Abstract
Toxoplasma gondii is an obligate intracellular parasite that can invade any nucleated cell of warm-blooded animals. During infection, T. gondii disseminates as a fast replicating form called the tachyzoite. Tachyzoites convert into a slow-growing encysted form called the bradyzoite by a signaling process that is not well characterized. Within animals, bradyzoite cysts are found in the central nervous system and muscle tissue and represent the chronic stage of infection. Conversion to bradyzoites can be simulated in tissue culture by CO2 starvation, using medium with high a pH, or the addition of interferon gamma (IFNγ). Bradyzoites are characterized by the presence of a cyst wall, to which the lectin Dolichos biflorus agglutinin (DBA) binds. Fluorescently labeled DBA is used to visualize the cyst wall in parasites grown in human foreskin fibroblasts (HFFs) that have been exposed to low CO2 and high pH medium. Similarly, parasites residing in murine bone marrow-derived macrophages (BMMs) display a cyst wall detectable by DBA after the BMMs are activated with IFNγ and lipopolysaccharide (LPS). This protocol will demonstrate how to induce conversion of T. gondii to bradyzoites using a high pH growth medium with low CO2 and activation of BMMs. Host cells will be cultured on coverslips, infected with tachyzoites and either activated with addition of IFNγ and LPS (BMMs) or exposed to a high pH growth medium (HFFs) for three days. Upon completion of infections, host cells will be fixed, permeabilized, and blocked. Cyst walls will be visualized using rhodamine DBA with fluorescence microscopy.
Protocol
1. Preparation of human foreskin fibroblasts (HFF)-coated coverslips
Place a sterile round glass coverslip on the bottom of the wells of a 24-well tissue culture plate.
To harvest HFFs from a confluent 150cm2 flask, rinse the flask twice with 1X PBS and add 2.5 ml of 0.025% trypsin-EDTA. Incubate flask at 37°C for 5-10 minutes.
Tapping the side of the flask against the palm of your hand can aid in detaching the cells from the flask. Once cells have released from the flask, add 150 ml of HFF medium (Dulbecco's Modified Eagle Medium [DMEM] with 10% FBS, 2 mM L-glutamine, and 1% penicillin-streptomycin) and use the medium to rinse the flask and collect the cells.
Dispense 1 ml of cells per well with the coverslips.
Allow cells to become confluent at 37°C, 5% CO2.
2. Preparing L929 conditioned medium (CM) for BMC development
L929 is a murine aneuploid fibrosarcoma cell line that secretes macrophage colony stimulating factor after long periods of confluency. This secretion enables the CM from L929 cells to be used to develop mouse bone marrow cells into macrophages in cell culture1.
Once L929 cells are confluent, let them incubate for an additional 7-9 days until they appear spherical and begin lifting off the flask. Collect supernatant and pellet any detached cells at 425 x g for 10 minutes. This supernatant is the CM.
One 150 cm2 flask of L929 cells yields 30 ml of CM, which generates 150 ml of BMC medium. BMC medium is composed of 10% FBS, 20% CM, 1% penicillin-streptomycin, and 1% L-glutamine in DMEM.
3. Isolation of bone marrow and cell culture of BMCs
Sacrifice one C57BL/6 mouse by CO2 asphyxiation and surface sterilize with 70% ethanol.
Expose the peritoneum by lifting the abdominal skin near the leg and cutting with scissors. Avoid puncturing the peritoneal cavity. Cut down to the leg to expose the tibia and the femur. Cut muscle tissue from the leg bones using scissors.
To remove the femur, cut once below the knee and once near the hip. Place the bones in a bacteriological Petri dish containing cold 1X PBS. Do not use tissue culture treated Petri dishes for these steps because the BMCs will adhere permanently. Use a scalpel to scrape off extra muscle tissue. Cut the bone just above the knee to expose the marrow.
Repeat steps (b) and (c) to collect bone marrow from the second femur.
Use a 10cc syringe filled with cold 1X PBS and a 25 gauge needle to dislodge the bone marrow into a 50 ml conical tube. The bone should appear pure white once marrow is removed.
Pass the PBS/marrow mixture through a 22 gauge needle to break up any bone marrow aggregates.
Spin the mixture at 425 x g for 10 minutes, and remove the supernatant.
Resuspend the pellet in 8 ml of BMC medium. Add 1 ml of cell suspension and 9 ml BMC medium to 8 bacteriological Petri dishes.
Incubate at 37°C, 5% CO2.
On day 5, add 10 ml BMC medium to each plate. By day 7, the cells will be fully developed. BMCs may be passaged for 1-2 weeks after maturation.
