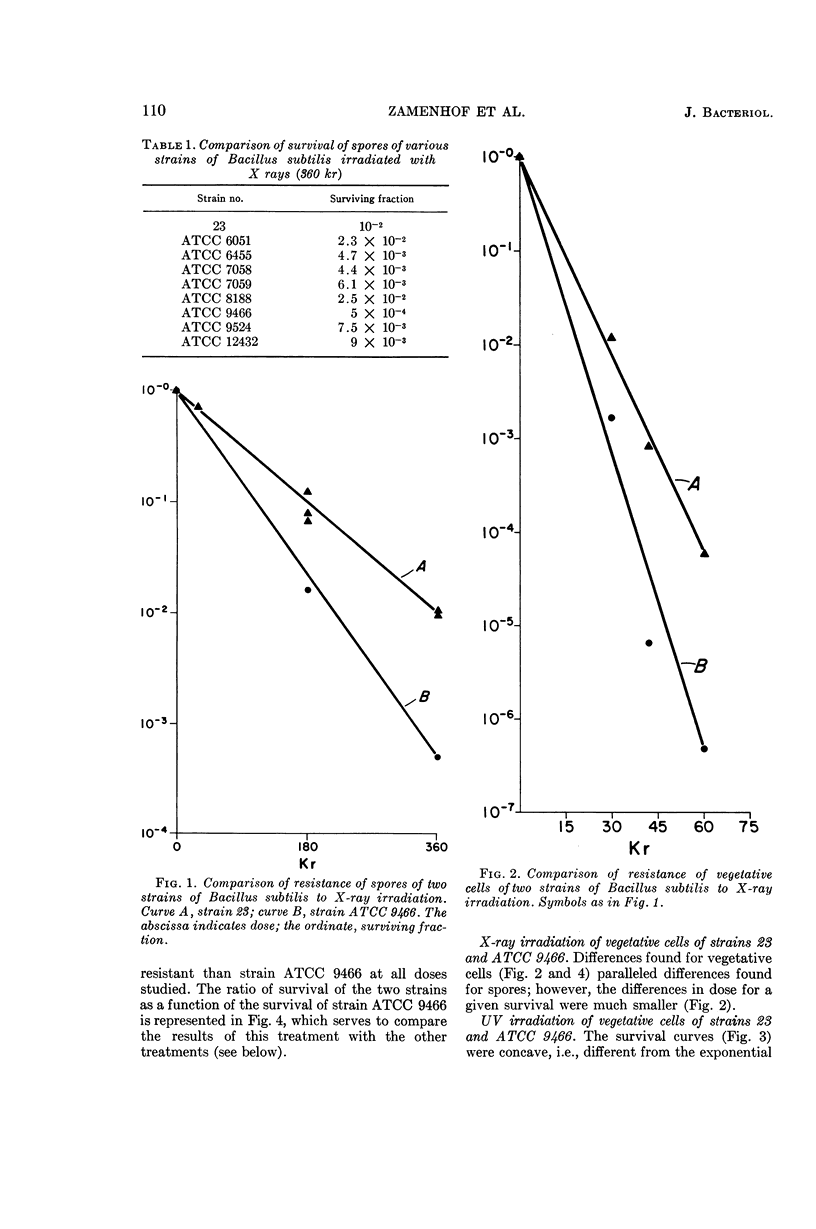
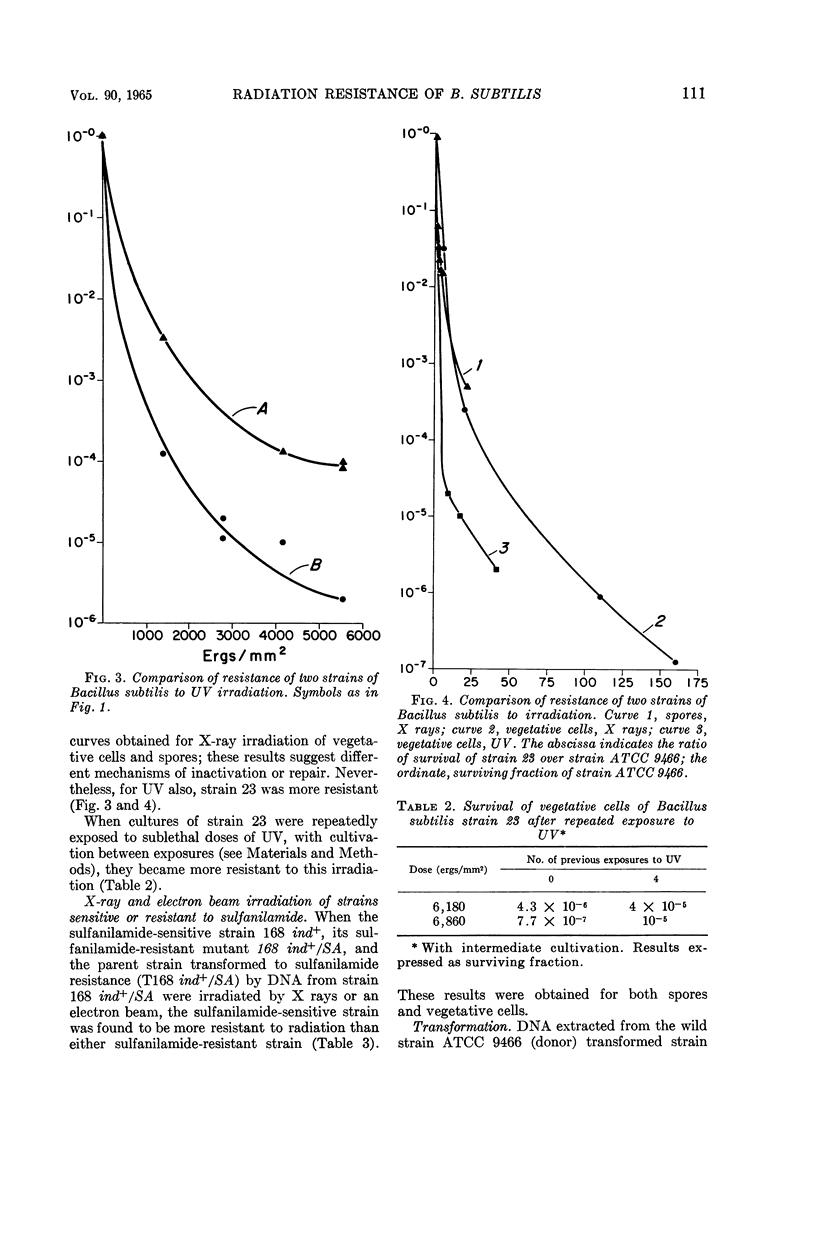
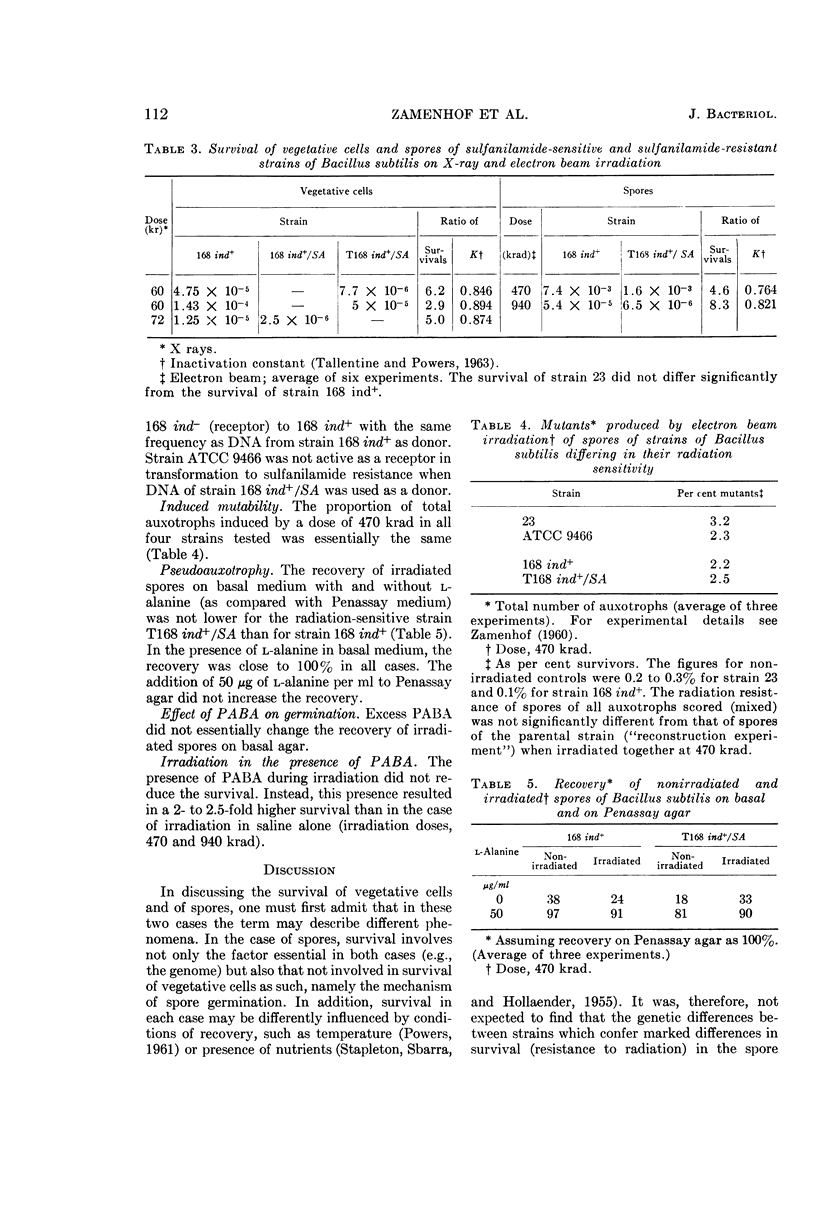
Abstract
Zamenhof, Stephen (University of California, Los Angeles), Hela Bursztyn, T. K. Ramachandra Reddy, and Patrice J. Zamenhof. Genetic factors in radiation resistance of Bacillus subtilis. J. Bacteriol. 90:108–115. 1965.—A study of several wild cross-transformable strains of Bacillus subtilis revealed differences in the resistance of their spores to X rays. Closer study of two such strains revealed differences of the same type when vegetative cells were exposed to X rays or to ultraviolet light (UV). Cell cultures repeatedly exposed to sublethal doses of UV (with cultivation between exposures) became more resistant to UV, presumably by enrichment in a more UV-resistant mutant. A sulfanilamide-resistant mutant of one strain (vegetative cells and spores) was less resistant to ionizing radiation; this sensitivity was transferable by transformation. No difference in radiation-induced mutability could be demonstrated in any of the strains studied. It is concluded that, at least in the cases studied, (i) the differences in radiation resistance of spores of different strains are not just a result of a superimposition of a common spore resistance mechanism(s) but rather are an amplification of genetically determined resistance differences in vegetative cells of these strains; (ii) sulfanilamide-resistance locus (p-aminobenzoic acid overproduction locus) is one of the loci of radiation sensitivity; (iii) no evidence was obtained