SUMMARY
Human and bovine factor Va (FVa) function similarly in the activation of prothrombin but differently in the activation of prethrombin-1 (Pre-1). Pre-1 activation with human FVa proceeds at about 22 percent of the rate with bovine FVa. The dependencies of initial rates on the FVa and Pre-1 concentrations indicate that the differential activity is expressed in kcat differences, rather than differences in the assembly of prothrombinase or the Km value of the substrate. The heavy and light chains of both species of FVa were separated and interspecies hybrids were constructed in the presence of Ca++. Studies of the activation of Pre-1 with these hybrids indicate that the species difference can be attributed specifically to the heavy chain of FVa. Analyses of the reactions by SDS-PAGE indicated that cleavage at Arg271 occurs at about the same rate with both species of FVa, but cleavage at Arg320 with human FVa is specifically retarded. A major difference in primary structure between the human and bovine FVa heavy chains comprises 10 residues at COOH-terminus, adjacent to the negatively charged hirudin-like DYDYQ sequence. These residues have pI values of 12.5 and 4.26 in human and bovine FVa, respectively. The lower value would complement the negatively charged DYDYQ sequence but the higher value would counteract it. Thus, we suggest that the differences in the COOH-terminus of the heavy chain are responsible for the differences in Pre-1 activation, and that it specifically influences cleavage at Arg320 in Pre-1.
INTRODUCTION
Factor Va (FVa) is a two subunit protein that functions as a non-enzymatic cofactor in prothrombin activation (1,2). It is derived factor V by limited proteolysis, catalyzed by thrombin or FXa (3–6). The subunits of FVa are designated the heavy chain (105 kDa) and the light chain (74 kDa) (7,8). These come, respectively, from the amino (NH2-) and carboxy (COOH-) termini of factor V (7–9). The heavy chain of human FVa spans residues 1–709 and contains an A1 and an A2 domain. The light chain of human FVa spans residues 1546–2196 and contains an A3, a C1 and a C2 domain (10). The heavy and light chains can be dissociated by Ca++ chelators and the individual chains can be isolated (3,7). Neither chain alone is functional, but activity can be regained by recombining the chains with Ca++ (3,7,11).
Prothrombin is a 72 kDa vitamin K-dependent protein (12). It has 3 domains that are designated, from the NH2-terminus, fragment-1 (22 kDa), fragment-2 (13 kDa) and prethrombin-2 (Pre-2) (38 kDa). Two respective cleavages catalyzed by FXa between fragment-2 and Pre-2 (Arg271) and within a disulfide loop in the Pre-2 domain (Arg320) generate thrombin (12,13). In addition, thrombin catalyzes cleavages of the bond joining fragment-1 and fragment-2. After activation, fragment-1 and fragment-2 are liberated as activation fragments. Fragment-1 confers upon prothrombin Ca++ and procoagulant phospholipid binding properties (12,14). These interactions are mediated by the ten gamma carboxyglutamic acid residues within fragment-1. Fragment-2 comprises a triple disulfide bonded structure referred to as a kringle. It mediates an interaction between prothrombin and FVa (13). Prethrombin-1 (Pre-1) is a truncated form of prothrombin lacking fragment-1 (12).
Prothrombinase consists of a 1:1 complex of FXa (serine protease) and FVa on a procoagulant surface (1,2,15). These components catalyze prothrombin activation 300,000-fold faster than FXa alone (16). In model systems, the surface can be provided by negatively charged phospholipid vesicles, whereas the physiologic surface is provided by activated platelets or monocytes and lymphocytes (17,18). FVa binds to both the surface and FXa, and thereby concentrates the protease with the substrate at the catalytic surface (19). This effect enhances the rate by approximately 100-fold (16,20). In addition, FVa, through an unknown mechanism, enhances the turnover number of the reaction by 3000-fold (16,20). These effects together account for the 300,000-fold increase in rate that occurs with the combination of the surface and FVa (20).
Studies of the kinetics of activation of prothrombin in the absence of phospholipid showed that the process conforms to a mechanism in which FVa forms a binary complex with either FXa or prothrombin, through interactions mediated by both the light and heavy chains of FVa (15,21–25). Either of these binary complexes can interact with the third component (FXa or prothrombin) to form a ternary FXa·FVa·prothrombin complex (23). Thrombin is then generated from prothrombin within this complex.
The heavy chain of FVa contains a hirudin-like sequence near the COOH-terminus, DYDYQ (695–699), that has been identified as a potential prothrombin interaction site and a determinant of the prothrombin activation pathway (26,27). Gerads et al. showed that cleavage of the heavy chain with an enzyme purified from the venom of the snake Naja naja oxiana resulted in a >9-fold increase in Km for prothrombin activation (28). Subsequently, Bakker et al. showed that the removal of residues 683–709 of the heavy chain by the same enzyme caused a 9-fold or greater increase in the dissociation constant for the FXa-FVa complex on a phospholipid membrane surface, regardless of whether FXa or FVa were limiting (29). Camire et al. used cathepsin G or human neutrophil elastase to remove the COOH terminal of the heavy chain. They found similar increases in the dissociation constant for the interaction between FVa and FXa on the membrane surface (30). Hirbawi et al. recently utilized two mutants of FVa, one missing residues 680–709 of the COOH-terminus of the heavy chain, and the other having DYDY (695–698) substituted with four alanine residues (31). Both mutants showed significant decreases in the clotting activity. These mutants, however, showed increases in the apparent kcat values for prothrombin activation when assessed in assays using purified reagents and thrombin-specific chromogenic substrates. A similar increase in kcat also was reported by Gerads et al. (28) and Camire et al. (30). Hirbawi et al. also showed that the mutants promoted meizothrombin formation normally, but were specifically defective in conversion of meizothrombin to thrombin (31). These observations, however, are at odds with a study by Toso and Camire in which various mutants of human FVa with truncated COOH-termini of the heavy chain, while defective in Pre-1 activation, were as functional as wild-type FVa in prothrombin activation (32).
The present studies were motivated by unexpected observations made when measuring thrombin generation in mixtures of bovine and human FXa, FVa, prothrombin and Pre-1. The results indicated that prothrombin is a good substrate for prothrombinase regardless of the species of the proteins. Pre-1, however, was efficiently converted only when bovine FVa was included in the prothrombinase complex, regardless of the species of FXa or Pre-1. These results were provocative since the sequences of the human and bovine FVa heavy and light chains, respectively, are 84 and 86 percent identical (8,9) and, therefore, similar if not identical function was expected. The studies below were undertaken to determine whether the differences in function between bovine and human FVa with respect to Pre-1 activation can be attributed to differences in Km, kcat or prothrombinase assembly, whether the differences can be specifically localized to the heavy or light chains of FVa, and whether they rationally correlate with differences in primary structure.
