Abstract
Background & Aims
It is recommended that patients with chronic hepatitis C virus (HCV) genotype 3 infections receive 24 weeks of treatment. A rapid virologic response (RVR, at week 4) predicts a sustained virologic response (SVR), although not all patients with an RVR achieve an SVR. We explored the relationships among hepatic steatosis, level of HCV RNA, relapse, and RVR in a phase 3 randomized controlled trial of 932 patients infected with HCV genotype 2 (n = 427) or 3 (n = 505) who received 24 weeks of therapy with interferon-α.
Methods
In patients with an RVR (HCV RNA <43 IU/mL), the presence of an SVR was modeled using multivariate logistic regression as a function of age, sex, weight, body-mass index, insulin resistance, steatosis, and levels of γ-glutamyl transpeptidase, alanine transaminase, liver fibrosis, and baseline HCV RNA.
Results
RVR, SVR, and relapse rates among patients with HCV genotype 3 were 79.6%, 79.2%, and 15.6%, respectively; corresponding rates among patients with HCV genotype 2 were 86.7%, 84.3%, and 10.1%. An RVR had high predictive value for an SVR in patients with HCV genotypes 2 (88.9%) and 3 (88.1%). The strongest independent predictors of relapse in patients with genotype 3 and an RVR were steatosis (odds ratio 3.0; P=.003) and HCV RNA ≥400,000 IU/mL (2.5; P=.04). Relapse rates in patients with steatosis were 17.4% and 20.9% for low and high baseline levels of HCV RNA, respectively; corresponding rates in those without steatosis were 2.5% and 8.8%.
Conclusions
Steatosis was associated with significantly higher rates of relapse, irrespective of viral load, in patients infected with HCV genotype 3 who had an RVR. Further studies are needed to determine if longer treatment durations are effective in patients with an RVR and these risk factors.
Keywords: Liver disease, response to therapy, prognosis, recurrence
Chronic hepatitis C virus (HCV) infection is a global health problem with an estimated prevalence of 2%, representing 120 to 130 million people.1 There is significant geographic variation in the prevalence and distribution of HCV genotypes. Although HCV genotype 1 predominates in Europe, North and South America, and Japan, genotype 3 accounts for 35% to 55% of chronic HCV infections in regions such as the Indian subcontinent, Southeast Asia, and Australasia.2,3 In comparison with other genotypes, genotype 3 is also associated with a higher incidence of hepatic steatosis and rapid progression of liver fibrosis.2,4–6 Furthermore, emerging direct-acting antiviral therapies, such as telaprevir, appear to have reduced efficacy in genotype 3, indicating that pegylated interferon (IFN)-α (Peg-IFNα) and ribavirin are likely to remain the standard-of-care for now. Current recommended therapy for genotype 2 or 3 is 24 weeks of Peg-IFNα and a fixed-dose of ribavirin (800 mg/d). Although sustained virologic response (SVR) rates for genotypes 2 and 3 (range 70% to 85%) are higher than for genotype 1, the rates observed for genotype 3 are consistently lower than for genotype 2.7–11 To tailor therapy, a shorter duration of treatment (12 to 16 weeks) has been explored, although an increase in relapse rates has been observed, most notably in patients who did not achieve a rapid virologic response at week 4 (RVR).8–10 Conversely, lengthening treatment duration and weight-based ribavirin may be beneficial for patients with genotype 3 who do not achieve an RVR.12–14 Very few studies, however, have been powered to assess host-viral factors associated with relapse in patients with genotype2 and 3 separately.15
Hepatitis C virus genotype 3 is associated with the presence of hepatic steatosis, with rates of up to 70% that are consistently higher than those reported in other genotypes.4 The impact of underlying steatosis on treatment outcomes in genotype has been explored in a number of studies with variable results. Several studies have suggested no association between steatosis and SVR in genotype,16,17 particularly when HCV-RNA levels18 or insulin resistance19 were considered. Other reports, however, have indicated the absence of steatosis as a significant predictor of SVR in these patients.7 This may relate to cohort differences in steatosis grading and prevalence, treatment regimens, or HCV-RNA thresholds, or failure to consider host metabolic factors such as insulin resistance. Of note, however, the association of steatosis with RVR in genotype 2 or 3 has not been clearly established. The aim of the present study was to further explore the inter-relationship between steatosis, HCV-RNA levels, and relapse in the context of RVR in a well-characterized cohort of patients with chronic HCV genotype 2 or 3 enrolled in a phase 3 trial.