To split BMCs, remove the medium and add 5 ml of cold 1X PBS to each Petri dish. Incubate at 4°C for 30 minutes, until the cells begin to lift. Rinse the BMCs off the plate with a sterile transfer pipette.
Pool the BMCs in a 50 ml conical tube and pellet at 425 x g for 10 minutes.
Resuspend the pellet in 10 ml BMC medium and count on a hemocytometer. Seed 2x105 BMCs per well in a 24-well plate with round glass coverslips on the bottom. Let the BMCs adhere overnight before infecting with T. gondii. Excess BMCs can be reseeded on Petri dishes for later use.
4. Infecting cells with T. gondii
Infect 25 cm2 flask of confluent HFFs with 2x106T. gondiiand grow until cells begin to lyse (about 2-3 days).
Use a cell scraper to remove the infected host cell monolayer from the tissue culture flask then release the parasites from the host cells by passing the dislodged monolayer through a 27-gauge needle.
Use a hemocytometer to determine the number of parasites in the medium.
Infect 105 parasites per well with coverslip of HFF monolayers from Protocol 1, or BMCs from Protocol 3. Let the parasites invade for 3 hours at 37°C, 5% CO2.
5. Initiating T. gondii bradyzoite development by environmental stresses
- Bradyzoite development in HFFs with medium pH increase and CO2 starvation
- Prepare development medium. Development medium contains RPMI 1640 without bicarbonate, 1% FBS, 1% penicillin-streptomycin, and 42 mM HEPES. pH to 8.0 and filter sterilize.
- Remove DMEM from infected HFFs, rinse with 1X PBS, and add 1ml of development medium.
- Incubate for 3 days at 37°C with ambient air.
- Activation of BMCs
- Prepare medium to activate BMCs. Activation medium is BMC medium supplemented with 100U/ml IFN-γ and 100 ng/ml LPS. Sonicate LPS in a water bath for 2 minutes before use to disrupt aggregates.
- Remove BMC medium from wells and replace with 1 ml of activation medium.
- Incubate at 37°C, 5% CO2 for 3 days.
6. Immunofluorescence detection
Rinse infected wells three times with 1X PBS.
Fix the monolayer with 200ul of 3% formaldehyde for 20 minutes.
Remove fixative and rinse wells with 1X PBS.
Add 200μl of 0.1 M glycine to wells and let sit for 5 minutes.
Remove glycine and rinse wells with 1X PBS.
Add 250 μl of 3% BSA/0.2% TritonX-100 in 1X PBS to permeabilize and block the monolayer for 30 minutes.
Remove blocking solution and rinse wells with 1X PBS.
Add 50 μl of a 1:250 dilution of rhodamine Dolichos biflorus agglutinin in 3% BSA/0.2% Triton X-100 in 1X PBS.
Cover plate and place on platform shaker at room temperature for 1 hour
Wash the coverslips 3 times with 0.2% Triton X-100 in 1X PBS for 5 minutes each on a platform shaker.
Mount coverslips using VectaShield mounting medium containing 4'6-diamidino-2-phenylindole (DAPI).
Stained T. gondiican be visualized using a fluorescent microscope with a filter appropriate for rhodamine at a magnification of 100x.
7. Representative results
Figure 1 shows representative DBA staining of T. gondii in activated BMMs and HFFs under pH stress. Both show DBA staining around parasite containing vacuoles, indicating the presence of cyst wall components. The activated BMM image shows DBA staining that is consistent with the surface of the vacuole. The cross section of T. gondii in pH stressed HFFs shows the cyst wall with no internal structures stained.
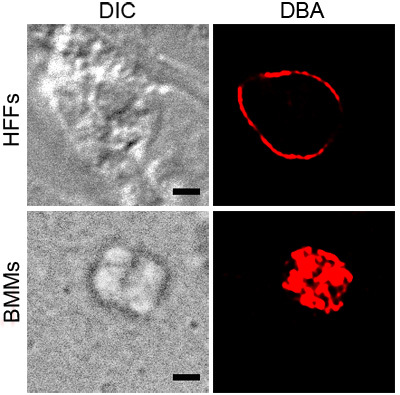 Figure 1. DBA staining of stressed T. gondii. Intracellular parasites under stress conditions, activated BMMs or pH stressed HFFs, were stained with rhodamine conjugated DBA (red). Differential interference contrast (DIC) shows the outline of the cyst and the black scale bar equals 2 μM.