MATERIALS AND METHODS
Proteins and Reagents
Human and bovine coagulation factor V (4), prothrombin and factor X were isolated as described previously (33) with modifications (34). FXa (35) and thrombin (36) were prepared as described previously. The fluorescent thrombin inhibitor dansylarginine N-(3-ethyl-1,5-pentanediyl)amide (DAPA) was prepared according to Nesheim, et al. (37). Vesicles of phosphatidyl-L-choline: phosphatidyl-L-serine (75% PC/25% PS (PCPS)) were prepared as described (38). Human Pre-1 was prepared by a modification of the procedure of Mann (12), as follows. Prothrombin (5.0mg/ml, 69μM, 12.0ml) in 0.017 imidazole, 0.144M NaCl, pH7.4 was incubated at 22°C with thrombin (5.0 NIH units/ml, 48nM) for 7.5 hours. The thrombin inhibitor D-phenylanyl-prolyl-arginyl chloromethyl ketone (PPAck, Calbiochem) then was added (50nM final) and the solution was dialyzed against 0.02M Tris-HCl, 0.15M NaCl, pH 7.4 at 4°C overnight (4 liters × 3). The dialyzed sample then was added at 22°C to a column of DEAE cellulose (20ml bed volume, 2 × 10cm) equilibrated in the sample buffer. The column was washed with the starting buffer and 5 ml fractions were collected. Pre-1 and residual inactive thrombin were recovered in the wash, which was diluted 2:3 with water and added to a column of SPC-50 Sephadex (40ml bed volume, 3 × 12cm) equilibrated in the sample buffer. Pre-1 was obtained in the flow through and was precipitated by dialysis against ammonium sulfate (80% saturation). A Pre-1 pellet, obtained by centrifugation, was resuspended in 1–1.5ml of the supernatant and stored at 4°C. Prior to use an aliquot of the suspension was centrifuged, the supernatant was discarded, and the pellet was dissolved in the appropriate buffer and dialyzed. The extinction coefficients and molecular weights were: FVa 1.74, 150,000 (11); FVa light chain 2.23, 74,000 (11); FVa heavy chain 1.24, 94,000 (11); FXa 1.16, 43,000 (39); human prothrombin 1.38, 72,000 (12); human Pre-1 1.64, 50,000 (12); human thrombin 1.83, 37,000 (40,41).
Conversion of human prothrombin and prethrombin-1 to thrombin with human or bovine factor Va
Human or bovine FVa (10nM), human FXa (1.0nM), CaCl2 (2.0mM), PCPS vesicles (20μM phospholipid) and DAPA (3.0μM) were prepared in 0.02M Tris-HCl, 0.15M NaCl, 1% polyethylene glycol 8000 or 0.01% Tween-80 to minimize protein adsorption (1.6ml total volume). The solution then was placed in a cuvette thermostated at 22°C. Substrate conversion was initiated by adding a 30μL aliquot of concentrated prothrombin in Ca++ (2.0mM). Thrombin formation was monitored continuously by the enhanced fluorescence of the DAPA-thrombin complex using a Perkin Elmer Model MPF-66 spectrofluorometer. The excitation and emission wavelengths were 335nm and 545nm, respectively, and a 430nm filter was used in the emission beam. The signal was calibrated by monitoring some of the reactions to completion. The fluorescence intensity of the human meizothrombin/DAPA complex is about 70 percent greater than that of the thrombin/DAPA complex (42). SDS-PAGE results (see below) indicated that at maximum, meizothrombin was 10 percent of thrombin in the initial parts of the reactions. Therefore, the rate of thrombin formation might be overestimated by as much as 7 percent. Because this is a relatively small effect, no efforts were made to correct signals for small amounts of meizothrombin (35,42,43) present transiently during activation reactions.
Similar experiments were performed using human Pre-1 (1.4μM) as the substrate, in the presence of human or bovine FVa (10nM), CaCl2 (2.0mM), PCPS vesicles (20μM phospholipid) and DAPA (3.0μM) prepared in 0.02M Tris-HCl, 0.15M NaCl, 0.01% Tween-80, pH 7.4. The excitation and emission wavelengths were 280nm and 545nm, respectively, and a 515nm cutoff filter was used in the emission beam. Substrate conversion was initiated by the addition of 2.5nM FXa. Prothrombin at various concentrations, fully activated to thrombin, was used as the standard to quantify the thrombin/DAPA complex generated in the reactions.
Separation and reconstitution of the heavy and light chains of bovine and human factor Va
Human factor V (0.1mg/ml) in 10.0ml of 0.02M Tris-HCl, 0.15M NaCl, 5mM CaCl2, pH 7.4 and was incubated at 37°C with thrombin (19nM) for 20 minutes. PPAck then was added (1μM final). The sample was diluted with an equal volume of water and was applied to a 1.0 × 2.5cm all glass column filled with QAE-cellulose (diethyl-(2-hydroxypropyl)-aminoethyl Cellulose, Sigma Chemical Co., St. Louis, MO) equilibrated at 22°C with the sample buffer. The contents of the column were washed with the same buffer. Then one bed volume of the buffer with EDTA (5mM) rather than Ca++ was added and the flow was stopped for 2.0 hours to dissociate the heavy and light chains of FVa on the column. Flow then was resumed with the same buffer and 10, 1.5ml fractions were collected in glass tubes. Elution then was continued in the same buffer containing 0.3M NaCl. Two peaks were identified by absorbance at 280nm. The first was obtained upon resumption of flow and contained the light chain. The second was obtained upon elution with 0.3M NaCl and contained the heavy chain. The bovine heavy and light chains were similarly isolated except prior chromatography of factor V on QAE-cellulose was used to remove minor contaminants. Bovine factor V (10mg) was dissolved in 10.0ml of 0.02M imidazole, 5mM CaCl2, pH 6.5. This solution was added to QAE cellulose equilibrated at 22°C in the same imidazole buffer in an all glass column (1.1 × 5.0cm). The contents of the column were washed with the equilibration buffer and factor V was eluted in a gradient of (0 to 0.3M) NaCl in the same buffer. The total volume of the gradient was 45ml and 1.5ml fractions were collected. The ten peak fractions, identified by absorbance at 280nm, were pooled and incubated at 37°C. Thrombin was added (19nM) and incubation was continued for 20 minutes. PPAck then was added (1.0μM). Three volumes of 0.02M imidazole, 5.0mM CaCl2, pH 6.5 then were added. The QAE-cellulose in the column used for the chromatography of factor V was washed with 0.02M imidazole, 5.0mM CaCl2, 1.0M NaCl, pH 6.5 and was re-equilibrated 0.02M imidazole, 5.0mM CaCl2, pH 6.5. The activated, diluted sample of factor V was applied to the column at 22°C and a wash with the equilibration buffer was applied. The buffer then was changed to 0.01M Tris-HCl, 0.075M NaCl, 2.5mM CaCl2, pH 7.5 (5–10 column volumes were added). One bed volume of the same buffer with EDTA (5.0mM) rather than Ca++ then was added and flow was stopped for 2 hours in order to dissociate the heavy and light chains of FVa on the column. Flow was then resumed and the heavy and light chains were recovered exactly as described above for their human counterparts. The fractions eluted from the QAE-cellulose containing human or bovine heavy and light chains were stored at 4°C. The isolated chains thus were stored in 0.01M Tris-HCl, 5.0mM EDTA, pH7.5 with NaCl at 0.075M (light chain) and 0.3M (heavy chain). The concentrations of stock solutions of the human chains were typically 0.5μM (light chain) and 0.9μM (heavy chain). The corresponding concentrations of the stock solutions of the bovine chains were 3.9μM (light chain) and 9.1μM (heavy chain). Specimens of FVa were prepared by diluting aliquots of the stock solutions containing heavy and light chains from both species in 0.02M Tris-HCl, 0.15M NaCl, pH 7.4 at 37°C and adding Ca++ (10mM). Clotting assays in factor V-deficient human plasma (4) were used to monitor reassembly. Clotting times decreased over the interval 0–20 minutes after the addition of Ca++ and were stable for at least 3 hours thereafter. The final concentrations (μM) of the heavy and light chains of the reconstituted species of FVa were: (human heavy, human light; 0.55, 0.24), (bovine heavy, bovine light; 0.39, 0.5); (human heavy, bovine light; 0.55, 0.55) and (bovine heavy, human light; 0.50, 0.18). The nominal FVa concentration was equated with the less concentrated chain in all cases.