Materials and Methods
Study Design
Overall, 932 treatment-naïve patients with chronic HCV genotype 2 (n = 427) or 3 (n = 505) were enrolled in a global, phase 3, randomized, active controlled study (ACHIEVE-2/3; ClinicalTrials.gov identifier NCT00411385) of albinterferon alfa-2b vs Peg-IFNα-2a (Pegasys; Hoffmann-La Roche, Inc, Nutley, New Jersey), plus fixed-dose ribavirin (Ribasphere; Three Rivers Pharmaceuticals, Warrendale, Pennsylvania; 800 mg/d), for 24 weeks.20 Study design, inclusion and exclusion criteria, and final study results have been reported previously.20 Hepatitis C virus-RNA levels were assessed by real-time polymerase chain reaction (CE-marked COBAS® Ampliprep/COBAS® Taqman® HCV Test, Roche Diagnostics, Basel, Switzerland). The limit of quantitation for this assay was 43 IU/mL and the limit of detection 15 IU/mL (testing performed by LabCorp, Raritan, New Jersey). The primary efficacy endpoint was SVR, defined as HCV-RNA level <15 IU/mL at week 48. Rapid virologic response was defined as HCV-RNA <43 IU/mL.
Pretreatment percutaneous liver biopsies were centrally evaluated for METAVIR fibrosis stage (F), METAVIR inflammatory activity, and hepatic steatosis grade by a central blinded pathologist. Steatosis was graded as the percentage of hepatocytes containing macrovesicular fat droplets: grade 0, 0%–5%; grade 1, 6%–30%; grade 2, 31%–60%; and grade 3, ≥61%.
Statistical Methods
The presence of relapse was modeled using multivariate logistic regression implementing a backward elimination approach. Potential predictors predefined in the study analysis plan included sex; weight ≥75 kg; body mass index (BMI) ≥30 kg/m2; age ≥45 years; alanine aminotransferase >15x the upper limit of normal (ULN); γ-glutamyl transpeptidase (GGT) ≤ ULN; advanced fibrosis (F3–4); Asian region vs the rest of the world; baseline HCV RNA ≥400,000 IU/mL; insulin resistance (homeostasis model assessment–insulin resistance index >2); and steatosis as a dichotomous variable (grade ≥1 or >5% fat). When treatment group effects were added to the modeling, these results remained unchanged. Pooled data from each of the 3 treatment groups were, therefore, used for the analysis. All P values reported are 2-sided; a significance level of .05 was used for all analyses. All analyses were performed using SAS®9 (SAS Institute Inc., Cary, North Carolina) and R (version 1.9.1) statistical software, and are presented in the intention-to-treat population.
Results
Patient Demographics
The characteristics of the study population according to HCV genotype are presented in Table 1. Most patients with genotype 3 were Caucasian (75%), while Asian patients comprised nearly half of the genotype 2 population (44%). Patients with genotype 3 were predominantly men (63%) and younger in age than those with genotype 2 (mean age ± standard deviation [SD] 40.4 ± 10 vs 49.3 ± 10.8 years; P <.001). As expected, there was a higher prevalence of hepatic steatosis in patients with genotype 3 vs 2 (46% vs 27%; P <.001). Although body weight was higher in patients with genotype 3 vs 2 (mean ± SD 75.8 ± 17.1 vs 72.8 ± 17.9 kg; P = .01), there were no differences between genotypes for BMI or homeostasis model assessment–insulin resistance. The 2 genotypes were similar with respect to baseline viral load (mean ± SD 6.0 ± 0.9 log10 IU/mL for both genotypes) and presence of F3–4 (11%–12%).
Table 1.
