Abstract
Human embryonic stem cells (hESCs) have an unlimited capacity for self-renewal, and the ability to differentiate into cells derived from all three embryonic germ layers (1). Directed differentiation of hESCs into specific cell types has generated much interest in the field of regenerative medicine (e.g., (2-5)), and methods for determining the in vivo fate of selected or manipulated hESCs are essential to this endeavor. We have adapted a highly efficient teratoma formation assay for this purpose. A small number of specifically selected hESCs is mixed with undifferentiated wild type hESCs and Phaseolus vulgaris lectin to form a cell pellet. This is grafted beneath the kidney capsule in an immunodeficient mouse. As few as 2.5 x 105 hESCs are needed to form a 16 cm3 teratoma within 8-12 weeks. The fate of the originally selected hESCs can then be determined by immunohistochemistry. This method provides a valuable tool for characterizing tissue-specific reagents for cell-based therapy.
Keywords: Cellular Biology, Issue 42, stem cell biology, human embryonic stem cells, differentiation, teratoma, renal capsule
Protocol
The written protocol below is based on published parameters reported by the authors, with some modifications. The protocol is performed with approval by the UCSF Institutional Animal Care and Use (IACUC) and Stem Cell Research Oversight (SCRO) Committees.
I. Culture of human embryonic stem cells (hESCs)
Grow hESCs in standard 6-well (3.5 cm diameter wells) plates.
At least 12 hours prior to passaging cells, seed mouse embryonic fibroblast (MEF; obtained from CF1 day e12.5 timed-mated mice, irradiated at 1000 Ci) feeder cells onto plates coated with 1% gelatin (6,7). MEFs should be plated at 50-60% confluence, or 4 x 105 cells per 3.5 cm diameter well (Figure 1).
Passage cells when colonies have reached ~70% confluence (Figure 2).
To passage the cells, aspirate KSR growth medium (6) from the wells and replace with 0.5 ml medium containing Collagenase IV in KSR at a concentration of 1 mg/ml.
Incubate plates with Collagenase IV at 37°C/5%CO2 for 5 minutes or until edges of colonies begin to lift from the plate.
Remove Collagenase IV from the plate, add 0.5 ml of KSR to each well, and manually scrape cells to fully remove from the plate.
Collect the cell suspension from each well, place in a 15 ml conical tube, and allow cells to settle by gravity for 5 minutes.
Remove the supernatant from the tube, leaving the cells and medium in a total volume of ~300μl.
Using a 200 μl micro-pipettor set at full volume, gently pipette the cell suspension 5-6 times to break up the colonies.
Bring the cell suspension to a total volume of 9 ml per original well of cells using KSR medium (i.e., if one well is harvested, the tube should contain 9 ml). Supplement the medium with recombinant human basic fibroblast growth factor (bFGF) to a concentration of 12.5 ng/ml.
After washing the new wells containing MEF cells (see step 2) with Dulbecco's phosphate buffered saline (DPBS) to remove all serum, add 3 ml of the cell suspension to each well and incubate the plates at 37°C/5%CO2.
Change KSR medium with bFGF 24 hours after passage, and every 24-48 hours until cells are ready to be passaged again.
II. Preparation of hESC pellet for grafting
At 24 hours before grafting, add ROCK inhibitor to the KSR medium to a final concentration of 10μM to increase cell viability (8). One well of cells at ~70% confluence (5 x 105 cells) will create two pellets, with two pellets inserted per kidney capsule.
To prepare cell pellets for grafting (9), incubate the ROCK inhibitor-treated cultures with Collagenase IV for 5 minutes at 37°C/5%CO2.
Aspirate the Collagenase IV from the well and replace with 1 ml DPBS with 10% penicillin/streptomycin (pen/strep).
Manually scrape the colonies from the plate, rinse the well with an additional 1 ml DPBS with pen/strep, and transfer each 1ml aliquot of cell suspension to a 1.5 ml microfuge tube.
Spin microfuge tubes briefly at 8,000 x g, aspirate the supernatant, and resuspend the pellets in 500μl DPBS with pen/strep.
To help bind the cells together for transplant, add 100μl 1 mg/ml Phaseolus vulgaris lectin (PHA) in DPBS to each tube.