Determination of Km and kcat values for the activation of human prethrombin-1 by prothrombinase containing either human or bovine factor Va
Pre-1 (0 to 25μM) was incubated with human or bovine FVa (10nM), CaCl2 (2.0mM), PCPS vesicles (20μM phospholipid) and DAPA (3.0μM) prepared in 0.02M Tris-HCl, 0.15M NaCl, 0.01% Tween-80, pH 7.4. Each reaction mixture was equilibrated in a microcuvette in a Perkin-Elmer lambda 50B spectrofluorimeter. The reactions were started by adding FXa (2.5nM), and thrombin generation was monitored by the fluorescence of the DAPA-thrombin complex. The excitation and emission wavelengths were 350nm and 545nm, respectively. Excitation at 350nm was used in order to minimize the internal filter effect at high Pre-1 concentrations. Slit widths of 5nm and 10nm were used in the excitation and emission paths, respectively, and a 430nm cut off filter was used in the emission beam. Initial rates of thrombin formation were fitted to the Michaelis-Menten equation by non-linear regression analyses using the NONLIN module of SYSTAT (SYSTAT, Evanston, IL).
Analysis of human prothrombin or prethrombin-1 activation by prothrombinase containing human or bovine factor Va by SDS-PAGE
Human prothrombin or Pre-1 (1.4μM) with either human or bovine FVa (10nM), CaCl2 (2.0mM), PCPS vesicles (20μM) and DAPA (3.0μM) in 0.02M HEPES, 0.15M NaCl, pH 7.4, 0.01% (v/v) Tween-80 was activated by adding FXa (2.5nM with Pre-1 or 1.0nM with prothrombin) (14). Aliquots were removed at various times and added to acetic acid (final concentration 0.134N). The solvent was removed with a SpeedVac, the dried samples were dissolved in gel sample buffer, and the components were resolved by 10–14.5% SDS-PAGE. The gels were fixed in 50% methanol, 20% ethanol, and 6% trichloroacetic acid for at least 2 hours and were stained with Coomassie Blue and destained. The gels then were dried using BioDesign GelWrap (BioDesign Inc., New York) and scanned using a CanoScan model 5000F (Canon Canada, Mississauga, Ontario). Densitometry was carried out to determine the concentration of each band.
RESULTS
Comparison of the activation of human prothrombin and prethrombin-1 by the prothrombinase complex with either human or bovine factor Va
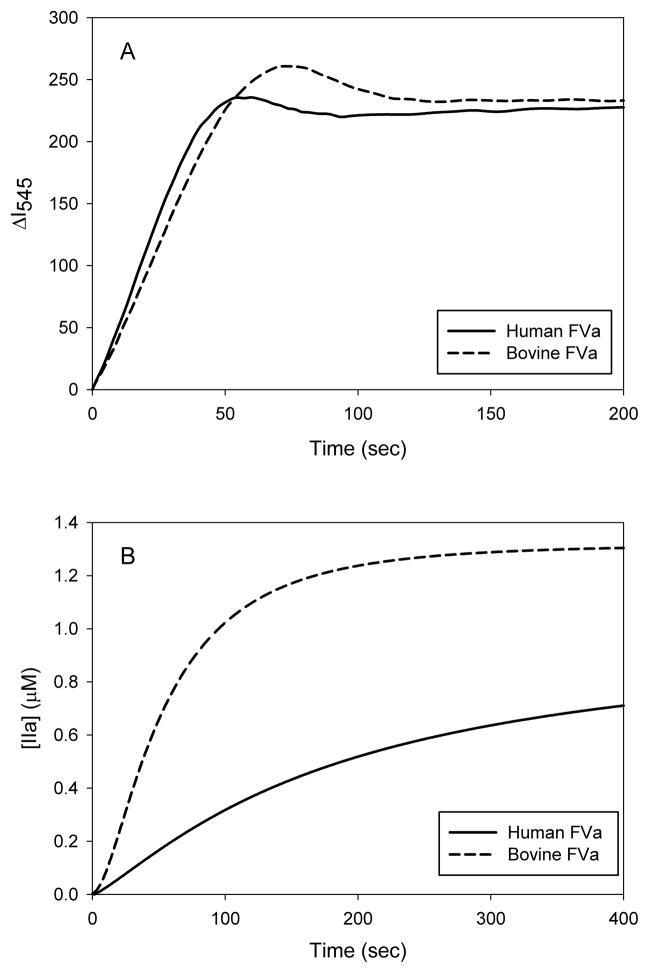
Time courses of prothrombin activation with human or bovine FVa are shown in Figure 1a. The initial rate in both instances was 26 mol thrombin/sec/mol FXa. The full time courses exhibited differences, however, in the amplitudes and durations of the transient meizothrombin peaks (35,42), which suggests species differences in the kinetics meizothrombin processing. In contrast, substantial species differences are evident with Pre-1 activation (Fig. 1b). With bovine FVa, thrombin formation occurred at an initial rate of 5.7 mol thrombin/sec/mol FXa and the reaction was complete within 5 minutes. With human FVa, however, the initial rate of the reaction was only 1.3 mol thrombin/sec/mol FXa. Although the data are not shown, the Pre-1 activation was rapid with bovine FVa and slow with human FVa, regardless of the species of FXa or Pre-1. Thus, both the similarities of human prothrombin conversion and the differences in human Pre-1 conversion exhibited by human or bovine FVa can be attributed specifically to the two species of FVa.
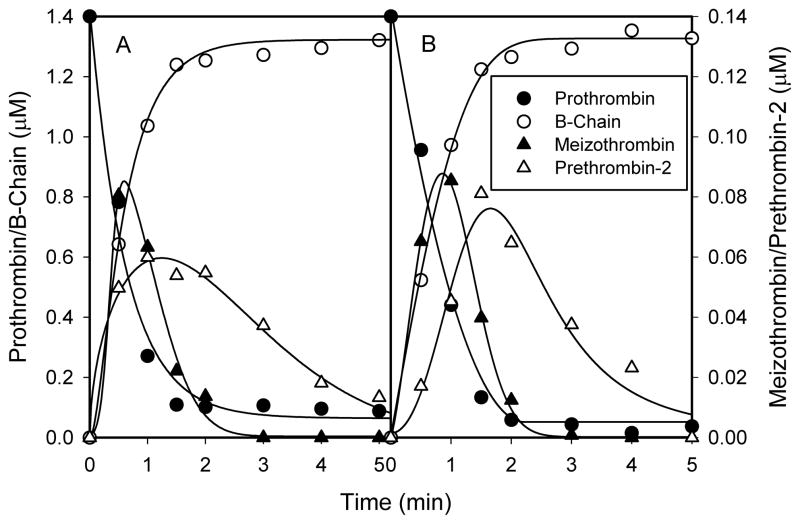
Figure 1. Time course of the prothrombinase-catalyzed activation of prothrombin and prethrombin-1 in the presence of human or bovine factor Va.
(A) Human prothrombin (1.0μM) was activated in 0.02M Tris-HCl, 0.15M NaCl, 2.0mM CaCl2, 20μM PCPS, 3.0μM DAPA, and 1.0nM FXa at 22°C. Thrombin formation was monitored by the fluorescence intensity of the DAPA-Thrombin (and meizothrombin) complex (λex=335nm, λem=545nm). Since a meizothrombin transient was present during prothrombin activation the values of the vertical axis are expressed in arbitrary units. (B) Human Pre-1 (1.4μM) was activated in 0.02M Tris-HCl, 0.15M NaCl, 2.0mM CaCl2, 20μM PCPS, 3.0μM DAPA, and 2.5nM factor Xa at 25°C. Thrombin formation was monitored by the fluorescence intensity (λex=280nm, λem=545nm) and quantified. Both prothrombin and Pre-1 were converted to thrombin in the presence of human or bovine FVa at a concentration of 10nM.
