, Santa Cruz, CA) and visualized on a Li-Cor Odyssey near-infrared imaging system using an Alexa Fluor 750 secondary antibody. Densitometry analysis was performed using Quantity One software (Bio-rad, Foster City, CA).
Immunohistochemistry (IHC)
IHC was performed as described by our group 10,22. To unmask post-synaptic PSD-95 labeling, sections were digested in pepsin (1 mg/mL in 0.2 N HCl at 37°C) for 5 minutes prior to antigen retrieval, using a published protocol 11. Sections were washed, incubated with 3% normal donkey serum (DS) in PBS containing 0.1% Triton X-100, followed by a 2h incubation with an anti-PSD-95 polyclonal antibody (1:100; Cell Signaling Technology, Beverly, MA) or NeuN (1:100; Millipore) diluted in blocking buffer containing 3% DS, and then incubated with an Alexa Fluor secondary antibody (Invitrogen, Carlsbad, CA, 1:200). Omission of primary antibody served as a negative controls. At least six to ten alternate sections per mouse were analyzed per group. Sections were imaged using a LSM510 Meta confocal laser microscope, as described by our group 10,22.
Assessment of neurological injury
To determine the effect of TBI on neurological outcome, the open-field activity test was conducted, as described by our group 40. Briefly, mice were placed in a 14 × 14 inch black box that was divided into a 2 × 2 inch square grid (49 squares in total). Open field activity, as determined by the number of crosses within a 3 minute trial, was measured by at least two investigators who were blinded to experimental conditions. The two-trial novel object recognition task was also performed in which a mouse was placed in an enclosed box with two identical objects were placed within a 4 inch diameter circle, located a set distance apart. The mouse was then removed from the environment for a set amount of time and one of the two previously used (familiar) objects was replaced with a novel object that was different from the familiar object in shape, texture, and appearance. The mouse’s behavior upon exposure to the novel object was then recorded. This test is based on the natural tendency of mice to investigate a novel object rather than a familiar one, which reflects the use of learning and recognition memory processes.
Statistical analysis
The effects of treatments were analyzed using a Student’s t-test or One-Way Analysis of Variance (ANOVA) following by Student Newman Keul’s post-hoc test. At least 5 animals per group were included for all analyses. Results are expressed as mean ± SEM. A p<0.05 was considered to be statistically significant.
RESULTS
Delayed reduction in hippocampal PSD-95 expression
Western blotting of PSD-95, a scaffolding protein that is abundantly expressed within excitatory synapses, was performed to determine the effect of TBI on the number of synapses in the hippocampus. In contrast to the pericontusional cortex, which exhibited a dramatic reduction in PSD-95 expression, significant differences in PSD-95 expression was not observed within the hippocampus for the first three days post-TBI (FIGURE 1A, B). Conversely, a dramatic reduction in hippocampal PSD-95 staining was observed by one week after injury (46.6 ± 5.2% of sham; p<0.05 vs. sham), a finding that is in line with the visualization of hippocampal injury at day 7 using cresyl violet staining (FIGURE 1A).
FIGURE 1. Delayed reduction in hippocampal PSD-95 expression following experimental TBI.

(A) Representative data indicating a time-dependent reduction in the expression of PSD-95 within the whole hippocampus of mice following a moderate TBI. PSD-95 expression was normalized to β-actin, to control for equal protein loading. Brain sections stained with cresyl violet are provided to further document hippocampal injury by day 7 post-injury. (B) Quantification of Western blotting data in (A) by densitometry. Data are expressed as the ratio of PSD-95/β-actin and are presented as mean ± SEM. * p<0.05 vs. sham-operated control mice.
