Abstract
Bioengineered mouse models have become powerful research tools in determining causal relationships between molecular alterations and models of cardiovascular disease. Although molecular biology is necessary in identifying key changes in the signaling pathway, it is not a surrogate for functional significance. While physiology can provide answers to the question of function, combining physiology with biochemical assessment of metabolites in the intact, beating heart allows for a complete picture of cardiac function and energetics. For years, our laboratory has utilized isolated heart perfusions combined with nuclear magnetic resonance (NMR) spectroscopy to accomplish this task. Left ventricular function is assessed by Langendorff-mode isolated heart perfusions while cardiac energetics is measured by performing 31P magnetic resonance spectroscopy of the perfused hearts. With these techniques, indices of cardiac function in combination with levels of phosphocreatine and ATP can be measured simultaneously in beating hearts. Furthermore, these parameters can be monitored while physiologic or pathologic stressors are instituted. For example, ischemia/reperfusion or high workload challenge protocols can be adopted. The use of aortic banding or other models of cardiac pathology are apt as well. Regardless of the variants within the protocol, the functional and energetic significance of molecular modifications of transgenic mouse models can be adequately described, leading to new insights into the associated enzymatic and metabolic pathways. Therefore, 31P NMR spectroscopy in the isolated perfused heart is a valuable research technique in animal models of cardiovascular disease.
Keywords: Medicine, Issue 42, cardiac physiology, high energy phosphate, phosphocreatine, ATP
Protocol
For these experiments, two separate systems are utilized simultaneously. For acquisition of 31P spectra, a Bruker 14T magnet is interfaced with the Avance III console and a computer equipped with TopSpin V2.1 software. For assessment of cardiac function, a custom built heart perfusion system is interfaced with the PowerLab 4/30 data acquisition, equipped with the LabChartPro 6 software for data analysis.
On the day of the experiment, 1 liter of Krebs-Henseleit buffer is prepared as follows: 0.5mM EDTA, 5.3mM KCl, 1.2mM MgSO4, 118mM NaCl, and 25mM NaHCO3. The mixture is then bubbled with 5% CO2/95% O2 for 10-15 minutes prior to the addition of 2mM CaCl2. Finally, substrates, in the form of 10 mM glucose and 0.5mM pyruvate, are added.
Temperature regulation during the experiment is critical. Heated circulators are used to maintain the temperature between 37.0 - 37.5°C while the heart is inside the magnet. Temperature is monitored for the duration of the experiment using a fiber optic temperature probe.
Perfusion pressure and left ventricular pressure are monitored via pressure transducers attached to a data acquisition system and displayed using the included software. These are calibrated with a standard sphygmomanometer prior to the experiment. In addition, pressure lines are flushed adequately in order to remove all air bubbles.
A standard sample of 150 mM sodium phosphate (which is equivalent to the ionic strength of the KH buffer) is used to "calibrate" the probe prior to the insertion of the heart. This facilitates the signal and decreases the time necessary to begin the acquisition period once the heart is positioned within the probe.
To reduce coagulation, the mouse is injected with 200 Units of heparin I.P. After 5 minutes, sodium pentobarbital (175 mg/kg) I.P. is given.
The heart is rapidly excised (with lungs and thymus intact) and arrested in ice cold KH buffer.
While kept on ice, the lungs are quickly removed. The lobes of the thymus are identified and gently peeled back to expose the aorta. The thymus is removed. The aorta is then isolated by carefully removing any surrounding tissue.
Micro suturing forceps are used to gently hold both walls of the aorta to expose the lumen. The aorta is carefully placed onto the cannula made from 0.965 mm OD polyethylene tubing (PE50). The aorta is held in place with a micro vessel clamp while sutures are quickly tied around the aorta. The clip is removed and the forceps are used to carefully check that the cannula is above aortic root. Additional ties are added as necessary to hold the heart in place.
Any extra tissue is removed using forceps and microscissors. A small incision is made into the left auricle. A 0.61 mm OD polyethylene tubing (PE10) is carefully inserted through the left atrium, LV cavity, and out through the apex while gently holding the heart. The excess tubing is trimmed.