Prethrombin-1 activation with preparations of factor Va reassembled from various combinations of human and bovine heavy and light chains
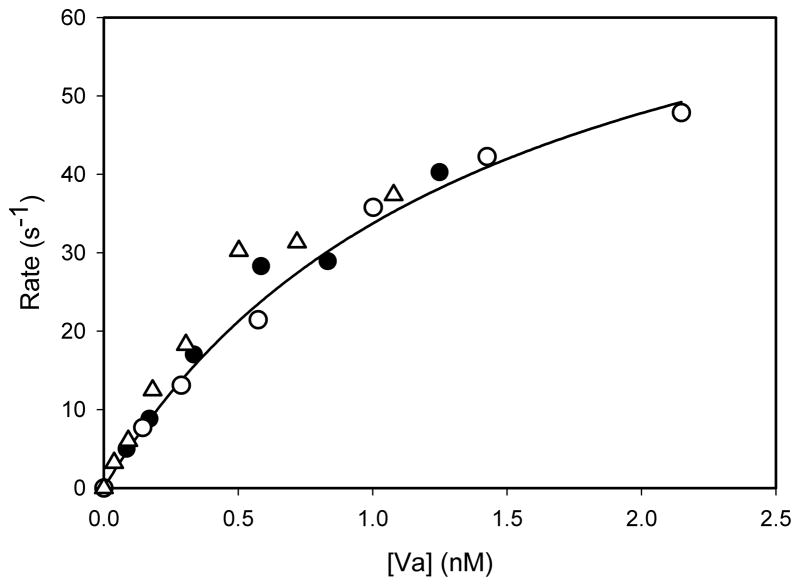
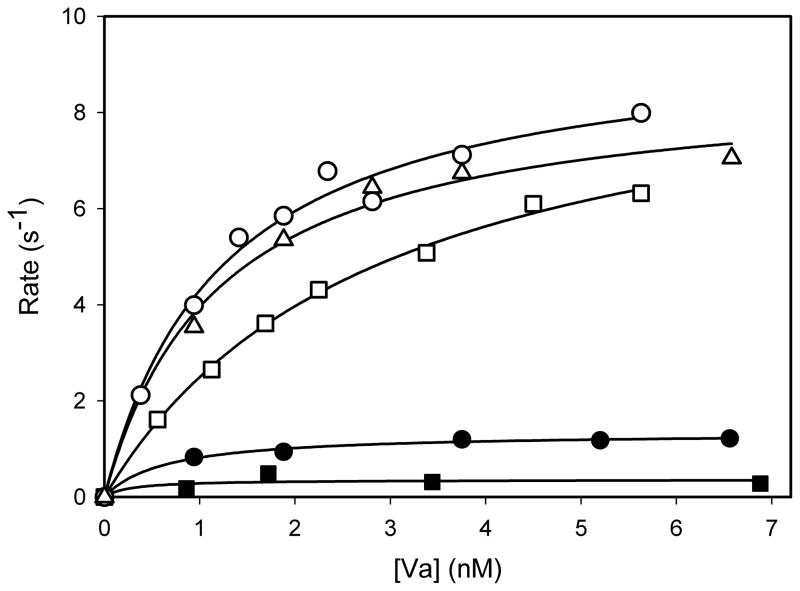
The isolated heavy and light chain preparations were analyzed by SDS-PAGE, and the bands were detected by silver staining. The heavy chains showed a single band while the light chains displayed the characteristic doublet (data not shown). The results indicated that the procedure yielded electrophoretically homogeneous chains from both species. The isolated chains then were recombined to produce preparations of FVa comprising various combinations of human and bovine heavy and light chains. Their contributions to the activation of both prothrombin and Pre-1 were investigated by measuring the initial rate of thrombin generation at various concentrations of the preparations of FVa. With prothrombin, very similar results were obtained with human FVa, bovine FVa, and human FVa reassembled from the heavy and light chains (Fig. 2). In each case, saturation in rates was exhibited with respect to the concentration of FVa. In contrast, with Pre-1, a clear distinction exists between those preparations that contained heavy chain of bovine FVa compared to those that contained the heavy chain of human FVa (Fig. 3). Bovine FVa and a preparation comprising reassembled bovine heavy and light chains yielded very similar concentration-versus-rate profiles and a rate at saturation of 7–8 mol thrombin/sec/mol FXa. A preparation comprising the bovine heavy chain and the human light chain yielded the same rate at saturation, although the concentration dependence suggests that a slightly higher concentration of this FVa is required to achieve saturation. The titration with human FVa also exhibited saturation, with a half-maximal concentration similar to that of bovine FVa. The maximum rate, however, was only one tenth of that obtained with bovine FVa. A preparation comprising the human heavy chain and the bovine light chain also showed saturation, but the maximum rate was only about 25 percent of that exhibited by human FVa. All data were analyzed by non-linear regression analyses for their ability to fit the binding equation r=r(max)*[Va]/(K1/2+[Va]), where r = initial rate, K1/2= apparent dissociation constant for the assembly of FVa in the prothrombinase complex and r(max) is the maximum rate at saturation with FVa. The results of these analyses are listed in Table 1. Very similar results were obtained with all preparations of FVa when prothrombin was the substrate. The apparent K1/2 values ranged from 0.5 to 0.9nM, and the rates at saturation ranged from 51 to 67 mol thrombin/sec/mol FXa. With Pre-1, saturation also was observed and similar values of K1/2, ranging from 0.5 to 2.9nM, were obtained with all forms of FVa. Bovine FVa, reconstituted bovine heavy and light chains, and the combination of the bovine heavy chain and the human light chain exhibited saturation rates of 8.6, 8.7 and 9.7 mol thrombin/sec/mol FXa, respectively. In contrast, human FVa and the combination of the human heavy chain and the bovine light chain yielded, respectively, saturation rates of 1.2 and 0.3 mol thrombin/sec/mol FXa. Although rates of Pre-1 activation exhibited saturation at similar concentrations of both species of FVa, the rates at saturation were 7.2-fold higher with bovine FVa compared to those with human FVa. In addition, the differential in the rate at saturation can be attributed specifically to the heavy chain of FVa.
Figure 2. The dependence of initial rates of prothrombin activation on the concentration of various preparations of factor Va.
The initial rates of activation of human prothrombin (1.0μM) were measured at 22°C by DAPA fluorescence in the presence of human FXa (1.0nM), PCPS vesicles (20μM phospholipid), CaCl2 (2.0mM), DAPA (3.0μM) and the indicated concentrations of human FVa (closed circles), bovine FVa (open circles), or a preparation of human FVa reassembled from its isolated heavy and light chains (open triangles). Rates are expressed as mol thrombin/sec/mol FXa.
Figure 3. The dependence of initial rates of prethrombin-1 activation on the concentration of various preparations of factor Va.
The initial rates of activation of human Pre-1 (1.0μM) were determined under conditions such as those described in the legend of Figure 2. The indicated data were obtained with preparations of FVa that included bovine FVa (open circles); bovine FVa reassembled from its heavy and light chains (open triangles); FVa reassembled from the bovine heavy chain and the human light chain (open squares); human FVa(closed circles); and FVa reassembled from the human heavy chain and the bovine light chain (closed squares).
Table 1. Maximum rates and factor Va concentrations required for half-maximal rates of human prethrombin-1 activation with various forms of factor Va included in the prothrombinase complex.