that the differences in radiation resistance of cells or spores can be ascribed to differences in radiation resistance of their deoxyribonucleic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., COPELAND J. C. Genetic analysis of radiation response in Escherichia coli. Genetics. 1962 Jun;47:701–712. doi: 10.1093/genetics/47.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALPER T. LETHAL MUTATIONS AND CELL DEATH. Phys Med Biol. 1963 Nov;8:365–385. doi: 10.1088/0031-9155/8/4/301. [DOI] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. RELEASE OF ULTRAVIOLET LIGHT-INDUCED THYMINE DIMERS FROM DNA IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Feb;51:293–300. doi: 10.1073/pnas.51.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS M. The irradiation of bacterial spores with low-voltage electrons. Arch Biochem Biophys. 1954 Feb;48(2):469–481. doi: 10.1016/0003-9861(54)90364-1. [DOI] [PubMed] [Google Scholar]
- DAVIS N. S., SILVERMAN G. J., MASUROVSKY E. B. RADIATION-RESISTANT, PIGMENTED COCCUS ISOLATED FROM HADDOCK TISSUE. J Bacteriol. 1963 Aug;86:294–298. doi: 10.1128/jb.86.2.294-298.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPHRATI-ELIZUR E., SRINIVASAN P. R., ZAMENHOF S. Genetic analysis, by means of transformation, of histidine linkage groups in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Jan 15;47:56–63. doi: 10.1073/pnas.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREER S. Studies on ultraviolet irradiation of Escherichia coli containing 5-bromouracil in its DNA. J Gen Microbiol. 1960 Jun;22:618–634. doi: 10.1099/00221287-22-3-618. [DOI] [PubMed] [Google Scholar]