Baseline Characteristics of Patients With Chronic Hepatitis C Virus Genotype 2 or 3
| Genotype 2 (n = 427) | Genotype 3 (n = 505) | P valuea | |
|---|---|---|---|
| Sex, n (%) | .004 | ||
| Male | 228 (53) | 317 (63) | |
| Female | 199 (47) | 188 (37) | |
|
| |||
| Race, n (%) | <.001 | ||
| Caucasian | 211 (49) | 377 (75) | |
| Asian | 187 (44) | 116 (23) | |
| Black/African heritage | 13 (3) | 4 (1) | |
| Other | 16 (4) | 8 (2) | |
|
| |||
| Age | |||
| Mean ± SD, y | 49.3 ± 10.8 | 40.4 ±10.0 | <.001 |
| <45 y, n (%) | 128 (30) | 317 (63) | <.001 |
| ≥45 y, n (%) | 299 (70) | 188 (37) | |
|
| |||
| Weight | |||
| Mean ± SD, kg | 72.8 ± 17.9 | 75.8 ± 17.1 | .01 |
| <75 kg, n (%) | 269 (63) | 263 (52) | <.001 |
| ≥75 kg, n (%) | 158 (37) | 242 (48) | |
|
| |||
| BMI | |||
| Mean ± SD, kg/m2 | 26.1 ± 5.0 | 26.0 ± 4.9 | .79 |
| <30 kg/m2, n (%) | 340 (80) | 410 (81) | .55 |
| ≥30 kg/m2, n (%) | 87 (20) | 95 (19) | |
|
| |||
| HOMA-IR | n =362 | n = 402 | |
| Mean ± SD | 3.0 ± 2.8 | 3.4 ± 4.5 | .12 |
| Insulin resistant, n (%)b | 178 (49) | 209 (52) | .44 |
|
| |||
| Steatosis, n (%)c | 115 (27) | 232 (46) | <.001 |
|
| |||
| ALT, n (%) | |||
| >1.5x ULN | 187 (44) | 285 (56) | <.001 |
| ≥1.5x ULN | 240 (56) | 220 (44) | |
|
| |||
| GGT, n (%) | |||
| ≤ULN | 328 (77) | 363 (72) | .087 |
| >ULN | 99 (23) | 142 (28) | |
|
| |||
| HCV RNA | |||
| Mean ± SD, log10 IU/mL | 6.0 ± 0.9 | 6.0 ± 0.9 | .96 |
| ≥400,000 IU/mL, n (%) | 302 (71) | 347 (69) | .51 |
| <400,000 IU/mL, n (%) | 125 (29) | 158 (31) | |
|
| |||
| Fibrosis | n =424 | n = 498 | |
| F3–4, n (%) | 50 (12) | 54 (11) | .65 |
| F0–2, n (%) | 374 (88) | 444 (89) | |
ALT, alanine transaminase; BMI, body mass index; f, fibrosis stage; GGT, γ-glutamyl transpeptidase; HCV, hepatitis C virus; HOMA-IR, homeostasis model of assessment–insulin resistance; SD, standard deviation; ULN, upper limit of normal.