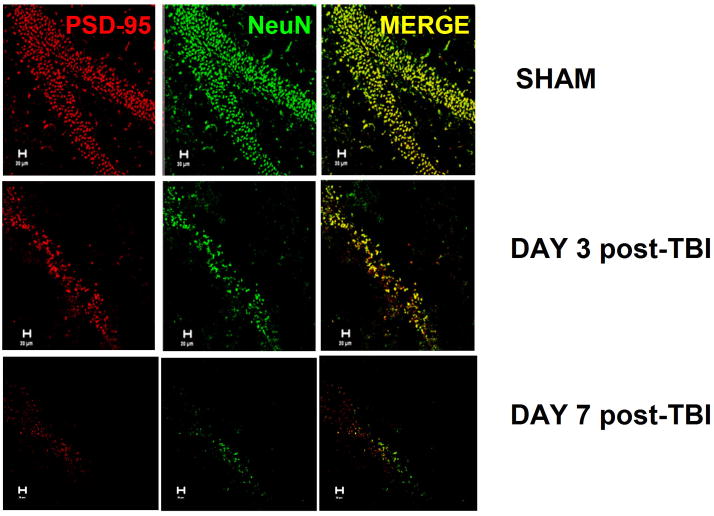
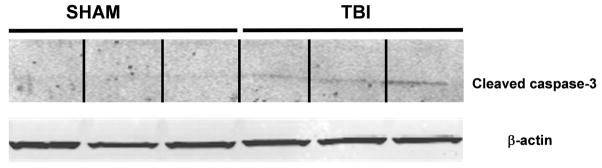
Spatial information cannot be ascertained from Western blotting; thus, to establish whether the reduction in PSD-95 expression was restricted to individual regions of the hippocampus or whether this effect was widespread, immunolocalization of PSD-95 was performed at various time points following TBI. Hippocampal injury was not evident in sham-operated mice, which showed a characteristic pattern of staining for NeuN, a neuron-specific marker, and abundant PSD-95 staining (FIGURE 2). Whereas changes in NeuN or PSD-95 immunostaining were not observed for the first 48 hours post-injury, a significant reduction in immunoreactivity for both markers was observed by 3 days post-injury, a time point which preceded the reduction by Western blotting. Consistent with the immunoblotting data, PSD-95 immunostaining was devoid by day 7 post injury. Interestingly, NeuN immunoreactivity was also lost at this time point, suggesting either a loss of antigenicity following delayed injury or significant cell death (FIGURE 2). In support of this notion, increased activity of the pro-apoptotic mediator, caspase-3, paralleled the loss of PSD-95 at day 7 post-TBI (FIGURE 3), but not at earlier time points (data not shown). These data suggest loss of PSD-95 may precede the activation of apoptotic signaling cascades.
FIGURE 2. Spatial distribution of hippocampal PSD-95 following TBI.
Immunoreactivity for NeuN (neuron-specific marker) and PSD-95 was performed within the hippocampus of sham or TBI (day 3 or 7 post-injury) mice. Immunoreactivity for both PSD-95 and NeuN was dramatically attenuated throughout the entire hippocampus at both time points following TBI, as compared to sham-operated control mice. Data are representative of 4–6 mice/group.
FIGURE 3. Activation of caspase-3 within the hippocampus at day 7 post-TBI.
Representative data indicating an increase in the expression of cleaved caspase-3, a marker of apoptotic injury, within the whole hippocampus of mice following TBI. Cleaved caspase-3 expression was normalized to β-actin to control for equal protein loading.