Incubate the cell/PHA suspension at room temperature for 5 minutes, then spin for 2 minutes at 8,000 x g. Rotate the tubes 180° within their rotor wells and spin again for 2 minutes at 8,000 x g.
Carefully aspirate the supernatant leaving the pellet intact.
Dislodge the cell pellets from the tubes by gently pipetting 200μl KSR next to the edge of the pellet until it slowly lifts up.
Once dislodged, immediately transfer the pellets to 0.4μm MILLICELL inserts placed in 3.5 cm diameter wells containing 3 ml KSR, and incubate for 2 hours to overnight at 37°C/5%CO2.
III. Renal capsule grafting
Perform all animal procedures in accordance with institutional guidelines using appropriate aseptic technique.
Anesthetize SCID beige mice (CB17.Cg-PrkdcscidLyst bg/Crl), aged 8-12 weeks, using a combination of inhaled 3% isoflurane/0.5%O2 and intraperitoneal tribromoethanol (Avertin; 25 mg/kg freshly prepared). Two cell pellets per mouse will be engrafted using described techniques (10).
After placing the mouse on a heating pad to prevent hypothermia, shave the incision area, then disinfect it with chlorhexidine. Make a 2.5 cm incision through the skin just off center but parallel to the spine.
Keeping the incision area moist with sterile saline, make a smaller incision through the muscle, just below the ribs and ~1 cm from the spine. The incision should be just large enough to pull the kidney through, but small enough that the kidney will not slip back into the abdominal cavity without force.
Pull the kidney gently through the muscle incision using a glass Pasteur pipette that has been pulled into the shape of a blunt, slightly curved hook.
Wet the kidney with sterile DPBS to prevent the capsule from drying out. This may be necessary to repeat throughout the procedure, as the capsule is very fragile.
Using Dumont #5 forceps, gently pull the capsule up from the kidney and make a 2 mm incision using Vannas spring scissors.
With another glass pipette that has been pulled into a very fine, blunted probe, make a small pocket between the capsule and the kidney.
Using a large-orifice pipette tip placed on a disposable transfer pipette, take a single cell pellet from the MILLICELL insert. Make sure to take as little extra media along as possible, as excess media can interfere with the pellet transfer.
While holding up the edge of the incision to create the pocket, gently place the pellet next to the opening and push it into the pocket toward one pole of the kidney with the pulled pipette.
Repeat the process with a second pellet. Place this under the capsule of the same kidney, but toward the opposite pole.
Carefully place the capsule back down onto the pellets. The pellets should stay within the pocket, and no sutures are necessary to close the capsule.
Gently push the kidney back into the abdominal cavity and close the muscle wall using 4-0 silk sutures (or smaller) with attached reverse-cutting needle. The incision should be small enough that only a single suture is necessary.
Close the skin with a series of 4-5 interrupted stitches, using the same suture material.
Before placing the mouse back in its cage, administer 0.05 mg/kg buprenorphine (11,12) and 5 mg/kg ketoprofen (12,13) as short-term and long-term analgesics, respectively. Controlled substances should only be used in accordance with institutional guidelines.
Transfer the animal back to its cage and allow it to recover from anesthesia on a heating pad. This should take about 20-30 minutes.
Once the mouse is fully ambulatory, it can be returned to the animal housing facility.
IV. Teratoma harvest, fixation and sectioning
At 8-12 weeks post-surgery, there should be a barely palpable teratoma near the kidney. Large, fluid-filled cysts will often develop as well, starting at 6-8 weeks. These may necessitate an earlier harvest if they cause distress to the animal. Since teratomas grow at slower rates than cysts, however, you will want to wait as long as possible to obtain a teratoma of sufficient size for analysis (i.e., at least 8 weeks).
Remove the kidney, teratoma, and any surrounding cysts as a block of tissue from the abdominal cavity using the same surgical approach by which the kidney was originally accessed (see Section III, steps 1-4 above).
Place the entire block of excised tissue into a tube containing 10% buffered formalin.
Allow the teratoma to fix overnight at 4°C.
The following day, remove the teratoma from the tube. Dissect away any cysts and as much kidney as possible without damaging the teratoma itself (Figure 3). Larger teratomas may be cut in half to facilitate dissection and embedding.