Data such as those indicated in Figure 4 were fit by non-linear regression analysis to the equation rate = rate(max)· [Va]/(K1/2+[Va]). kmax is rate(max)/[factor Xa]. Values of kmax and K1/2 are indicated ± S.E.M. The various preparations of factor Va are: hFVa, human FVa; bFVa, bovine FVa; hH + hL and bH + bL, human and bovine FVa, respectively, reassembled from their isolated chains; hH + bL, FVa reassembled from the human heavy and bovine light chains; and bH + hL, FVa reassembled from the bovine heavy and human light chains.
| Substrate | Factor Va | K1/2 (nM) | kmax (s−1) |
|---|---|---|---|
| Prothrombin | hFVa | 0.9±0.4 | 63±15 |
| Prothrombin | hH+hL | 0.7±0.1 | 62±7 |
| Prothrombin | bFVa | 0.7±0.1 | 51±2 |
| Prothrombin | bH+bL | 0.9±0.3 | 63±7 |
| Prothrombin | hH+bL | 0.5±0.1 | 67±6 |
|
| |||
| Prethrombin-1 | bFVa | 0.9±0.2 | 8.6±0.5 |
| Prethrombin-1 | bH+hL | 2.9±0.2 | 9.7±0.4 |
| Prethrombin-1 | hFVa | 0.5±0.1 | 1.2±0.1 |
| Prethrombin-1 | hH+bL | 0.7±0.3 | 0.3±0.1 |
Kinetics of prothrombin and prethrombin-1 activation by the prothrombinase complex with human or bovine factor Va
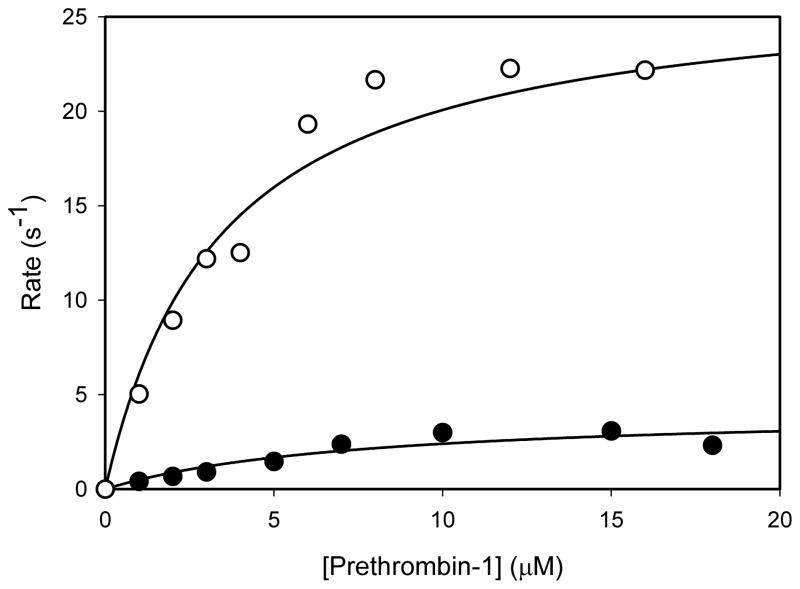
The kinetics of prothrombin activation were very similar with bovine and human FVa (data not shown). With human FVa the Km and kcat values were 0.53±0.05μM, and 92±3 s−1, respectively; with bovine FVa the corresponding values were 0.66±0.07μM and 88±4 s−1. Results with Pre-1 are shown in Figure 4. As these data indicate, saturation of rates is obtained with respect to concentration of Pre-1 with both bovine and human FVa. The Km values were 5.7±1.9μM and 3.4±0.7μM with human or bovine FVa, respectively. The kcat values were 4.2±0.1 and 27.0±1.5 (mol thrombin/sec/mol FXa) with human or bovine FVa, respectively. The catalytic efficiencies (kcat/Km) for thrombin generation from Pre-1 by prothrombinase were 7.9±0.5μM−1s−1 with bovine FVa and 0.7±0.2μM−1s−1 with human FVa. Thus, these data indicate an approximate 11-fold decrease in catalytic efficiency for Pre-1 activation with human FVa compared to bovine FVa. Furthermore, the data suggest that prothrombinase complexes with human or bovine FVa bind Pre-1 similarly, but convert bound substrate to product very differently.
Figure 4. The substrate concentration dependence of prethrombin-1 activation in the presence of human (closed circles) or bovine (open circles) factor Va.
Initial rates of activation of human Pre-1 (indicated as mol thrombin/sec/mol FXa) were measured at 22°C by DAPA fluorescence at initial concentrations of prethrombin-1 indicated by the units of the horizontal axis. The data were fit by non linear regression analysis to the equation v=v(max)[Pre-1]/(Km+[Pre-1]) to yield the kcat and Km values.
Analysis by SDS-PAGE of human prothrombin or prethrombin-1 activation in the presence of human or bovine factor Va
The data of Table 1 and Figure 4 indicate that the relatively slow rates of Pre-1 activation in the presence of human FVa are expressed through a reduction in kcat for thrombin formation and not through deficient substrate binding or prothrombinase assembly. Because two cleavages of Pre-1 (and prothrombin) are required to form thrombin, the measurements of the generation of thrombin do not indicate whether the reduced rate of catalysis is reflected in the cleavage of the substrate Pre-1 to form the intermediate Pre-2 or in cleavage of the latter to form thrombin. Thus, the progress of the reaction was monitored by SDS-PAGE, which can distinguish Pre-1, Pre-2, and thrombin.
With prothrombin as the substrate, the time courses of prothrombin, Pre-2, meizothrombin, and the B-chain were similar, in the presence of either human or bovine FVa (Fig. 5). Prothrombin was consumed at initial rates of 20.6s−1 and 19.8s−1 with human or bovine FVa, respectively. The initial rates of B-chain accumulation were 20.1s−1 and 19.0s−1 for human or bovine FVa, respectively. The rates of B-chain accumulation for both human and bovine FVa match their respective prothrombin consumption rates, suggesting that the majority of prothrombin consumption resulted from the initial cleavage at Arg320. With human FVa, a 0.088μM meizothrombin peak occurred at 30 seconds, whereas with bovine FVa, a 0.117μM peak occurred at 1.0 minute. Pre-2 time courses with human or bovine FVa were similar, in that they both peaked at 2 minutes at concentrations of 0.052μM and 0.071μM, respectively, and both were nearly consumed by 5 minutes.
Figure 5. The time courses of human prothrombin, meizothrombin, prethrombin-2 and the B-chain during prothrombin activation by prothrombinase in the presence of A) human or B) bovine factor Va.
Prothrombin (1.4μM) was incubated with either human or bovine FVa (10nM), CaCl2 (2.0mM), PCPS vesicles (20μM) and DAPA (3.0μM) in 0.02M HEPES, 0.15M NaCl, pH 7.4 and0.01% (v/v) Tween-80, and its activation was initiated by the addition of FXa (1.0nM). Samples were withdrawn at various times and subjected to 10–14.5% SDS-PAGE. The gels were fixed in 50% methanol, 20% ethanol, and 6% trichloroacetic acid for at least 2 hours and were stained with Coomassie Blue and destained. The time courses were determined by quantitative densitometry.