Delayed cognitive deficits following moderate TBI in mice
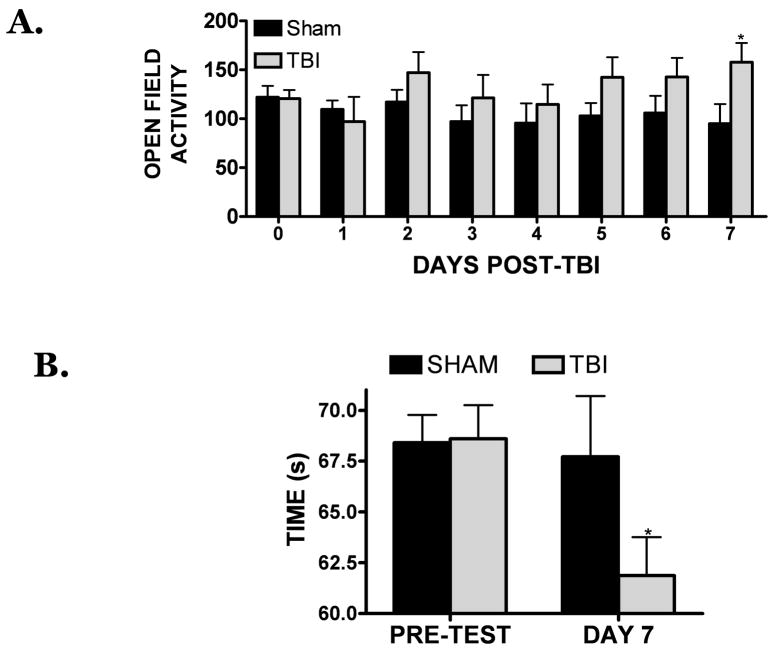
PSD-95 exhibited a delayed reduction within the hippocampus following TBI; however, it remained unclear whether these changes were associated with neurological demise. To establish whether PSD-95 levels correlated with cognitive deficits, the open-field activity test and the two-object novel recognition test were performed. Both tests are widely applied and permit the evaluation of learning and memory and hippocampal function. Following TBI, open field activity was increased between days 2–6 post-injury, although these differences failed to reach statistical significance. On day 7 post-injury open field activity was significantly increased (consistent with hippocampal impairment) while PSD-95 expression declined (FIGURE 1A, 4A). The hippocampus is important for spatial memory, but is also implicated in recognition memory 4. Thus, the time spent exploring a novel object is considered a sensitive test of memory functioning and may relate to hippocampal integrity. Consistent with this assertion, the time spent exploring a novel object was significantly reduced by day 7 post-TBI, further suggestive of functional deficits within the hippocampus (FIGURE 4B). Together, these findings indicate the loss of PSD-95 directly correlates with cognitive demise and indicate a relationship between hippocampal structure and functional outcome following TBI.
FIGURE 4. Delayed neurological deficits following TBI.
(A) Open-field activity testing was performed in sham-operated or TBI-induced mice at various time point following TBI. Activity, as measured by the average number of crosses per trial, was recorded. (B) Novel object recognition test of memory was performed in sham-operated or in mice at day 7 post-TBI, when PSD-95 expression was lowest. The average time spent exploring a novel object, a measure of recognition memory, was recorded and compared with sham-operated control mice. For all studies, n=6–8 mice/group and * was p<0.05 vs. sham.
DISCUSSION
The manifestation of cognitive deficits following head trauma confound rehabilitation and contribute to a poor quality of life. Remediation of learning and memory deficits remains a significant goal of TBI research; unfortunately, many promising therapies for TBI are limited by a narrow therapeutic window. The present report documents a delayed reduction in the hippocampal expression of PSD-95, an abundant protein within excitatory synapses, following TBI. Notably, the loss of PSD-95 directly correlated with a reduction in cognitive function, suggesting a possible cellular mechanism which could, at least in part, explain the neurological deficits observed weeks and even months after initial trauma. The timing of these delayed secondary changes may permit the institution of therapy in most, if not all patients.
Neuronal loss, even in the absence of elevated ICP, is observed within the hippocampus in over 80% of fatal human TBI 20,21 and apoptotic neurons are observed in the human hippocampus up to one year following the initial trauma 41. Prior studies have shown that hippocampal LTP, which contributes to learning and memory formation, was disrupted seven days following lateral fluid percussion in rats 35. Although the precise cellular mechanisms underlying these actions were not established, a reduction in NMDA potentials, attenuated glutamate-induced excitatory currents, and decreased expression of α-calcium calmodulin kinase II (αCAMKII) were observed 35. In the present report, PSD-95 expression was maximally reduced throughout the hippocampus at a time point that paralleled impaired LTP in rats. Notably, overexpression of PSD-95 mimicked several key aspects of LTP and experience-driven synaptic potentiation, including the enhancement of AMPA-R mediated transmission and translocation of GluR1 into synapses, whereas a dominant negative PSD-95 construct blocked LTP in brain slice cultures and attenuated experience-driven plasticity via a reduction in AMPA-mediated currents 8. Although the mechanisms underlying LTP disruption following TBI remain poorly understood, our findings showing a time-dependent reduction in hippocampal PSD-95 expression may permit the future characterization of these processes.