- Process the teratoma using a standard paraffin-fixation technique. Automated units are available (e.g., Shandon Hypercenter):
I. 80% Flex alcohol 2 hours II. 80% Flex alcohol 1 hour III. 85% Flex alcohol 1.5 hours IV. 95% Flex alcohol 1 hour V. 100% Flex alcohol 1 hour VI. 100% Flex alcohol 1 hour VII. 100% Flex alcohol 1 hour VIII. Clear-Rite 3 1 hour IX. Clear-Rite 3 1 hour X. Clear-Rite 3 1 hour XI. Paraffin (TissuePrep) 2 hours XII. Paraffin (TissuePrep) 2 hours
V. Immunohistochemistry and analysis
Once embedded in paraffin, section the teratoma in 5μm serial slices using a rotary microtome, and float sections onto slides.
Before staining, bake the slides for at least one hour.
- Stain the slides with hematoxylin and eosin using standard methods to identify tissue structures representative of each of the three embryonic germ layers (Figure 4):
I. Xylene wash 3-5 minutes II. Xylene 3-5 minutes III. 100% alcohol 3-5 minutes IV. 100% alcohol 3-5 minutes V. 95% alcohol 3-5 minutes VI. 80% alcohol 3-5 minutes VII. Water wash (several changes) 2 minutes VIII. Gill's Hematoxylin #3 2-5 minutes IX. Water wash (several changes) until water runs clear X. Scott's water (to blue nuclei) 3 minutes or more XI. Water wash (several changes) 1-2 minutes XII. Eosin Y 1 minute XIII. Water wash (several changes) 30 seconds XIV. 80% alcohol 1 minute XV. 95% alcohol 2 minutes XVI. 95% alcohol 2 minutes XVII. 100% alcohol 3 minutes XVIII. 100% alcohol (clean) 3 minutes XIX. Xylene 2 minutes or more XX. Xylene 2 minutes or more Mount a coverslip on each slide with Permount.
VI. Representative Results
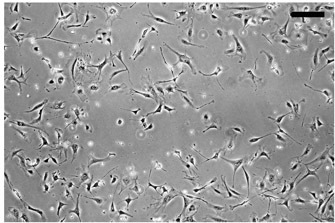 Figure 1. Plating of irradiated CF1 mouse embryonic fibroblasts as a feeder layer for culturing undifferentiated hESCs. MEFs are plated at ~50% confluence, as shown here, or 4 x 105 cells per 3.5 cm diameter well in a 6-well culture dish. Bar, 100 μm.
Figure 1. Plating of irradiated CF1 mouse embryonic fibroblasts as a feeder layer for culturing undifferentiated hESCs. MEFs are plated at ~50% confluence, as shown here, or 4 x 105 cells per 3.5 cm diameter well in a 6-well culture dish. Bar, 100 μm.
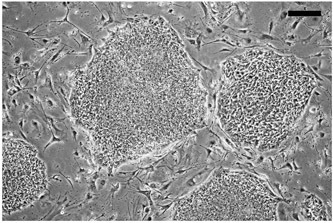 Figure 2. Example of subconfluent culture of undifferentiated hESCs on a MEF feeder layer. hESCs are grown on MEF feeder layers until ~70% confluent, as shown here, then passaged as described. Bar, 100 μm.
Figure 2. Example of subconfluent culture of undifferentiated hESCs on a MEF feeder layer. hESCs are grown on MEF feeder layers until ~70% confluent, as shown here, then passaged as described. Bar, 100 μm.
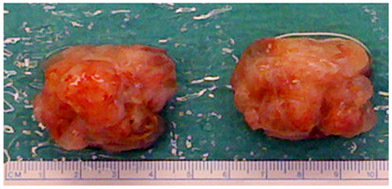 Figure 3. Example of explanted teratomas. Following explantation, the teratoma, kidney, and any accessory tissue are fixed as a block in formalin, then the kidney and accessory tissue are carefully trimmed away before embedding in paraffin.
Figure 3. Example of explanted teratomas. Following explantation, the teratoma, kidney, and any accessory tissue are fixed as a block in formalin, then the kidney and accessory tissue are carefully trimmed away before embedding in paraffin.