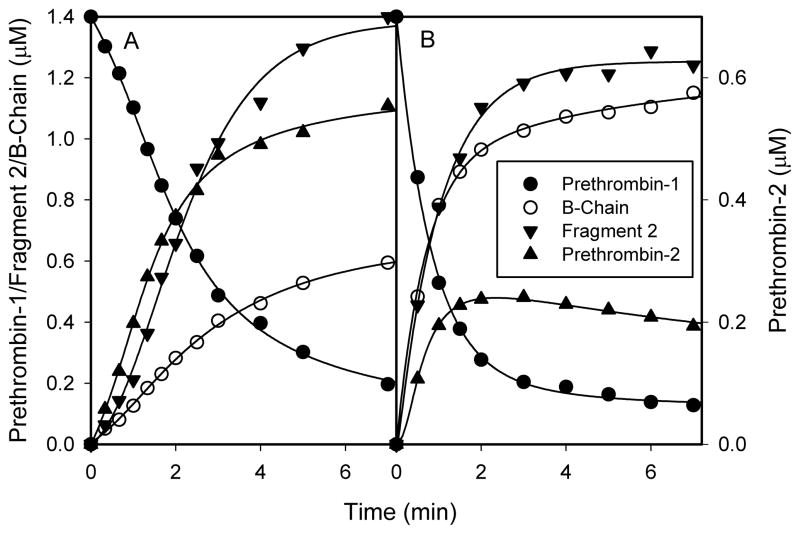
The initial rate of Pre-1 consumption with human FVa (2.32s−1) was 3.8-fold lower than with bovine FVa (8.81s−1) (Fig. 6). With bovine FVa, the Pre-1 level approached a plateau comprising 85 percent total consumption at 3 minutes, whereas with human FVa, it approached a plateau comprising 86 percent total consumption at 7 minutes. With human FVa, Pre-2 accumulated at an initial rate of 1.50s−1 and approached the plateau of 0.62μM at 7 minutes. With bovine FVa, Pre-2 accumulated at an initial rate of 1.84s−1, peaked at 0.24μM at 2 minutes, and slowly declined thereafter. With human FVa, B-chain accumulated at an initial rate of 0.99s−1 for the first three minutes, and continued to accumulate at a slower rate for the remainder of the time. With bovine FVa, B-chain accumulated at an initial rate of 8.61s−1, and approached a plateau of 1.2μM at 5 minutes. Accumulation of fragment-2, which indicates the cleavage at Arg271 in either Pre-1 or meizothrombin-des-fragment-1, also was quantified. The initial rates of fragment-2 accumulation were 1.71s−1 and 7.09s−1 for human or bovine FVa, respectively.
Figure 6. The time courses of human prethrombin-1, prethrombin-2, fragment 2 and the B-chain during prethrombin-1 activation by prothrombinase in the presence of A) human or B) bovine factor Va.
Pre-1 (1.4μM) was incubated with either human or bovine FVa (10nM), CaCl2 (2.0mM), PCPS vesicles (20μM) and DAPA (3.0μM) in 0.02M HEPES, 0.15M NaCl, pH 7.4 and 0.01% (v/v) Tween-80, and its activation was initiated by the addition of FXa (2.5nM). Samples were withdrawn at various times, and were analyzed as in Figure 5.
The rate of Pre-2 consumption at the end of the reaction with bovine FVa was only 0.077s−1, which is very small relative to the initial rate of Pre-1 consumption (8.81s−1). Therefore, one can assume that the rate of Pre-2 accumulation is, to a very good approximation, equal to the rate of cleavage of Pre-1 at Arg271. Accordingly, the initial rates of cleavage of Pre-1 at Arg271 were 1.50s−1 and 1.84s−1 for human and bovine FVa, respectively. The initial rates of cleavage at Arg320 were calculated by subtracting the rates of Pre-2 accumulation from rates of Pre-1 consumption, and values of 0.82s−1 and 6.97s−1 were found for human and bovine FVa, respectively. These calculations indicate that human and bovine FVa process cleavage at Arg271 very similarly, but cleavage at Arg320 very differently when Pre-1 is the substrate. All of the rates are summarized in Table 2.
Table 2.
The initial rates of prethrombin-1 consumption and intermediate/product accumulation measured by SDS-PAGE analysis (Fig. 6).
| Rate (s−1)
|
||
|---|---|---|
| Human factor Va | Bovine factor Va | |
| Prethrombin-1 | 2.32 | 8.81 |
| B-chain | 0.99 | 8.61 |
| Fragment-2 | 1.71 | 7.09 |
| Prethrombin-2 | 1.50 | 1.84 |
|
| ||
| a Arg271 Cleavage | 1.50 | 1.84 |
| b Arg320 Cleavage | 0.82 | 6.97 |
As measured by the initial rate of Pre-2 accumulation.
Difference between the initial rate of Pre-1 consumption and Pre-2 accumulation.
DISCUSSION
These studies show that bovine and human FVa function very similarly in prothrombin activation but very differently Pre-1. The differences can be attributed specifically to the heavy chains of the two species. The effect is exhibited primarily in kcat rather than Km or Kd for prothrombinase assembly and is specifically due to deficient catalysis of Arg320 cleavage.
As shown in Figure 7, the heavy chains of bovine and human FVa consist of two homologous repeated domains, designated A1 and A2, that comprise the bulk of the primary structure, plus two other regions of sequence that complete the primary structure. The A1 and A2 domains are highly homologous, both to one another within and across species, and to corresponding domains in the heavy chain of coagulation factor VIII, the A3 domains in the light chains of FVa and factor VIII(a), and the A1, A2, and A3 domains of ceruloplasmin (8–10,44). The A1 domain spans amino acid residues 1–303 in the heavy chains of both species and share 84.5% and 94.7% sequence homology and similarity, respectively. This is followed by thirteen amino acids in both species (residues 304–316) that differ only at positions 308, 311, and 314 (L->P, I->L, and E->D, human -> bovine). This is followed by the A2 domain. The A2 domain of the bovine heavy chain (residues 317–660) is four residues longer than the A2 domain of the human heavy chain by virtue of the lack of an arginine residue corresponding to position 439 in the human sequence, and the replacement of threonine residue corresponding to position 560 in the human sequence with the six residues NFTLPA at positions 559–564 in the bovine sequence. This latter insert has no counterpart in the human sequence. The A2 domains of the two species share 88.7% and 97.1% sequence homology and similarity, respectively. The A2 domain of the heavy chains of both species is followed by 53 amino acids, spanning residues 661–713 in the bovine heavy chain and residues 657–709 in the human heavy chain. In contrast to the high degree of similarity exhibited by the other regions of sequence of the two species, these latter 53 residues display species differences at 21 of the 53 positions when the sequences are aligned by discounting the glycine at position 667 of the bovine sequence and discounting the glutamate at position 691 of the human sequence.
Figure 7. Comparison of amino acid sequence between human and bovine factor Va heavy chain.
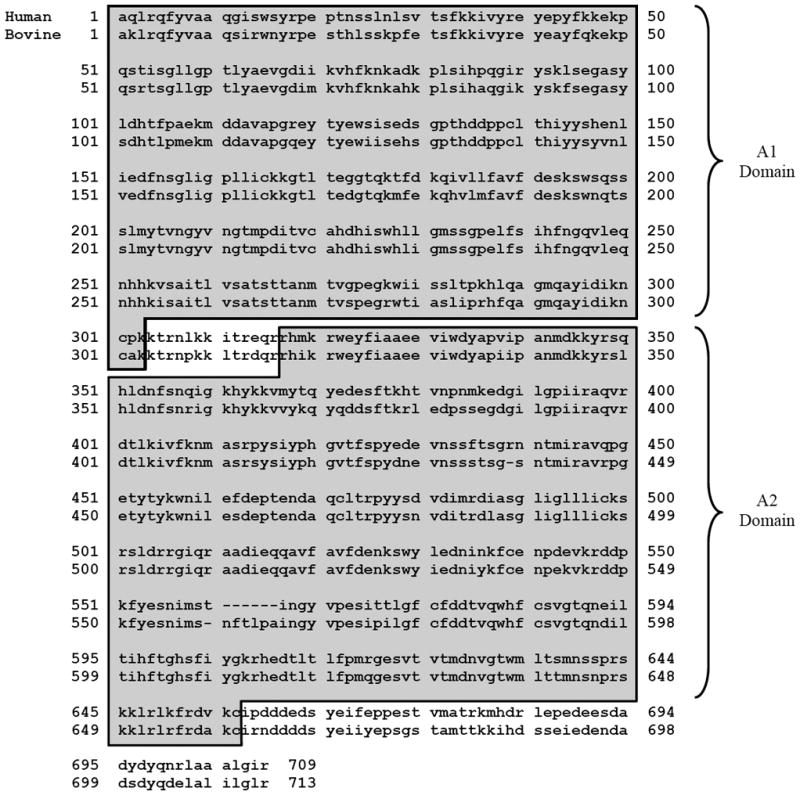
The heavy chain comprises of the A1 domain that spans residues 1 to 303, followed by a thirteen residue connecting region, followed by the A2 domain that spans residues 317 to 656 in human and residues 317 to 660 in bovine, and finally the carboxy-terminal end region that spans residues 657 to 709 in human and residues 661 to 713 in bovine. The hirudin-like peptide D(Y/S)DYQ (695–699 in human and 699–703 in bovine) is located in this carboxy-terminal region. Human Factor V sequence pubmed accession #AAB59532; Bovine Factor V sequence pubmed accession #AAA30512.