2-sided P value for comparison of genotypes obtained from Pearson chi-square or t test.
Defined as HOMA-IR >2.
Defined as fat grade >5% on baseline biopsy or HOMA-IR score >4.5 and triglyceride level >180 mg/dL if biopsy data were missing.
Virologic Response
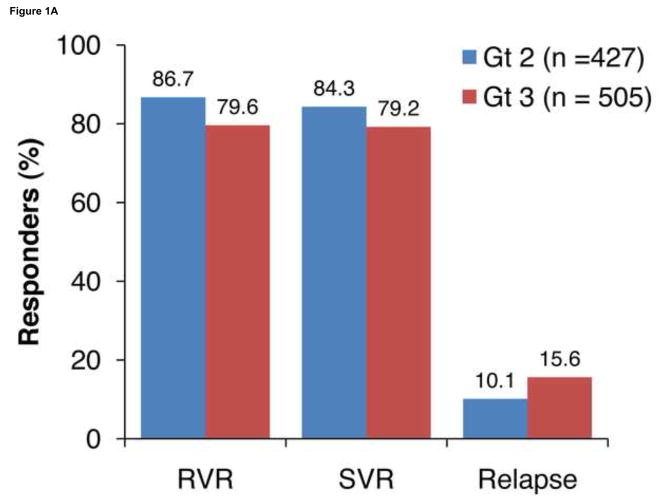
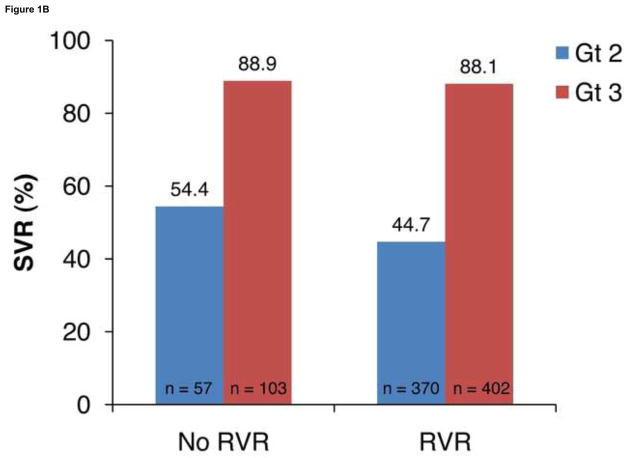
Virologic response rates (RVR, SVR, and relapse) in all patients are shown in Figure 1A. Overall, SVR rates were lower in patients with HCV genotype 3 vs 2 (79.2% vs 84.3%; P = .04); this was a result of lower RVR rates with genotype 3 vs 2 (79.6% vs 86.7%; P = .004). Further, relapse rates were higher with genotype 3 vs 2 (15.6% vs 10.1%; P = .001). The SVR rates in patients with an RVR were high in both genotypes 2 and 3 (88.9% and 88.1%, respectively; Figure 1B).
Figure 1.
Virologic response rates in genotypes (Gt) 2 and 3. A, rapid virologic response at week 4 (RVR), sustained virologic response (SVR), and relapse rates. B, SVR rates in patients with and without RVR.
Independent Predictors of Relapse
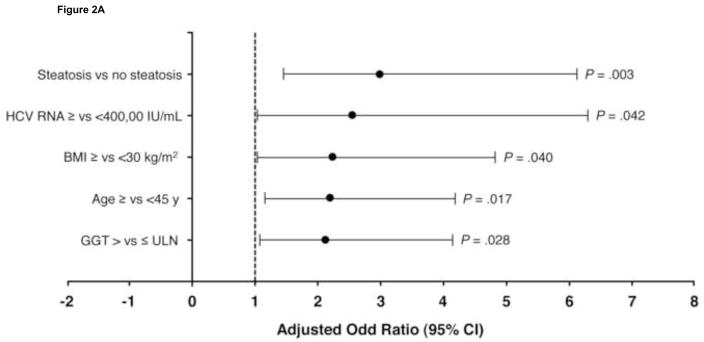
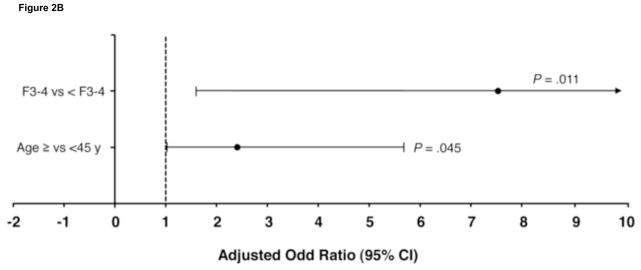
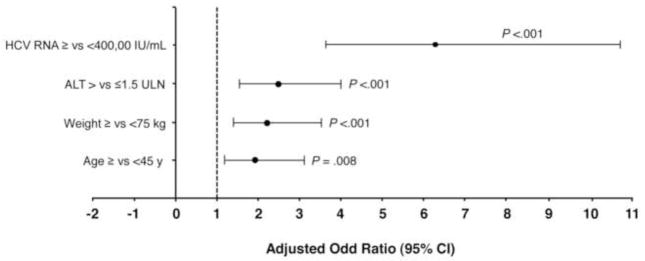
There were 402 patients with HCV genotype 3 who achieved an RVR. Multivariate modeling in these patients indicated that hepatic steatosis (>5%) was the strongest independent predictor of relapse (odds ratio [OR] 3.0 [95% confidence interval (CI) 1.5–6.1]; P = .003; Figure 2A). Other predictors were HCV-RNA level ≥400,000 IU/mL (OR 2.5 [95% CI 1.0–6.3]; P = .04), BMI ≥30 (2.2 [1.0–4.8]; P = .04), age ≥45 years (2.2 [1.2–4.2]; P = .02), and GGT >ULN (2.1 [1.1–4.1; P = .03). In the 103 patients with genotype 3 without an RVR, F3–4 (OR 7.6 [95% CI 1.6–35.8]; P = .01) and age ≥45 (2.4 [1.0–5.7]; P = .04) were the independent predictors of relapse (Figure 2B).
