Abstract
Whole embryo culture (WEC) technique has been developed in 1950's by New and his colleagues, and applied for developmental biology 1. Although development and growth of mammalian embryos are critically dependent on the function of the placenta, WEC technique allows us to culture mouse and rat embryos ex vivo condition during limited periods corresponding to midgestation stages during embryonic day (E) 6.5-E12.5 in the mouse or E8.5-E14.5 in the rat 2, 3, 4. In WEC, we can directly target desired areas of embryos using fine glass capillaries because embryos can be manipulated under the microscope. Therefore, rodent WEC is very useful technique when we want to study dynamic developmental processes of postimplanted mammalian embryos. Up to date, several types of WEC systems have been developed 1. Among those, the rotator-type bottle culture system is most popular and suitable for long-term culture of embryos at midgestation, i.e., after E9.5 and E11.5 in the mouse and rat, respectively 1. In this video protocol, we demonstrate our standard procedures of rat WEC after E12.5 using a refined model of the original rotator system, which was designed by New and Cockroft 5, 6, and introduce various applications of WEC technique for studies in mammalian developmental biology.
Protocol
1. Setting up the WEC system
Joint a 0.22 μm membrane filter to the inlet silicon tube within WEC system a).
Open the valve of a gas cylinder containing O2 (95%) and CO2 (5%). Flow the gas mixture into the drum via the bubbling bottle containing autoclaved water.
Adjust the flow volume of gas mixture to 50 cc.
Insert the out let silicon tube into a water bottle and check out the flow of gas mixture.
Rotate the drum at the speed of 20 rpm/min.
2. Preparation of culture medium
Thaw rat immediately-centrifuged (IC) serum at 37.0°C b). Add D-glucose (2 mg/ml) into thawed serum in an autoclaved 100 ml beaker. Culture medium should be prepared at the primary culture level (e.g., using a hood).
Add antibiotic-antimycotic (100X) liquid to the serum with 1: 400 dilution.
Vaporize remaining isoflurane (due to anesthesia of rats during collecting blood) from the serum for 20 minutes at room temperature (RT) c).
Sterilize the culture medium using a 0.45 μm membrane filter.
Pour 3.0 ml of the medium in each culture bottle and seal the top of each bottle with a silicon plug, covered with aluminum foil (see ref. 14) d).
Prepare three or four disposal Petri dishes containing Tyrode's saline e).
3. Anesthesia of the rat and isolation of the uterus
Anesthetize a timed-pregnant rat deeply with inhalational or parenteral anesthetics.
Clean the abdominal area of the anesthetized pregnant rat with 70% ethanol.
Pick up the skin with forceps and exteriorize the abdominal wall by cutting the skin with large scissors f).
Pick up the abdominal wall with a pair of forceps and make U-shaped large longitudinal incisions on the abdominal wall with large scissors up to the thoracic level g).
Bear away the intestine to the left side with a pair of forceps and expose one end of the uterus connected to the ovary.
Pick up the end of the uterus with a pair of forceps and cut the position between the uterus and ovary with large scissors.
Take out the uterus from the pregnant rat by cutting the fat around the uterus until the other end of the uterus.
Transfer the uterus to a Petri dish containing Tyrode's saline.
Euthanize the rat by excessive administration of anesthetics or cervical dislocation after removing the uterus.
4. Removal of embryos from the uterus
Briefly rinse the uterus in Tyrode's saline and transfer it to the second Petri dish with a pair of fine forceps h).
Under a binocular microscope, pick up the uterine wall with a pair of fine forceps and cut the uterine wall at the side opposite to the mesometrium connecting with blood vessels using ophthalmic straight scissors.
Insert the tip of ophthalmic straight scissors into the space between the uterine wall and decidua. Cut the uterine wall longitudinally along the antimesometrial side.
Clump the decidua at the mesometrial side and separate conceptuses from the uterus using two pairs of fine forceps i).
Transfer conceptuses to the third Petri dish with a sterilized glass pipette with a suitable diameter.
5. Dissection of rat embryos
Insert one tip of the microforceps into the decidua and make a transverse incision on the decidua around the conceptus with two pairs of forceps.
Insert the tips of the forceps into the decidua at the placental side and make two longitudinal incisions on the decidua to the top.
Remove the decidua at the placental side by ripping out that with two pairs of forceps. Remove the remaining decidua with two pairs of forceps.
Pick up Reichert's membrane, make a horizontal incision on it, and separate the membrane from the conceptus at the anti-placental side.
Make a small hole on the yolk sac near the head of the embryo with the two pairs of forceps, and cut the yolk sac with a pair of ophthalmic curved scissors. It is important not to damage major blood vessels.
Pick up the amnion at the site around the head of the embryo with the two pairs of fine forceps, and gently pull out the embryo from the yolk sac by tearing the amnion h). The body of the embryo should be pulled out of the yolk sac to increase the supply of oxygen for the embryo at the midgestation stage.