The functional differences observed in the current work can be attributed to one or more of the differences in primary structure between the two species of FVa. If one assumes that regions of substantial differences in sequence account for these functional differences, likely candidates comprise the inserts and deletions in the A2 domains and the regions spanning the 53 residues at the COOH-termini of the heavy chains. Recent studies have suggested that the pentapeptide sequence DYDYQ in the heavy chain of human FVa at positions 695–699 may be involved in the interaction of prothrombin with FVa in prothrombinase assembled on PCPS vesicles (27). A similar pentapeptide sequence DSDYQ is present in the heavy chain of bovine FVa at positions 699–703. These sequences of residues start 34 residues from the COOH-terminal end of the A2 domains in both species. These sequences are followed at their COOH-termini by ten amino acids, the last of which is the COOH-terminal residue of the heavy chain. In the human heavy chain, the sequence is NRLAAALGIR, whereas in the bovine heavy chain it is DELALILGLR. These are similar or identical except for the first two amino acids, which are NR and DE in human and bovine heavy chains, respectively. These two residues profoundly affect the calculated pI values of the two sequences. In human FVa, the pI value of the ten amino acids is 12.5, whereas in bovine FVa, it is 4.26. With bovine FVa, the net negative charge of these ten residues would augment the negative charge of the DSDYQ pentapeptide and perhaps specifically facilitate the interaction with Pre-1 required for cleavage at Arg320. Conversely, with human FVa, the net positive charge of these ten residues would partially negate the negative charge of the DYDYQ pentapeptide, and thereby would not facilitate the interaction with Pre-1. This is supported by the observations that the initial rates of Pre-2 accumulation, which reflects cleavage at Arg271, were nearly identical between the two species, whereas cleavage at Arg320 was 8.5-fold faster with bovine FVa.
Toso and Camire recently published studies of prothrombin and Pre-1 activation by prothrombinase containing recombinant human FVa variants truncated from the COOH-terminus of the heavy chain (32). They found that the truncation of as many as 51 residues had no effect on prothrombin activation. In contrast, truncation of the ten COOH-terminal residues increased the Km for Pre-1 activation by 7-fold or more, with little effect on kcat. Their results imply that the ten amino acids of the COOH-terminus of the human FVa heavy chain influence the Km of Pre-1 activation, but not the kcat. Our results suggest that replacement of the ten amino acids of human FVa heavy chain with those of the bovine heavy chain do not affect Km, but increase kcat 6.4-fold. Both studies suggest that the identity of the last 51 residues of the heavy chain may influence the kinetics of Pre-1 activation.
Since both species of FVa share a common physiologic property (enhanced activation of prothrombin) but differ substantially in a functional variant of that property (enhanced activation of the prothrombin derivative, Pre-1), the two properties together should be very useful in efforts to correlate the structure of FVa with its function and to consequently further characterize the mechanism by which prothrombin is activated by the prothrombinase complex.
| 1. What is known on this topic | 2. What this paper adds |
|---|---|
|
|
Acknowledgments
The authors wish to acknowledge the efforts of Tom Abbott and P. Michael Cook in preparing some of the proteins used in this work.
The abbreviations used are
- DYDYQ
hirudin-like pentapeptide sequence found at position 695–699 of human factor Va heavy chain
- DSDYQ
pentapeptide sequence found at position 699–703 of bovine factor Va heavy chain
- PCPS
phosphatidyl-L-choline (75%), phosphatidyl-L-serine (25%)
- DAPA
dansylarginine-N-(3-ethyl-1,5-pentanediyl)amide
- FVa
factor Va
- FXa
factor Xa
- Pre-1
prethrombin-1
- Pre-2
prethrombin-2
- PPAck
D-Phe-Pro-Arg chloromethyl ketone
Footnotes
This work was supported by grant MT-9781 from the Canadian Institutes of Health Research (to MEN) and by grant HS4441 from the Heart and Stroke Foundation of Canada (to MEN). This work was also supported by a Heart and Stroke Foundation of Canada Doctoral Research Award (to PYK).
References
- 1.Mann KG, Jenny RJ, Krishnaswamy S. Cofactor proteins in the assembly and expression of blood clotting enzyme complexes. Ann Rev Biochem. 1988;57:915–56. doi: 10.1146/annurev.bi.57.070188.004411. [DOI] [PubMed] [Google Scholar]
- 2.Mann KG, Nesheim ME, Church WR, et al. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76:1–16. [PubMed] [Google Scholar]
- 3.Esmon CT. The subunit structure of thrombin-activated factor V isolation of activated factor V, separation of subunits, and reconstitution of biological activity. J Biol Chem. 1979;254:964–73. [PubMed] [Google Scholar]
- 4.Nesheim ME, Katzmann JA, Tracy PB, Mann KG. Factor V. Methods Enzymol. 1981;80:249–74. doi: 10.1016/s0076-6879(81)80023-7. [DOI] [PubMed] [Google Scholar]
- 5.Nesheim ME, Mann KG. Thrombin-catalyzed activation of single chain bovine factor V. J Biol Chem. 1979;254:1326–34. [PubMed] [Google Scholar]
- 6.Foster WB, Nesheim ME, Mann KG. The factor Xa-catalyzed activation of factor V. J Biol Chem. 1983;258:13970–7. [PubMed] [Google Scholar]
- 7.Nesheim ME, Foster WB, Hewick R, Mann KG. Characterization of factor V activation intermediates. J Biol Chem. 1984;259:3187–96. [PubMed] [Google Scholar]
- 8.Jenny RJ, Pittman DD, Toole JJ, et al. Complete cDNA and derived amino acid sequence of human factor V. Proc Natl Acad Sci USA. 1987;84:4846–50. doi: 10.1073/pnas.84.14.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinto ER, Esmon CT, Mann KG, MacGillivray RT. The complete cDNA sequence of bovine coagulation factor V. J Biol Chem. 1992;267:2971–8. [PubMed] [Google Scholar]
- 10.Mann KG, Kalafatis M. Factor V: a combination of Dr Jekyll and Mr Hyde. Blood. 2003;101:20–30. doi: 10.1182/blood-2002-01-0290. [DOI] [PubMed] [Google Scholar]
- 11.Krishnaswamy S, Russell GD, Mann KG. The reassociation of factor Va from its isolated subunits. J Biol Chem. 1989;264:3160–8. [PubMed] [Google Scholar]
- 12.Mann KG. Prothrombin. Methods Enzymol. 1976;45:123–56. doi: 10.1016/s0076-6879(76)45016-4. [DOI] [PubMed] [Google Scholar]
- 13.Owen WG, Esmon CT, Jackson CM. The conversion of prothrombin to thrombin; 1. Characterization of the reaction products formed during the activation of bovine prothrombin. J Biol Chem. 1974;249:594–605. [PubMed] [Google Scholar]