A deflated water-filled balloon is inserted through the atrium into the LV and is held in place using adhesive tape or sutures. The peristaltic pump speed is gradually increased to provide sufficient flow to the heart. Until the heart is place into the the NMR probe, the heart will continue to be perfused with constant flow equivalent to approximately 2 ml/min. The LV balloon is inflated with a small volume using a micrometer syringe to verify that the LV pressure transducer is functioning.
The heart is carefully inserted into a 10 mm NMR tube. A wide bore "spinner" is used to help guide the tube into the proper position within the probe. The entire apparatus is then firmly attached to the "umbilical cord" with adhesive tape.
The umbilical cord is slowly lowered into the upper bore of magnet until the heart/NMR tube is inside the coil of the 10 mm NMR probe.
Once the heart is in the proper position within the probe, the peristaltic pump flow is adjusted in order to achieve a perfusion pressure of 80mmHg. (Remember, that up to this point the heart was perfused with a constant flow of approximately 2 ml/min). The perfusion pressure is then maintained by enabling the "hold" mechanism on the pump controller. The heart is then allowed a 15-20 minute equilibration period. During that time, the volume of the LV balloon is adjusted to achieve an end-diastolic pressure of 8-10 mmHg.
During the equilibration period, it is necessary to optimize the spectrometer parameters in order to obtain the best possible phosphorous signal. This is accomplished by setting the radio pulse at the frequency at which the phosphorous nucleus resonates ("tuning") and making the magnetic field homogenous ("shimming").
After the equilibration period, multiple 31P NMR spectra can be obtained. The acquisition period for each spectrum is dependent on the field strength of the magnet, the size of the sample, and the signal to noise ratio required for a specific experiment. Spectra are obtained using a 14 Telsa magnet by averaging the signal obtained from 256 radio frequency pulses of 20 μs with a 60 degree flip angle and delays of 2.0 seconds. This experiment will require approximately 10 min.
Representative Results
From the data acquisition hardware and the LabChart software, several parameters of cardiac function can be measured throughout the experimental protocol. The typical measure of cardiac function, the left ventricular developed pressure (LVDevP), is obtained by subtracting the end-diastolic pressure (EDP) from the systolic pressure (Figure 1). This measure can vary depending on the strain of the mouse and the condition of the heart (i.e., pressure overload). However, in a normal C57BL6 mouse heart LVDevP is typically between 100-110 mmHg at fixed end-diastolic pressure of 8-10 mmHg. In addition, the LabChart program allows for the measurement of heart rate based on the cyclic measurements of the LV pressure waves. Again, this measure can vary but typical values are 350-400 bpm when hearts are allowed to beat at intrinsic rates. However, heart can be standardized using a pacing system where heart rate is kept at 420 bpm. In addition, measures of contractility (+dP/dt) and relaxation (-dP/dt) can be estimated using the first derivative of the LV pressure wave. During the experimental protocol it is easy to assess the Starling mechanism by incorporating a pressure-volume relationship. This is accomplished by making gradual increases in the LV balloon volume and noting the LVDevP as well as the EDP. These values can then be plotted as depicted in Figure 2. While the Starling curve is optimal, noting the volume necessary to achieve an EDP of 8-10 mmHg can give an indirect idea of LV chamber dimension. This can be used in models of aortic banding as hypertrophied hearts typically will require a smaller balloon volume while dilated hearts require a larger volume when compared to controls. Table 1 displays representative cardiac function data as acquired during the perfusion protocol.