6. Whole embryo culture
Check damages on the placenta, yolk sac, and the condition of heart beating and blood circulation.
Being aware of not stretching the umbilical cord, transfer the embryo to a culture bottle with a sterilized glass pipette. Carry over of Tyrode's saline to culture bottles should be as little as possible.
Remove a non-hole silicon plug from the rotator drum. Connect the culture bottle with a plug with a hole to the rotator drum of the WEC system perpendicularly. Be careful not to put force on the drum in unsuitable directions since the rotator drum is a very fine equipment. The bottles containing the dissected embryo are transferred to culture one by one; opening of the front door of the incubator should be minimized to avoid the temperature of the incubator is decreased j).
After 10 hr of culture, increase the flow volume of gas mixture up to 75 cc and up to 100 cc after 24 hr (Table 1).
The culture medium should be changed around 24 hr by transferring the cultured embryo into a new culture bottle with freshly prepared medium. Increase the flow volume up to 125 cc after 34 hr and up to 150 cc after 48 hr (Table 1)
Occasionally check the condition of the cultured embryos by counting heartbeat rate (i.e., 120-150/min is optimal) and by observing blood circulation. If required count the number of somites to judge the growth of embryos in Tyrode's saline. In normal development, one somite is added in two hours.
When WEC is finished, stop supply of gas mixture and disconnect the inlet tube and the membrane filter to avoid back flow into the inlet tube from the bubbling bottle.
7. Notes
The temperature inside the WEC system is constantly kept at 37.0-37.5°C.
100% rat IC serum is routinely used in our WEC for rat and mouse embryos, and kept at -20°C before use.
We purchase rat IC serum, which is collected from rats that are anesthetized with isoflurane.
Disposable bottles are also available from Ikemoto Rika. Bottles and silicon plugs should be autoclaved.
Dissection of embryos should be performed at RT because maintaining of embryos at low or high temperature during dissection affect subsequent development in WEC.
Tools for dissection are sterilized by dry heat.
The skin and abdominal wall should be cut separately to avoid contamination of hairs into a dish as possible. We change a pair of large scissors and forceps with a new pair of them when we cut the skin and isolate the uterus, respectively.
Using forceps whose tips are fine is very important to dissect tissues certainly. We manually sharpen the tips of forceps with a stone and machine oil.
We use one side of the forceps like a knife to avoid excess pressure to embryos.
When the temperature inside the WEC system decreases by opening the door repeatedly, we continue to turn on the light to keep temperature inside the system.
8. Representative results
Figure 1 shows procedures of dissection of the rat embryo and cultured rat embryos.
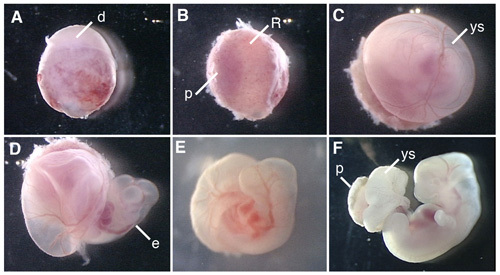 Figure 1. Whole embryo culture of the E12.5 rat embryo. (A) A conceptus dissected from the uterus of the pregnant rat. (B) Removal of the decidua at the placenta side. (C) Removal of Reichert's membrane. (D) Opening the yolk sac. (E) The rat embryo culturing in the bottle for 6 hr after the beginning of WEC. (F) The rat embryo cultured for 42 hr. d, decidua; R; Reichert's membrane; p, placenta; ys, yolk sac; e, embryo.
Figure 1. Whole embryo culture of the E12.5 rat embryo. (A) A conceptus dissected from the uterus of the pregnant rat. (B) Removal of the decidua at the placenta side. (C) Removal of Reichert's membrane. (D) Opening the yolk sac. (E) The rat embryo culturing in the bottle for 6 hr after the beginning of WEC. (F) The rat embryo cultured for 42 hr. d, decidua; R; Reichert's membrane; p, placenta; ys, yolk sac; e, embryo.
| 0 hr | 12 hr | 24 hr | 36 hr | 48 hr | |
| E12.5 rat embryo | 95% 50 cc | 95% 75 cc(10 hr) | 95% 100 cc | 95% 125 cc (34 hr) | 95% 150 cc |
Table 1. Optimal oxygen condition.
Discussion
There are two critical steps in rodent WEC for the success. First, the dissection procedure should be accurate not to damage embryos, especially blood vessels. Second, the procedure should be as quickly as possible because oxygen and nutrient are no longer supplied via the placenta after isolation from the uterus. This is critical for older embryos. In the case of rat WEC after E12.5, we should transfer dissected embryos into culture bottles within 30 minutes.