Figure 1. DBA staining of stressed T. gondii. Intracellular parasites under stress conditions, activated BMMs or pH stressed HFFs, were stained with rhodamine conjugated DBA (red). Differential interference contrast (DIC) shows the outline of the cyst and the black scale bar equals 2 μM.
Experiments on Animals
Experiments on animals were performed in accordance with the guidelines and regulations set forth by University of Wisconsin Animal Care and Use Committee.
Discussion
While the mechanism of bradyzoite development is not fully understood, molecular genetic analyses of T. gondii stage conversion in tissue culture has led to the discovery of genes that are involved in bradyzoite cyst formation2,3,4. The analyses also led to the observation that some bradyzoite markers are expressed in other prolonged stress conditions, including growth in activated macrophages5,6. The above methods describe how to grow T. gondii and induce development a DBA positive cyst wall structure in both BMCs and HFFs. These results highlight that the cyst development pathway can be induced by different stress stimuli.
Because T. gondii can grow in virtually any nucleated cell from warm-blooded hosts, many cell types can be used to grow T. gondii in vitro. HFFs were used in this protocol because they are a contact inhibited cell line. Since cell culture bradyzoite development takes three days, it is easier to use cell lines that will not overgrow. HFFs are primary cells that cannot be cultured indefinitely. The longer HFFs are passaged in tissue culture, the less tolerant they become to high pH medium, and thus the less unusable they become for bradyzoite switching. For optimal results, the host cells should be low passage and allowed to rest at least a week after becoming confluent. There are alternative methods for inducing development to bradyzoites that have different effects on the host cell7.
T. gondii thrives in many immune cell types. For some applications, BMCs are preferable to macrophage cell lines because they avoid artifacts that may have been introduced by immortalization8. BMCs are also useful for in vitro experiments because they have a flat morphology compared to RAW264.7 cells, making them more amenable to microscopy. The basic BMC culture method described here can be adapted for other purposes, including production of BMCs from other mouse strains9,10. The degree of activation seen with IFNγ and LPS may differ depending on the vendor and even the lot. It is therefore necessary to titrate the amounts of LPS and IFNγ used in the activation medium for optimal and consistent performance.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the University of Wisconsin-Madison Herman I. Shapiro Distinguished Graduate Fellowship (C.M.T.), NIH award AI072817 (A.M.P.) and American Heart Association Award 0840059N (L.J.K).
References
- Tomida M, Yamamoto-Yamaguchi Y, Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984;259(17):10978–10980. [PubMed] [Google Scholar]
- Matrajt M, Donald RG, Singh U, Roos DS. Identification and characterization of differentiation mutants in the protozoan parasite Toxoplasma gondii. Mol Microbiol. 2002;44:735–747. doi: 10.1046/j.1365-2958.2002.02904.x. [DOI] [PubMed] [Google Scholar]
- Singh U, Brewer JL, Boothroyd JC. Genetic analysis of tachyzoite to bradyzoite differentiation mutants in Toxoplasma gondii reveals a hierarchy of gene induction. Mol Microbiol. 2002;44:721–733. doi: 10.1046/j.1365-2958.2002.02903.x. [DOI] [PubMed] [Google Scholar]
- J P, Knoll LJ. Isolation of Toxoplasma gondii development mutants identifies a potential proteophosphogylcan that enhances cyst wall formation. Molecular and Biochemical Parasitology. 2010;169(2):120–123. doi: 10.1016/j.molbiopara.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Induction of Bradyzoite-Specific Toxoplasma gondii Antigens in Gamma Interferon-Treated Mouse Macrophages. Infection and Immunity. 1993;61(3):1141–1145. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HM, Bannai H, Xuan X, Nishikawa Y. Toxoplasma gondii Cyclophilin 18-Mediated Production of Nitric Oxide Induces Bradyzoite Conversion in a CCR5-Dependent Manner. Infection and Immunity. 2009;77(9):3686–3695. doi: 10.1128/IAI.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends in Parasitology. 2002;18(5):198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- Berghaus LJ, Moore JN, Hurley DJ, Vandenplas ML, Fortes BP, Wolfert MA, Boons GJ. Innate immune responses of primary murine macrophage-lineage cells and RAW 264.7 cells to ligands of Toll-like receptors 2, 3, and 4. Comp Immunol Microbiol Infect Dis. 2009 doi: 10.1016/j.cimid.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue DG, Scott-Weathers CF, Tobin CM, Knoll LJ. A patatin-like protein protects Toxoplasma gondii from degradation in activated macrophages. Mol Microbiol. 2007;63(2):482–496. doi: 10.1111/j.1365-2958.2006.05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infection and Immunity. 1994;62(5):1761–1777. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