The 31P NMR spectrometer will provide signals of phosphocreatine (PCr) and the three phosphates from ATP (γ-ATP, α-ATP, and β-ATP) as well as inorganic phosphate (Pi) as shown in Figure 2. Analysis of each of these peaks provides a value for the area under the curve. The amount of ATP is estimated by averaging the γ-ATP and β-ATP areas. (The α-ATP is not used because NAD molecules contribute to a unknown portion of the total signal). The energetic status of the heart is determined by the quotient of the PCr and ATP areas (PCr:ATP ratio). This value is typically 1.5 - 1.7 in a mouse heart supplied with glucose as the primary substrate. Although 31P NMR does not provide direct measures of ATP or PCr, the area of the peaks is proportional to the amount of the phosphorous containing compounds in the sample. Values for these signals can be estimated by using other methods. For example, direct measures of ATP by high-performance liquid chromatography (HPLC) in a cohort of hearts can yield an average concentration. This value can then be used to calibrate the average ATP areas observed in the spectra. The PCr concentration can be calculated based on the PCr area relative to the ATP area. It is also possible to estimate pH by analyzing the relative chemical shift of the inorganic phosphate (Pi) signal to the PCr signal.1 Using different radio pulse sequences, the creatine kinase reaction velocity or the ATP synthesis reaction velocity can also be measured.2
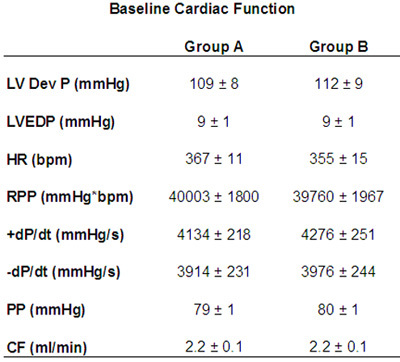 Table 1. Baseline cardiac function from isolated perfused hearts. LVDevP: left ventricular developed pressure; LVEDP: left ventricular end-diastolic pressure; HR: heart rate; RPP: rate pressure product; +dP/dt: first derivative LV pressure positive; -dP/dt: first derivative LV pressure negative; PP: perfusion pressure; CF: coronary flow.
Table 1. Baseline cardiac function from isolated perfused hearts. LVDevP: left ventricular developed pressure; LVEDP: left ventricular end-diastolic pressure; HR: heart rate; RPP: rate pressure product; +dP/dt: first derivative LV pressure positive; -dP/dt: first derivative LV pressure negative; PP: perfusion pressure; CF: coronary flow.
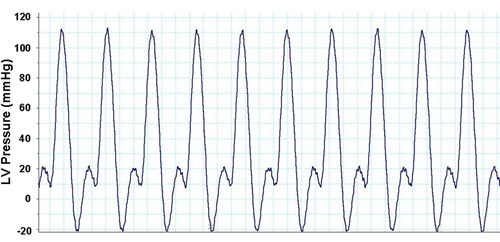 Figure 1. Representative LV pressure waves from LabChart Pro software.
Figure 1. Representative LV pressure waves from LabChart Pro software.
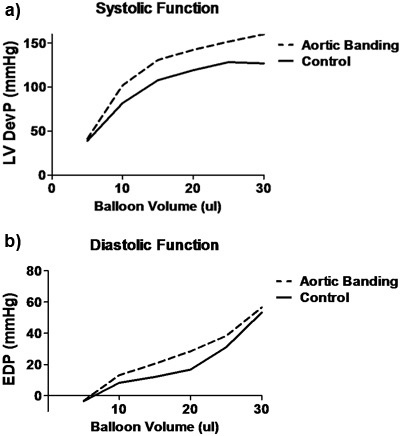 Figure 2. Representative Starling curves from control (solid line) and aortic banded (dotted-line) mice. A) Systolic function as represented by LVDevP over increasing LV volumes as determined by the volume of the LV balloon. B) Diastolic function as represented by EDP over increasing LV volumes as determined by volume of the LV balloon. LVDevP: left ventricular developed pressure (systolic minus diastolic pressure); EDP: end-diastolic pressure.
Figure 2. Representative Starling curves from control (solid line) and aortic banded (dotted-line) mice. A) Systolic function as represented by LVDevP over increasing LV volumes as determined by the volume of the LV balloon. B) Diastolic function as represented by EDP over increasing LV volumes as determined by volume of the LV balloon. LVDevP: left ventricular developed pressure (systolic minus diastolic pressure); EDP: end-diastolic pressure.
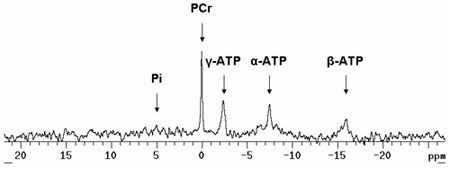 Figure 3. Representative 31P NMR spectra of isolated perfused mouse heart. Notice the relatively small Pi peak. In an aerobically perfused heart supplied with pyruvate or fatty acids in addition to glucose, this peak should be minimal. During periods of ischemia, this peak increases while the PCr peak decreases. Notice the shoulder to the right of the α-ATP peak. This is the contribution of NAD molecules. Pi: inorganic phosphate; PCr: phosphocreatine; ATP: adenosine triphosphate.