Figure 2.
Independent predictors of relapse in patients with hepatitis C virus (HCV) genotype 3 (A) with and (B) without rapid virologic response. BMI, body mass index; CI, confidence interval; F, fibrosis score; GGT, γ-glutamyl transpeptidase; ULN, upper limit of normal.
Given the importance of hepatic steatosis as a predictor of relapse in patients with HCV genotype 3 and an RVR, the association of baseline factors with steatosis was examined in these patients (Figure 3). High baseline HCV-RNA level was the strongest predictor of steatosis (OR 6.3 [95% CI 3.6–10.8]; P <.001). Other factors associated with steatosis included high baseline alanine transaminase (OR 2.5 [95% CI 1.6–4.0]), weight >75kg (2.2 [1.4–3.5]), and age ≥45years (1.9 [1.2–3.1]). When the multivariate modeling was extended for all patients with genotype 3 (irrespective of RVR status), these predictors remained the same.
Figure 3.
Independent predictors of hepatic steatosis in patients with hepatitis C virus (HCV) genotype 3 who achieved rapid virologic response. ALT, alanine transaminase; CI, confidence interval; ULN, upper limit of normal.
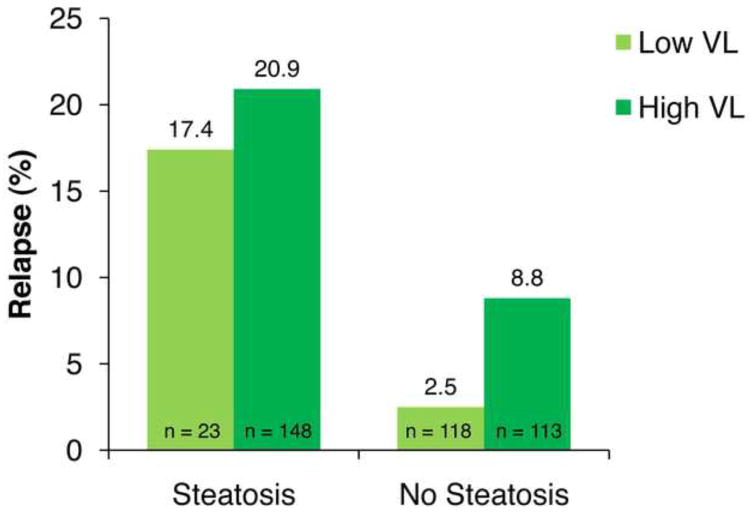
It is noteworthy that while viral load was the strongest predictor of hepatic steatosis, steatosis remained the strongest independent predictor of relapse when controlling for viral load and other factors. In patients with HCV genotype 3 and an RVR with low baseline HCV-RNA levels, relapse rates were 17.4% and 2.5% in patients with and without steatosis, respectively (P = .003; Figure 4); the corresponding rates in such patients with high baseline HCV-RNA levels were 20.9% and 8.9%, respectively (P = .008). These data suggest the importance of steatosis regardless of viral load with respect to elevated relapse rates.
Figure 4.
Relapse rates in patients with hepatitis C virus genotype 3 and rapid virologic response based on baseline viral load (VL) and hepatic steatosis.