- HILLS G. M. Chemical factors in the germination of spore-bearing aerobes; the effects of amino acids on the germination of Bacillus anthracis, with some observations on the relation of optical form to biological activity. Biochem J. 1949;45(3):363–370. doi: 10.1042/bj0450363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., BOYCE R. P., SIMSON E., THERIOT L. A genetic locus in E. coli K12 that controls the reactivation of UV-photoproducts associated with thymine in DNA. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2109–2115. doi: 10.1073/pnas.48.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATO M. L., BROWN G. M. MECHANISMS OF RESISTANCE OF ESCHERICHIA COLI TO SULFONAMIDES. Arch Biochem Biophys. 1963 Dec;103:443–448. doi: 10.1016/0003-9861(63)90435-1. [DOI] [PubMed] [Google Scholar]
- POWERS E. L. Reversibility of x irradiation-induced effects in dry biological systems. J Cell Comp Physiol. 1961 Dec;58(3):13–25. doi: 10.1002/jcp.1030580404. [DOI] [PubMed] [Google Scholar]
- RORSCH A., EDELMAN A., COHEN J. A. The gene-controlled radiation sensitivity in Escherichia coli. Biochim Biophys Acta. 1963 Feb 26;68:263–270. doi: 10.1016/0006-3002(63)90141-0. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., CARRIER W. L. THE DISAPPEARANCE OF THYMINE DIMERS FROM DNA: AN ERROR-CORRECTING MECHANISM. Proc Natl Acad Sci U S A. 1964 Feb;51:226–231. doi: 10.1073/pnas.51.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAPLETON G. E., SBARRA A. J., HOLLAENDER A. Some nutritional aspects of bacterial recovery from ionizing radiations. J Bacteriol. 