In this study, hippocampal injury was delayed by several days from the primary traumatic event, despite the presence of acute cellular necrosis and edema formation in the pericontusional cortex beginning immediately after injury. Although the mechanisms underlying delayed synaptic loss and cell death within the hippocampus remains unresolved, recent work suggests a prominent role for oxidative stress in promoting neuronal injury following experimental TBI in rats 1. Indeed, PSD-95 couples NMDA receptor activation with nitric oxide-mediated neurotoxicity, supporting a causative role for PSD-95 in neuronal excitotoxicity 34; however, this explanation may not fully explain why a focal cortical injury induces diffuse, widespread hippocampal damage several days after the initial injury. Notably, bilateral entorhinal cortical deafferentation induced dendritic atrophy in the hippocampus and potentiated cognitive morbidity following fluid percussion injury in rats via a NMDA-dependent manner 29, suggesting damage to the cortical projections which innervate the hippocampus may promote secondary injury within the hippocampus, resulting in cognitive decline.
CONCLUSION
The correlation between delayed loss of hippocampal synapses and behavioral deficits following TBI in mice may provide a cellular mechanism to explain the delayed learning and memory deficits in humans. Taken together, these findings provide a conceptual framework for testing novel therapeutic strategies to preserving synaptic integrity or increase synaptogenesis within the hippocampus.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (NS065172) and American Heart Association (BGIA2300135) to KMD and by a fellowship from the American Heart Association (PRE2250690) to MDL
Portions of this work were presented in poster form at National Neurotrauma Society Annual Meeting, Orlando, FL, July, 2008
Footnotes
CW and MDL performed the brain injury model, and immunohistochemistry. SRS performed the Western blotting studies. All authors contributed to data analysis and final manuscript editing. KMD and JV conceptualized the study and wrote the manuscript.
References
- 1.Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 2.Ashman TA, Cantor JB, Gordon WA, Sacks A, Spielman L, Egan M, et al. A comparison of cognitive functioning in older adults with and without traumatic brain injury. J Head Trauma Rehabil. 2008;23:139–148. doi: 10.1097/01.HTR.0000319930.69343.64. [DOI] [PubMed] [Google Scholar]
- 3.Bramlett HM, Green EJ, Dietrich WD. Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 1997;762:195–202. doi: 10.1016/s0006-8993(97)00387-9. [DOI] [PubMed] [Google Scholar]
- 4.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, et al. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Christoforidis GA, Slivka A, Mohammad Y, Karakasis C, Avutu B, Yang M. Size matters: hemorrhage volume as an objective measure to define significant intracranial hemorrhage associated with thrombolysis. Stroke. 2007;38:1799–1804. doi: 10.1161/STROKEAHA.106.472282. [DOI] [PubMed] [Google Scholar]
- 7.Dhandapani KM, Hadman M, De Sevilla L, Wade MF, Mahesh VB, Brann DW. Astrocyte protection of neurons: role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J Biol Chem. 2003;278:43329–43339. doi: 10.1074/jbc.M305835200. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Fink KB, Andrews LJ, Butler WE, Ona VO, Li M, Bogdanov M. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94:1213–1218. doi: 10.1016/s0306-4522(99)00345-0. [DOI] [PubMed] [Google Scholar]
- 11.Fukaya M, Watanabe M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: a study in the adult mouse brain. J Comp Neurol. 2000;426:572–586. [PubMed] [Google Scholar]
- 12.Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK. Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma. 1993;10:405–414. doi: 10.1089/neu.1993.10.405. [DOI] [PubMed] [Google Scholar]
- 13.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology. 2006;66:187–192. doi: 10.1212/01.wnl.0000194264.60150.d3. [DOI] [PubMed] [Google Scholar]