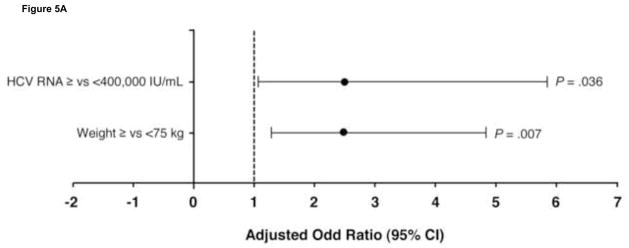
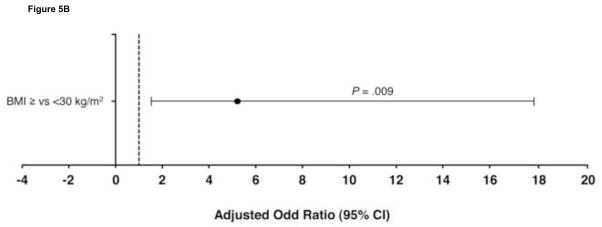
The predictors of relapse in patients with HCV genotype 2 are shown in Figure 5. In the 370 patients with genotype 2 who achieved an RVR, the strongest predictors for relapse were baseline HCV-RNA level ≥400,000 IU/mL (OR 2.5 [95% CI 1.1–5.8]; P = .036) and weight ≥75 kg (OR 2.5 [1.3–4.8]; P = .007). In the 57 patients with genotype 2 without an RVR, BMI ≥30 kg/m2 was the only significant predictor of SVR failure (OR 5.2 [1.5–17.7; P = .009). Further, SVR rates were significantly higher in these patients with a BMI < vs ≥30 kg/m2 (67% vs 28%; P = .006). This may reflect the higher proportion of Asian patients, as has been described previously.20 Most patients in this group (n = 54; 95%) had a high baseline HCV-RNA level.
Figure 5.
Independent predictors of relapse in patients with hepatitis C virus (HCV) genotype 2 (A) with and (B) without rapid virologic response. BMI, body mass index; CI, confidence interval.
Discussion
This study of a large well-characterized cohort of patients with chronic HCV genotypes 2 and 3 provides the first report that hepatic steatosis significantly increases the risk of relapse independently of HCV-RNA levels in patients with genotype 3 who achieve an RVR with IFN-based regimens. In patients with genotype 2 and an RVR, higher HCV-RNA levels and body weight were associated with relapse. Insulin resistance was not associated with relapse in patients with either genotype 2 or 3 irrespective of RVR status. As individualized or response-guided therapy for genotypes 2 and 3 evolves, these data highlight the need for a comprehensive approach considering genotype, RVR status, and steatosis when considering shortening or extending therapy for a given patient.
Several studies have shown that RVR consistently remains an important determinant of SVR in patients with HCV genotype 2 or 3.10,21–23 The most remarkable observation in the present study was the high relapse rate despite achieving an RVR in patients with genotype 3 and hepatic steatosis regardless of baseline HCV-RNA level. Several studies have indicated that steatosis is an independent predictor of virologic response only in patients with genotypes other than 3.16,17,24 This suggests that the underlying pathogenic mechanisms of steatosis differ between genotype 3 and other genotypes, and may influence response to IFN-based therapy.
Steatosis in genotype 3 is associated with intrahepatic HCV RNA25 and greater quasispecies diversity than in other genotypes.26 This study showed, however, that HCV-RNA levels had limited impact on relapse rate in patients with steatosis. The interactions between HCV genotype 3 and host-signaling pathways that affect response to therapy and/or impact liver fibrosis and steatosis are being actively explored. Mechanisms invoke altered IFNα signaling, and altered cytokine and gut hormones that are associated with virologic response in genotype 3.27,28 Recently, single nucleotide polymorphisms on a linkage disequilibrium block encompassing 2 IFNλ genes—encoding type III IFNλ2 (IL28A) and IFNλ3 (IL28B)—have been implicated in response to IFN-based therapy.29 The IL28B “responder” single nucleotide polymorphism has been associated with higher GGT levels and possibly liver steatosis.30 Although elevated GGT levels were a predictor of relapse in patients with genotype 3 and RVR in this study, there was no independent association of GGT with steatosis in these patients. Further, IL28B polymorphisms are associated with lower endogenous IFN responses, higher lipid levels in genotypes other than 3,31 and achievement of SVR in patients without RVR with genotypes other than 2 and 3.32 Thus IL28B polymorphisms alone are unlikely to explain the complex host-viral associations between steatosis and early and late IFN responses in genotype 3 that result in relapse following RVR. Polymorphisms in other host genes, such as patatin-like phospholipase domain-containing protein 3 or adiponutrin, were shown to influence the degree of fibrosis and steatosis in patients with chronic HCV, and should be explored further in the context of response to therapy.33,34
This study suggests that patients with HCV genotype 3 with hepatic steatosis who achieve an RVR have higher relapse rates. Although biopsy is deemed unnecessary in genotypes 2 and 3, there are no simple alternatives that can accurately assess steatosis, which appears to be an important determinant of relapse in patients with genotype 3 and an RVR. Studies are planned to further evaluate the roles of immunoregulatory cytokines and other host-viral responses in patients with genotype 3 and high viral loads who achieve an RVR and then relapse with or without steatosis.