1955 Jul;70(1):7–14. doi: 10.1128/jb.70.1.7-14.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLENTIRE A., POWERS E. L. MODIFICATION OF SENSITIVITY TO X-IRRADIATION BY WATER IN BACILLUS MEGATERIUM. Radiat Res. 1963 Oct;20:270–287. [PubMed] [Google Scholar]
- WOESE C. Further studies on the ionizing radiation inactivation of bacterial spores. J Bacteriol. 1959 Jan;77(1):38–42. doi: 10.1128/jb.77.1.38-42.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF B., HOTCHKISS R. D. Genetically modified folic acid synthesizing enzymes of pneumococcus. Biochemistry. 1963 Jan-Feb;2:145–150. doi: 10.1021/bi00901a026. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Genetics of Resistance to Radiation in ESCHERICHIA COLI. Genetics. 1947 May;32(3):221–248. doi: 10.1093/genetics/32.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S., DE GIOVANNI-DONNELLY R., HELDENMUTH L. H. Transfer, by transformation, of information determining mutation rates in Bacillus subtilis. Proc Natl Acad Sci U S A. 1962 Jun 15;48:944–947. doi: 10.1073/pnas.48.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S. Gene unstabilization induced by heat and by nitrous acid. J Bacteriol. 1961 Jan;81:111–117. doi: 10.1128/jb.81.1.111-117.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamenhof S. EFFECTS OF HEATING DRY BACTERIA AND SPORES ON THEIR PHENOTYPE AND GENOTYPE. Proc Natl Acad Sci U S A. 1960 Jan;46(1):101–105. doi: 10.1073/pnas.46.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]