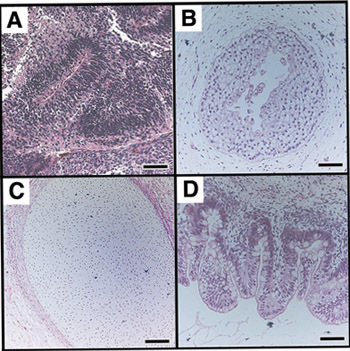 Figure 4. Teratomas derived from undifferentiated hESCs contain tissues representative of all three germ layers in vivo. A teratoma, such as one depicted in Figure 3, was sectioned and stained with hematoxylin and eosin to identify embryonic tissues. hESCs give rise to tissues derived from all three embryonic germ layers (ectoderm (A,B), mesoderm (C), endoderm (D). (A) Nascent neural tube structure. (B) Primitive squamous epithelium. (C) Cartilage surrounded by capsule of condensed mesenchyme. (D) Glandular intestinal structure. Bar, 100 μm. Images reprinted with permission of author.
Figure 4. Teratomas derived from undifferentiated hESCs contain tissues representative of all three germ layers in vivo. A teratoma, such as one depicted in Figure 3, was sectioned and stained with hematoxylin and eosin to identify embryonic tissues. hESCs give rise to tissues derived from all three embryonic germ layers (ectoderm (A,B), mesoderm (C), endoderm (D). (A) Nascent neural tube structure. (B) Primitive squamous epithelium. (C) Cartilage surrounded by capsule of condensed mesenchyme. (D) Glandular intestinal structure. Bar, 100 μm. Images reprinted with permission of author.
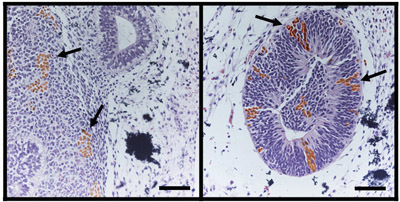 Figure 5. Teratoma analysis can be used to map the fate of tagged, undifferentiated hESCs. As previously published (9), a mixture of specifically tagged hESCs with untagged hESCs can be used to map the fate of the tagged cells in a teratoma formation assay. As shown here, undifferentiated hESCs expressing the surface marker, CD133, and tagged with enhanced green fluorescent protein (eGFP), were mixed with untagged, undifferentiated hESCs. These were used to form teratomas by renal capsule grafting. Immunohistochemical analysis of hematoxylin and eosin-stained teratoma sections with anti-eGFP antibody demonstrates the neuroectodermal fate of the CD133+ hESCs. Teratomas were processed as in Figure 4 and counterstained with anti-eGFP antibody (brown) to localize derivatives of CD133+GFP+ cells within tissues. CD133+-derived cells (arrows) were observed in tissues arising from embryonic ectoderm, specifically neural epithelium (left) and nascent neural tube-like structures (right). Bar, 100 μm. Images reprinted with permission of author.
Figure 5. Teratoma analysis can be used to map the fate of tagged, undifferentiated hESCs. As previously published (9), a mixture of specifically tagged hESCs with untagged hESCs can be used to map the fate of the tagged cells in a teratoma formation assay. As shown here, undifferentiated hESCs expressing the surface marker, CD133, and tagged with enhanced green fluorescent protein (eGFP), were mixed with untagged, undifferentiated hESCs. These were used to form teratomas by renal capsule grafting. Immunohistochemical analysis of hematoxylin and eosin-stained teratoma sections with anti-eGFP antibody demonstrates the neuroectodermal fate of the CD133+ hESCs. Teratomas were processed as in Figure 4 and counterstained with anti-eGFP antibody (brown) to localize derivatives of CD133+GFP+ cells within tissues. CD133+-derived cells (arrows) were observed in tissues arising from embryonic ectoderm, specifically neural epithelium (left) and nascent neural tube-like structures (right). Bar, 100 μm. Images reprinted with permission of author.
Gelatin
0.1% Porcine gelatin
- 99.9% DPBS
- Add gelatin to DPBS, incubate at 37°C for one hour or until all gelatin goes into solution, and filter sterilize (0.22μm) before use.
MEF medium
10% Fetal Bovine Serum (Qualified)
90% Dulbecco's Modified Essential Medium (D-MEM)
1x L-Glutamine
- 1x Pen/Strep
- Filter sterilize before use.