Acknowledgments
Supported by Human Genome Sciences, Inc., Rockville, Maryland, and Novartis Pharma AG, Basel, Switzerland.
Graham R. Foster has received funding from Hoffman-LaRoche Inc., Nutley, New Jersey and Novartis Pharma AG, Basel, Switzerland; Letha Healey, Erik Pulkstenis, and G. Mani Subramanian are employees of and own stock in Human Genome Sciences, Inc., Rockville, Maryland. John G. McHutchison has received research support from and is a consultant for HGS, Novartis, and Hoffmann-La Roche, Inc, Nutley, New Jersey. Mark S. Sulkowski has received consulting fees and research support from HGS and Roche. Stefan Zeuzem has received consulting fees from HGS and Novartis, and lecture fees from Novartis. David R. Nelson has received research grants from HGS and Novartis, and is a consultant for HGS.
Editorial support was provided by Geoff Marx of BioScience Communications, New York, NY, and was funded by Human Genome Sciences and Novartis.
Abbreviations used in this paper
- BMI
body mass index
- CI
confidence interval
- F
fibrosis stage
- GGT
γ-glutamyl transpeptidase
- HCV
hepatitis C virus
- IFN
interferon
- OR
odds ratio
- Peg-IFNα
pegylated IFNα
- RVR
rapid virologic response at week 4
- SD
standard deviation
- SVR
sustained virologic response
- ULN
upper limit of normal
Footnotes
Other authors have no potential conflicts of interest relevant to this article to declare.
Author Contributions: Samir R. Shah, Keyur Patel, Erik Pulkstenis, G. Mani Subramanian, John G. McHutchison, Mark S. Sulkowski, Stefan Zeuzem, and David R. Nelson were responsible for study concept and design; Samir R. Shah, Keyur Patel, Erik Pulkstenis, and G. Mani Subramanian were responsible for drafting the manuscript; Erik Pulkstenis was responsible for statistical analysis; all authors were responsible for data acquisition, analysis, and interpretation, and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Hissar SS, Goyal A, Kumar M, et al. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006;78:452–458. doi: 10.1002/jmv.20561. [DOI] [PubMed] [Google Scholar]
- 3.Lee CM, Lu SN, Hung CH, et al. Hepatitis C virus genotypes in southern Taiwan: prevalence and clinical implications. Trans R Soc Trop Med Hyg. 2006;100:767–774. doi: 10.1016/j.trstmh.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123–130. doi: 10.1136/gut.2005.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochud PY, Cai T, Overbeck K, et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009;51:655–666. doi: 10.1016/j.jhep.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Adinolfi LE, Utili R, Andreana A, et al. Serum HCV RNA levels correlate with histological liver damage and concur with steatosis in progression of chronic hepatitis C. Dig Dis Sci. 2001;46:1677–1683. doi: 10.1023/a:1010697319589. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Hultcrantz R, Bourliere M, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40:993–999. doi: 10.1016/j.jhep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Mangia A, Santoro R, Minerva N, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 9.von Wagner M, Huber M, Berg T, et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129:522–527. doi: 10.1016/j.gastro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman ML, Suter F, Bacon BR, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;357:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 11.Andriulli A, Mangia A, Iacobellis A, et al. Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment Pharmacol Ther. 2008;28:397–404. doi: 10.1111/j.1365-2036.2008.03763.x. [DOI] [PubMed] [Google Scholar]
- 12.Aghemo A, Rumi MG, Soffredini R, et al. Impaired response to interferon-alpha2b plus ribavirin in cirrhotic patients with genotype 3a hepatitis C virus infection. Antivir Ther. 2006;11:797–802. [PubMed] [Google Scholar]