- 14.Hoskison MM, Moore AN, Hu B, Orsi S, Kobori N, Dash PK. Persistent working memory dysfunction following traumatic brain injury: evidence for a time-dependent mechanism. Neuroscience. 2009;159:483–491. doi: 10.1016/j.neuroscience.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutchinson PJ, O’Connell MT, Rothwell NJ, Hopkins SJ, Nortje J, Carpenter KL. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–1557. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 16.Kawamata T, Katayama Y. Cerebral contusion: a role model for lesion progression. Prog Brain Res. 2007;161:235–241. doi: 10.1016/S0079-6123(06)61016-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 18.Kingston PA, Zufall F, Barnstable CJ. Widespread expression of olfactory cyclic nucleotide-gated channel genes in rat brain: implications for neuronal signalling. Synapse. 1999;32:1–12. doi: 10.1002/(SICI)1098-2396(199904)32:1<1::AID-SYN1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Kotapka MJ, Gennarelli TA, Graham DI, Adams JH, Thibault LE, Ross DT, et al. Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J Neurotrauma. 1991;8:247–258. doi: 10.1089/neu.1991.8.247. [DOI] [PubMed] [Google Scholar]
- 20.Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal human head injury without high intracranial pressure. J Neurotrauma. 1994;11:317–324. doi: 10.1089/neu.1994.11.317. [DOI] [PubMed] [Google Scholar]
- 21.Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal non-missile human head injury. Acta Neuropathol. 1992;83:530–534. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- 22.Laird MD, Sangeetha SR, Swift AE, Meiler SE, Vender JR, Dhandapani KM. Curcumin attenuates cerebral edema following traumatic brain injury in mice: a possible role for aquaporin-4? J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06630.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Jover-Mengual T, Wong J, Bennett MV, Zukin RS. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2006;103:19902–19907. doi: 10.1073/pnas.0609924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Skeberdis VA, Francesconi A, Bennett MV, Zukin RS. Postsynaptic density protein-95 regulates NMDA channel gating and surface expression. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathias JL, Mansfield KM. Prospective and declarative memory problems following moderate and severe traumatic brain injury. Brain Inj. 2005;19:271–282. doi: 10.1080/02699050400005028. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell WL, Dhillon K, Harper L, Espin J, MacIntosh TK, Smith DH, et al. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol. 2003;62:272–279. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka M, Chen XH, Pierce JE, Leoni MJ, McIntosh TK, Wolf JA, et al. Prolonged activation of NF-kappaB following traumatic brain injury in rats. J Neurotrauma. 1999;16:1023–1034. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- 28.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, et al. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 29.Phillips LL, Lyeth BG, Hamm RJ, Reeves TM, Povlishock JT. Glutamate antagonism during secondary deafferentation enhances cognition and axo-dendritic integrity after traumatic brain injury. Hippocampus. 1998;8:390–401. doi: 10.1002/(SICI)1098-1063(1998)8:4<390::AID-HIPO7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 30.Roche KW, Standley S, McCallum J, Dune Ly C, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 31.Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008;89:S69–76. doi: 10.1016/j.apmr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Sangeetha SR, Singh N, Vender JR, Dhandapani KM. Suberoylanilide hydroxamic acid (SAHA) induces growth arrest and apoptosis in pituitary adenoma cells. Endocrine. 2009;35:389–396. doi: 10.1007/s12020-009-9159-1. [DOI] [PubMed] [Google Scholar]
- 33.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzbach E, Bonislawski DP, Xiong G, Cohen AS. Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus. 2006;16:541–550. doi: 10.1002/hipo.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. J Neurol Neurosurg Psychiatry. 2005;76:129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tate DF, Bigler ED. Fornix and hippocampal atrophy in traumatic brain injury. Learn Mem. 2000;7:442–446. doi: 10.1101/lm.33000. [DOI] [PubMed] [Google Scholar]
- 38.Till C, Colella B, Verwegen J, Green RE. Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. 2008;89:S25–34. doi: 10.1016/j.apmr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakade C, King MD, Laird MD, Alleyne CH, Jr, Dhandapani KM. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid Redox Signal. 2009;11:35–45. doi: 10.1089/ars.2008.2056. [DOI] [PubMed] [Google Scholar]
- 41.Williams S, Raghupathi R, MacKinnon MA, McIntosh TK, Saatman KE, Graham DI. In situ DNA fragmentation occurs in white matter up to 12 months after head injury in man. Acta Neuropathol. 2001;102:581–590. doi: 10.1007/s004010100410. [DOI] [PubMed] [Google Scholar]