We have analyzed migration patterns of neural crest cells in the wild type and Pax6 mutant rat embryos by labeling small numbers of cells with fluorescent dye DiI or transplanting DiI-labeled cells in rat embryos of the wild type and mutant background 7. A fate map of the anterior neural plate has been drawn in the mouse from labeling neuroepithelial cells with DiI and culturing for 24 hr 8. WEC has also been used for transplantation of cells expressing GFP into the telencephalon of E12.5 rat embryos to examine cell autonomy in migration defects shown in the Pax6 mutant rat 9. Various inhibitors can be applied to the culture medium to examine signaling pathways involved in axis formation and cell cycle during development 10, 11. Furthermore, we can simply introduce expression plasmids and siRNA into developing brains of cultured embryos by electroporation to analyze gene functions 12,13,14. We also investigated behavior of neuroepithelial cells labeled with fluorescent protein in organ culture or slice culture following WEC (see ref. 14 and references therein) 14. Therefore, rodent WEC is very useful not only for analyzing cell lineage but also for elucidating gene functions by loss-of-function and gain-of-function experiments.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank Mr. Hajime Ichijo for video-recording and helpful advices for editing the video. We also thank Drs.Yuji Tsunekawa and Kaichi Yoshizaki for kind assistant for video-recording. This work is supported by KAKENHI on Young Scientist B and on Priority Areas- Molecular Brain Science from MEXT of Japan. We acknowledge the support of Global COE Program "Basic and Translational Research Center for Global Brain Science" from MEXT of Japan and The Core Research for Evolutional Science and Technology (CREST) from Japanese Science and Technology Corporation from Japanese Science and Technology Agency (JST).
References
- New DAT. Intoroduction. In mammalian postimplantation embryos. In: Copp AJ, Cockroft DL, editors. In Mammalian Postimplantation Embryos A Practical Approach. Oxford: IRL Press; 1990. pp. 1–14. [Google Scholar]
- Cockroft DL. Dissection and culture of post implantation embryos. In: Copp AJ, Cockroft DL, editors. In Mammalian Postimplantation Embryos A Practical Approach. Oxford: IRL Press; 1990. pp. 15–40. [Google Scholar]
- Morris-Kay GM. Essential Developmental Biology A Practical Approach. Oxford: IRL Press; 1993. Postimplantation mammalian embryos; pp. 55–66. [Google Scholar]
- Fujinaga M. In vitro culture of rodent embryos during the early postimplantation period. In: Tuan RS, Lo CW, editors. Developmental Biology Protocols. Totowa: Humana Press; 2000. pp. 53–76. [DOI] [PubMed] [Google Scholar]
- Eto K, Takakubo F. Improved development of rat embryos in culture during the period of craniofacial morphogenesis. J. Craniofac. Genet. Dev. Biol. 1985;5:351–355. [PubMed] [Google Scholar]
- New DA, Cockroft DL. A rotating bottle culture method with continuous replacement of the gas phase. Experientia. 1979;35:138–140. doi: 10.1007/BF01917926. [DOI] [PubMed] [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Eto K. Mammalian craniofacial embryology in vitro. Int. J. Dev. Biol. 1997;41:187–194. [PubMed] [Google Scholar]
- Inoue T, Nakamura S, Osumi N. Fate mapping of the mouse prosencephalic neural plate. Dev. Biol. 2000;219:373–383. doi: 10.1006/dbio.2000.9616. [DOI] [PubMed] [Google Scholar]
- Nomura T, Holmberg J, Frisen J, Osumi N. Pax6-dependent boundary defines alignment of migrating olfactory cortex neurons via the repulsive activity of ephrin A5. Development. 2006;133:1335–1345. doi: 10.1242/dev.02290. [DOI] [PubMed] [Google Scholar]
- Inoue YU, Asami J, Inoue T. Genetic labeling of mouse rhombomeres by Cadherin-6::EGFP-BAC transgenesis underscores the role of cadherins in hindbrain compartmentalization. Neurosci. Res. 2009;63:2–9. doi: 10.1016/j.neures.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Osumi N, Inoue T. Gene transfer into cultured mammalian embryos by electroporation. Methods. 2001;24:35–42. doi: 10.1006/meth.2001.1154. [DOI] [PubMed] [Google Scholar]
- Arai Y. Role of Fabp7, a downstream gene of Pax6, in the maintenance of neuroepithelial cells during early embryonic development of the rat cortex. J. Neurosci. 2005;25:9752–9761. doi: 10.1523/JNEUROSCI.2512-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Nomura T, Osumi N. Transferring genes into cultured mammalian embryos by electroporation. Dev. Growth Differ. 2008;50:485–497. doi: 10.1111/j.1440-169X.2008.01046.x. [DOI] [PubMed] [Google Scholar]