KSR (Knockout Serum Replacement) medium
20% KnockOut Serum Replacement
80% KnockOut D-MEM
1x L-Glutamine
1x Nonessential Amino Acids
0.1 mM β-mercaptoethanol/
- 12.5 ng/ml bFGF
- Filter sterilize.
- Store at 4°C, but warm to 37°C before adding to cells.
- Add bFGF immediately before use.
Collagenase IV
Dissolve 1 mg/ml in KSR medium by placing at 37°C for 1-2 hours or until all powder goes into solution.
Filter sterilize.
Avertin
0.25 g 2,2,2-tribromoethanol
- 0.25 ml 2-methyl-2-butanol
- Incubate at 37°C overnight to form 100% solution.
- Several hours before surgery, dilute to 2.5% working solution using DPBS and incubate at 37°C for at least 1 hour.
- Filter sterilize and aliquot.
- Tribromoethanol is highly light-sensitive, so protect from light at all stages of preparation and use.
- Prepare no more than 12-24 hours before use.
Buprenorphine
- 1 mg/ml stock solution in methanol
- Dilute to 0.015 mg/ml working solution using sterile H2O.
- Store at -20°C for up to 1 month.
Ketoprofen
- 0.5 mg/ml working solution in DPBS
- Add DPBS, incubate overnight at 37°C for 1-2 hours or until all powder goes into solution.
- Filter sterilize and store at 4°C.
Discussion
We have adapted a highly efficient teratoma formation assay for the purpose of mapping the fate of differentiating hESCs in an in vivo environment. A small number of specifically selected hESCs is mixed with undifferentiated wild type hESCs and Phaseolus vulgaris lectin to form a cell pellet. This is grafted beneath the kidney capsule in an immunodeficient mouse. The fate of the originally selected hESCs can then be determined by immunohistochemistry (Figure 5), as previously reported (9). This method provides a valuable tool for identifying and generating tissue-specific reagents for cell-based therapy.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a Comprehensive Research Grant from the California Institute for Regenerative Medicine (RC1-00104), a Public Health Service Grant (HL085377) from NHLBI, and a gift from the Pollin Foundation to H.S.B.
References
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Hoof DVan, D'Amour KA, German MS. Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Res. 2009;3:73–87. doi: 10.1016/j.scr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Li Z, Han Z, Wu JC. Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases. J Cell Biochem. 2009;106:194–194. doi: 10.1002/jcb.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safinia N, Minger SL. Generation of hepatocytes from human embryonic stem cells. Methods Mol Biol. 2009;481:169–180. doi: 10.1007/978-1-59745-201-4_14. [DOI] [PubMed] [Google Scholar]
- Nury D, Neri T, Puceat M. Human embryonic stem cells and cardiac cell fate. J Cell Physiol. 2009;218:455–459. doi: 10.1002/jcp.21631. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP, Wang S, Morton CC, McMahon AP, Powers D, Melton DA. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353–1356. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- Schatten G, Smith J, Navara C, Park JH, Pedersen R. Culture of human embryonic stem cells. Nat Methods. 2005;2:455–463. doi: 10.1038/nmeth0605-455. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- King FW, Ritner C, Liszewski W, Kwan HC, Pedersen A, Leavitt AD, Bernstein HS. Subpopulations of human embryonic stem cells with distinct tissue-specific fates can be selected from pluripotent cultures. Stem Cells Dev. 2009;18:1441–1450. doi: 10.1089/scd.2009.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Epithelio-mesenchymal interactions in primordial gland structures which become responsive to androgenic stimulation. Anat Rec. 1972;172:179–195. doi: 10.1002/ar.1091720206. [DOI] [PubMed] [Google Scholar]
- Kirsch JH, Klaus JA, Blizzard KK, Hurn PD, Murphy SJ. Pain evaluation and response to buprenorphine in rats subjected to sham middle cerebral artery occlusion. Contemp Top Lab Anim Sci. 2002;41:9–14. [PubMed] [Google Scholar]
- Lee-Parritz D. Analgesia for rodent experimental surgery. Isr J Vet Med. 2007;62:74–78. [Google Scholar]
- Julou L, Guyonnet JC, Ducrot R, Pasquet J. Some pharmacological and toxicological studies on ketoprofen. Rheumatol Rehabil. 1976. [DOI] [PubMed]