Figure 3. Representative 31P NMR spectra of isolated perfused mouse heart. Notice the relatively small Pi peak. In an aerobically perfused heart supplied with pyruvate or fatty acids in addition to glucose, this peak should be minimal. During periods of ischemia, this peak increases while the PCr peak decreases. Notice the shoulder to the right of the α-ATP peak. This is the contribution of NAD molecules. Pi: inorganic phosphate; PCr: phosphocreatine; ATP: adenosine triphosphate.
Discussion
31P NMR spectroscopy in the Langendorff-perfused isolated mouse heart provides reliable and reproducible data.3, 4 However, it is imperative that cannulation of the aorta and insertion of the LV balloon are done properly such to allow stable cardiac performance while inside the NMR tube. In addition, temperature regulation is paramount in order to achieve proper baseline function. One important factor in obtaining good, analyzable NMR spectra is increasing the signal to noise ratio. This can be achieved by ensuring optimal "tuning" and "shimming" on the sample. As mentioned in the protocol text, the use of a standard sample prior to the insertion of the heart can facilitate this. It is also helpful to have an adequate sized "sample". Hearts weighing less than 100 mg typically provide lower PCr and ATP signals so increases in acquisition time will be necessary to obtain good phosphorous spectra.
There are several ways to modify the existing protocol to garner additional information regarding cardiac function and energetics. In our laboratory, we have perfused hearts with mixed substrate buffers which can include the presence of different combinations of fatty acids (in low and high concentrations), lactate, ketones, and insulin. With the use of stable isotopes in the perfusion buffer (i.e., 13C labeled substrates), we possess the ability to estimate substrate utilization by the relative contribution of labeled acetyl CoA to the TCA cycle.5-7 For this application, we perform isotopomer analysis of 13C3- and 13C4- glutamate with 13C NMR spectroscopy. This requires freeze-clamping the heart at the end of the perfusion protocol and performing an extraction of the frozen tissue. This will be an additional experiment as the analysis requires the use of a different probe with separate setup parameters. Other applications include the substitution of glucose with deoxyglucose in the buffer while monitoring the time-dependent accumulation of 2-deoxyglucose phosphate in the heart using 31P NMR spectroscopy. This method allows for the measurement of myocardial glucose uptake.7, 8 In addition, our laboratory has analyzed cardiac function and energetics in perfusion protocols consisting of ischemia/reperfusion and high workload challenge.6, 8-10
In summary, 31P NMR spectroscopy in isolated mouse hearts is a technically challenging procedure requiring the use of sophisticated equipment. However, the data that it yields is invaluable to the researcher who wishes to analyze the function and energetics of bioengineered mouse models. For our laboratory, these techniques have been vital in our understanding of the consequences of a variety of stressors on cardiac function, energetics, and metabolism.1, 11, 12
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors would like to thank Lynne Spencer for her support during the NMR spectroscopy portion of the experiment. This work was supported by grants from the National Institutes of Health fund R01 HL059246, R01 HL067970, R01 HL088634 (to Dr. Tian) and F32 HL096284 (to Dr. Kolwicz).
References
- Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- Spindler M, Saupe KW, Tian R, Ahmed S, Matlib MA, Ingwall JS. Altered creatine kinase enzyme kinetics in diabetic cardiomyopathy. A(31)P NMR magnetization transfer study of the intact beating rat heart. J Mol Cell Cardiol. 1999;31:2175–2189. doi: 10.1006/jmcc.1999.1044. [DOI] [PubMed] [Google Scholar]
- Ingwall JS. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982;242:H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- Ingwall JS, Javadpour MM, Miao W. 31P NMR spectroscopy of the mouse heart. In: Hoit BD, Walsh RA, editors. Cardiovascular physiology in the genetically engineered. Boston: Kluwer; 2002. pp. 151–163. [Google Scholar]
- Luptak I, Balschi JA, Xing Y, Leone TC, Kelly DP, Tian R. Decreased contractile and metabolic reserve in peroxisome proliferator-activated receptor-alpha-null hearts can be rescued by increasing glucose transport and utilization. Circulation. 2005;112:2339–2346. doi: 10.1161/CIRCULATIONAHA.105.534594. [DOI] [PubMed] [Google Scholar]
- Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest. 2007;117:1432–1439. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]