- 14.Bukys MA, Orban T, Kim PY, et al. The interaction of fragment 1 of prothrombin with the membrane surface is a prerequisite for optimum expression of factor Va cofactor activity within prothrombinase. Thromb Haemost. 2008;99:511–22. [PubMed] [Google Scholar]
- 15.Pryzdial EL, Mann KG. The association of coagulation factor Xa and factor Va. J Biol Chem. 1991;266:8969–77. [PubMed] [Google Scholar]
- 16.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine factor V and factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–62. [PubMed] [Google Scholar]
- 17.Tracy PB, Mann KG. Prothrombinase complex assembly on the platelet surface is mediated through the 74,000-dalton component of factor Va. Proc Natl Acad Sci USA. 1983;80:2380–4. doi: 10.1073/pnas.80.8.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracy PB, Rohrbach MS, Mann KG. Functional prothrombinase complex assembly on isolated monocytes and lymphocytes. J Biol Chem. 1983;258:7264–7. [PubMed] [Google Scholar]
- 19.Krishnaswamy S, Jones KC, Mann KG. Prothrombinase complex assembly kinetic mechanism of enzyme assembly on phospholipid vesicles. J Biol Chem. 1988;263:3823–34. [PubMed] [Google Scholar]
- 20.Nesheim ME, Tracy RP, Mann KG. “Clotspeed”, a mathematical simulation of the functional properties of prothrombinase. J Biol Chem. 1984;259:1447–53. [PubMed] [Google Scholar]
- 21.Luckow EA, Lyons DA, Ridgeway TM, et al. Interaction of clotting factor V heavy chain with prothrombin and prethrombin 1 and role of activated protein C inregulating this interaction: analysis by analytical ultracentrifugation. Biochemistry. 1989;28:2348–54. doi: 10.1021/bi00431a055. [DOI] [PubMed] [Google Scholar]
- 22.Guinto ER, Esmon CT. Loss of prothrombin and of factor Xa-factor Va interactions upon inactivation of factor Va by activated protein C. J Biol Chem. 1984;259:13986–92. [PubMed] [Google Scholar]
- 23.Boskovic DS, Giles AR, Nesheim ME. Studies of the role of factor Va in the factor Xa-catalyzed activation of prothrombin, fragment 1. 2-prethrombin-2 and dansyl-L-glutamyl-glycyl-L-arginine-meizothrombin in the absence of phospholipid. J Biol Chem. 1990;265:10497–505. [PubMed] [Google Scholar]
- 24.Kalafatis M, Beck DO. Identification of a binding site for blood coagulation factor Xa on the heavy chain of factor Va. Amino acid residues 323–331 of factor V represent an interactive site for activated factor X. Biochemistry. 2002;41:12715–28. doi: 10.1021/bi026208+. [DOI] [PubMed] [Google Scholar]
- 25.Singh LS, Bukys MA, Beck DO, Kalafatis M. Amino acids Glu323, Tyr324, Glu330, and Val331 of factor Va heavy chain are essential for expression of cofactor activity. J Biol Chem. 2003;278:28335–45. doi: 10.1074/jbc.M300233200. [DOI] [PubMed] [Google Scholar]
- 26.Kalafatis M, Beck DO, Mann KG. Structural requirements for expression of factor Va activity. J Biol Chem. 2003;278:33550–61. doi: 10.1074/jbc.M303153200. [DOI] [PubMed] [Google Scholar]
- 27.Beck DO, Bukys MA, Singh LS, et al. The contribution of amino acid region Asp695-Tyr698 of factor V to procofactor activation and factor Va function. J Biol Chem. 2004;279:3084–95. doi: 10.1074/jbc.M306850200. [DOI] [PubMed] [Google Scholar]
- 28.Gerads I, Tans G, Yukelson LY, et al. Activation of bovine factor V by an activator purified from the venom of Naja naja oxiana. Toxicon. 1992;30:1065–79. doi: 10.1016/0041-0101(92)90052-7. [DOI] [PubMed] [Google Scholar]
- 29.Bakker HM, Tans G, Thomassen MC, et al. Functional properties of human factor Va lacking the Asp683 - Arg709 domain of the heavy chain. J Biol Chem. 1994;269:20662–7. [PubMed] [Google Scholar]
- 30.Camire RM, Kalafatis M, Tracy PB. Proteolysis of factor V by cathepsin G and elastase indicates that cleavage at Arg1545 optimizes cofactor function by facilitating factor Xa binding. Biochemistry. 1998;37:11896–906. doi: 10.1021/bi980520v. [DOI] [PubMed] [Google Scholar]
- 31.Hirbawi J, Bukys MA, Barhoover MA, et al. Role of the acidic hirudin-like COOH-terminal amino acid region of factor Va heavy chain in the enhanced function of prothrombinase. Biochemistry. 2008;47:7963–74. doi: 10.1021/bi800593k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toso R, Camire RM. Role of hirudin-like factor Va heavy chain sequences in prothrombinase function. J Biol Chem. 2006;281:8773–9. doi: 10.1074/jbc.M511419200. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj SP, Rapaport SI, Maki SL, Brown SF. A procedure for isolation of human protein C and protein S as by-products of the purification of factors VII, IX, X and prothrombin. Prep Biochem. 1983;13:191–214. doi: 10.1080/00327488308064248. [DOI] [PubMed] [Google Scholar]
- 34.Bajzar L, Fredenburgh JC, Nesheim ME. The activated protein C-mediated enhancement of tissue-type plasminogen activator-induced fibrinolysis in a cell-free system. J Biol Chem. 1990;265:16948–54. [PubMed] [Google Scholar]
- 35.Krishnaswamy S, Mann KG, Nesheim ME. The prothrombinase-catalyzed activation of prothrombin proceeds through the intermediate meizothrombin in an ordered, sequential reaction. J Biol Chem. 1986;261:8977–84. [PubMed] [Google Scholar]
- 36.Nesheim ME. A simple rate law that describes the kinetics of the heparin-catalyzed reaction between antithrombin III and thrombin. J Biol Chem. 1983;258:14708–17. [PubMed] [Google Scholar]
- 37.Nesheim ME, Prendergast FG, Mann KG. Interactions of a fluorescent active-site-directed inhibitor of thrombin: dansylarginine N-(3-ethyl-1,5-pentanediyl)amide. Biochemistry. 1979;18:996–1003. doi: 10.1021/bi00573a010. [DOI] [PubMed] [Google Scholar]
- 38.Barenholz Y, Gibbes D, Litman BJ, et al. A simple method for the preparation of homogeneous phospholipid vesicle. Biochemistry. 1977;16(12):2806–10. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 39.Fujikawa K, Legaz ME, Davie EW. Bovine factors X1 and X2 (Stuart Factor). Isolation and characterization. Biochemistry. 1972;11:4882–91. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- 40.Butkowski RJ, Elion J, Downing MR, Mann KG. Primary structure of human prethrombin 2 and alpha-thrombin. J Biol Chem. 1977;252:4942–57. [PubMed] [Google Scholar]
- 41.Fenton JW, II, Fasco MJ, Stackrow AB. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977;252:3587–98. [PubMed] [Google Scholar]
- 42.Krishnaswamy S, Church WR, Nesheim ME, Mann KG. Activation of human prothrombin by human prothrombinase. Influence of factor Va on the reaction mechanism. J Biol Chem. 1987;262:3291–9. [PubMed] [Google Scholar]
- 43.Kim PY, Nesheim ME. Further evidence for two functional forms of prothrombinase each specific for either of the two prothrombin activation cleavages. J Biol Chem. 2007;282:32568–81. doi: 10.1074/jbc.M701781200. [DOI] [PubMed] [Google Scholar]
- 44.Church WR, Jernigan RL, Toole J, et al. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci USA. 1984;81:6934–7. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]