- 13.Jacobson IM, Brown RS, Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 14.Mangia A, Dalgard O, Minerva N, et al. Ribavirin dosage in patients with HCV genotypes 2 and 3 who completed short therapy with peg-interferon alpha-2b and ribavirin. Aliment Pharmacol Ther. 2010;31:1346–1353. doi: 10.1111/j.1365-2036.2010.04290.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangia A. Individualizing treatment duration in hepatitis C virus genotype 2/3-infected patients. Liver Int. 2011;31:36–41. doi: 10.1111/j.1478-3231.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 16.Poynard T, Ratziu V, McHutchison J, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 17.Patton HM, Patel K, Behling C, et al. The impact of steatosis on disease progression and early and sustained treatment response in chronic hepatitis C patients. J Hepatol. 2004;40:484–490. doi: 10.1016/j.jhep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Torres M, Govindarajan S, Diago M, et al. Hepatic steatosis in patients with chronic hepatitis C virus genotype 2 or 3 does not affect viral response in patients treated with peginterferon alpha-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks. Liver Int. 2009;29:237–241. doi: 10.1111/j.1478-3231.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 19.Poustchi H, Negro F, Hui J, et al. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28–34. doi: 10.1016/j.jhep.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DR, Benhamou Y, Chuang WL, et al. Albinterferon alfa-2b was not inferior to pegylated interferon-alpha in a randomized trial of patients with chronic hepatitis C virus genotype 2 or 3. Gastroenterology. 2010;139:1267–1276. doi: 10.1053/j.gastro.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mangia A, Minerva N, Bacca D, et al. Determinants of relapse after a short (12 weeks) course of antiviral therapy and re-treatment efficacy of a prolonged course in patients with chronic hepatitis C virus genotype 2 or 3 infection. Hepatology. 2009;49:358–363. doi: 10.1002/hep.22679. [DOI] [PubMed] [Google Scholar]
- 22.Lagging M, Langeland N, Pedersen C, et al. Randomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infection. Hepatology. 2008;47:1837–1845. doi: 10.1002/hep.22253. [DOI] [PubMed] [Google Scholar]
- 23.Dalgard O, Bjoro K, Ring-Larsen H, et al. Pegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological response. Hepatology. 2008;47:35–42. doi: 10.1002/hep.21975. [DOI] [PubMed] [Google Scholar]
- 24.Negro F, Clement S. Impact of obesity, steatosis and insulin resistance on progression and response to therapy of hepatitis C. J Viral Hepat. 2009;16:681–688. doi: 10.1111/j.1365-2893.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 25.Rubbia-Brandt L, Quadri R, Abid K, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33:106–115. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 26.Asselah T, Martinot M, Cazals-Hatem D, et al. Hypervariable region 1 quasispecies in hepatitis C virus genotypes 1b and 3 infected patients with normal and abnormal alanine aminotransferase levels. J Viral Hepat. 2002;9:29–35. doi: 10.1046/j.1365-2893.2002.00327.x. [DOI] [PubMed] [Google Scholar]
- 27.Pazienza V, Clement S, Pugnale P, et al. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- 28.Zografos TA, Liaskos C, Rigopoulou EI, et al. Adiponectin: a new independent predictor of liver steatosis and response to IFN-alpha treatment in chronic hepatitis C. Am J Gastroenterol. 2008;103:605–614. doi: 10.1111/j.1572-0241.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 29.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 30.Abe H, Ochi H, Maekawa T, et al. Common variation of IL28 affects gamma-GTP levels and inflammation of the liver in chronically infected hepatitis C virus patients. J Hepatol. 2010;53:439–443. doi: 10.1016/j.jhep.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Li JH, Lao XQ, Tillmann HL, et al. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904–1911. doi: 10.1002/hep.23592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mangia A, Thompson AJ, Santoro R, et al. An IL28B polymorphism determines treatment response of hepatitis C virus genotype 2 or 3 patients who do not achieve a rapid virologic response. Gastroenterology. 2010;139:821–827. 827, e821. doi: 10.1053/j.gastro.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 33.Cai T, Dufour JF, Muellhaupt B, et al. on Behalf of the Swiss Hepatitis C Cohort Study Group. Viral genotype-specific role of PNPLA3, PPARG, MTTP and IL28B in hepatitis C virus-associated steatosis. J Hepatol. 2011 Jan 11; doi: 10.1016/j.jhep.2010.12.020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Valenti L, Rumi M, Galmozzi E, et al. Patatin-Like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology. 2010 Dec 13; doi: 10.1002/hep.24123. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]