Abstract
Background
Human papillomaviruses (HPV) constitute one of the most prevalent sexually transmitted infections and are the etiological agents for invasive cervical cancer, the predominant cancer among women in Botswana. However, the prevalence of HPV genotypes in Botswana has yet to be reported
Methods
139 endocervical swabs were taken at baseline from HIV-1 infected, HSV-2 seropositive women enrolled in a longitudinal cohort study designed to assess the influence of herpes simplex virus-2 (HSV-2) infection on genital tract shedding of HIV-1. Extracted DNA was evaluated for the presence of low-risk and high-risk HPV using the Roche Linear Array.
Results
Genotyping identified HPV in 95 of 139 women of which 61/95 were infected with high-risk HPV and 56/95 with low-risk HPV. The median number of genotypes was 2 (IQR: 1–4). The most prevalent HPV genotype in HIV-infected women was HPV 58. Abnormal cervical cytology was detected in 87/127 women and was associated with contemporaneous HPV infection (RR=1.43, 95% CI: 1.05–1.93) (p=0.02).
Conclusions
HPV prevalence was high among HIV-infected women with infection by multiple genotypes being widespread. The associations attributed to specific oncogenic HPV subtypes and cervical squamous intraepithelial lesions presented here provide critical information to inform future vaccine policy within Botswana.
Keywords: HIV, Human Papillomavirus, co-infection, cervical cancer
Background
Human papillomaviruses (HPV) constitute one of the most prevalent sexually transmitted infections and are considered the etiological agents for invasive cervical cancer (ICC). The incidence rate of cervical cancer in Botswana is estimated at 22.2 per 100,000 in 2008, making it the most prevalent cancer among women in Botswana and the second in terms of morality after Kaposi sarcoma [WHO/ICO, 2010]. The success of the national HIV treatment program in Botswana has led to increased access to antiretroviral treatment (ART) for a number of HIV-infected women although a consequence of a prolonging life expectancy is the increased frequency of malignancies. Despite the high incidence of ICC, the prevalence of HPV genotypes in Botswana has yet to be reported.
Based on sequence variation within the L1 capsid protein of HPV subtypes, the papillomavirus family has been grouped into high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 58, 59, 68, 73 and 82); low-risk non-oncogenic types that lead to the development of non-malignant lesions (6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 and 89); and those with limited epidemiological data that are classed as probable oncogenic types (26, 53, 66) [Munoz et al., 2003; Schiffman et al., 2009].
HPV shares a number of common behavioral risk factors with HIV, with HIV-infected women, particularly those with a CD4 count <200 cells/μl or an HIV viral load >100,000 copies/ml, presenting with multiple concurrent HPV infections that display a broader range of genotypes, compared to HIV-uninfected women [Strickler et al., 2005]. CD4 T cell activity is important for resolution of genital HPV infection, and the decline of cell-mediated immunity in untreated HIV-infected women results in an increased likelihood of progression to ICC [Coleman et al., 1994; Stanley, 2008].
Recent studies have associated HPV infection with an increased risk for HIV acquisition and possibly transmission [Auvert et al., 2010; Averbach et al., 2010; Smith et al., 2010; Smith-McCune et al., 2010; Veldhuijzen et al., 2010]. It is difficult to determine causality from observational studies, and an apparent explanation for the potential association is the transmission of both viruses from a co-infected partner. However, independent studies have mapped initial infection with HPV, and possibly its ensuing immunological clearance, with subsequent HIV infection, rather than solely demonstrating a higher incidence of HPV infection in HIV-infected individuals [Averbach et al., 2010].
Two HPV vaccines are now available that induce protective antibodies against the L1 capsid protein of oncogenic HPV 16 and 18, with one of the vaccines also protecting against low-risk HPV 6 and 11. It is important to establish the distribution of HPV genotypes among HIV-infected women and those with cervical neoplasia in Botswana in order to assess the potential effectiveness of these bivalent and quadrivalent vaccines. The aim of this study was to determine the prevalence of HPV infection in HIV-infected women in Botswana, the frequency of HPV genotypes and their relationship with cervical squamous intraepithelial lesions.
Methods
Study population
The “Basadi” Treatment Study is a longitudinal cohort study designed to evaluate the influence of herpes simplex virus-2 (HSV-2) infection on genital tract shedding of HIV-1 in the context of ART.
Potentially eligible participants (women ≥18 yrs age with advanced HIV-infection, preparing to initiate ART through Botswana's National ART Program) were recruited at government clinics in Gaborone, Botswana at the time of their ART pre-initiation visit. Potential participants were excluded if they were pregnant or planned to become pregnant while on study, had previously received ART, or had taken any antiretrovirals or antivirals within 30 days prior to enrolment. Consenting potential study subjects were evaluated (and treated as necessary) at the screening visit for sexually transmitted infections (STIs) per the government's syndromic approach to the diagnosis and treatment of STIs.
Between September 5, 2005, and November 22, 2007, 220 HIV-1 / HSV-2 co-infected women with advanced HIV infection were enrolled in the study. The study was conducted at the Infectious Disease Care Clinic (IDCC) on the grounds of Princess Marina Hospital in Gaborone, Botswana. The study was approved by the Institutional Review Boards of Botswana and the Harvard School of Public Health. All patients provided written informed consent for the collection of samples and subsequent analysis. Study visits occurred at the time of ART initiation and at 1, 3, and 6 months after ART initiation. Genital exams (including collection of vaginal swab, cervico-vaginal lavage, and endocervical swab) were conducted at each study visit. Study visits occurring when the participant was menstruating were re-scheduled after menses ceased. Pap smears were provided for all participants beginning at the screening visit and were collected after the cervico-vaginal lavage and vaginal and cervical swabs were obtained, but were deferred to the next visit in the presence of blood or abnormal discharge that might be associated with menses or an STI.
Pap smears were obtained by collecting exfoliated cells from the transformation zone of the cervix using a cytobrush. The cells were transferred directly to a slide and fixed using the conventional technique. Cytologic analysis was conducted at the Botswana's National Health Laboratory in Gaborone, Botswana. The Bethesda System of reporting was utilized.
Endocervical swabs (ECSs) were obtained by placing a dry Dacron swab into the cervical canal and rotating 360° once. The swab was immediately placed into a dry cryovial and sealed, and then transferred to a −70°C freezer within six hours of collection.
DNA extraction
139 ECSs were available for HPV genotyping. ECSs were thawed at room temperature and placed in a lysis solution in the presence of protease as outlined in the manufacturer's protocol for the QIAamp DNA Blood Mini Kit (Qiagen). The swab was incubated at 56°C for 10 minutes then placed in a Swab Extraction Tube System (S.E.T.S) (Roche) so that any absorbed lysis buffer and associated DNA could be recovered. The S.E.T.S. was spun briefly at 13,000RPM and the flow-through was added to the original lysate before following the remainder of the manufacturer's protocol.
Human Papillomavirus genotyping
Linear Array HPV genotyping test (Roche Diagnostics Ltd) was carried out as previously described [Woo et al., 2007]. A 100 μl reaction comprised 50 μl of DNA and 50 μl working master mix containing MgCl2, KCl, Amplitaq Gold DNA polymerase, uracil-N-glycosilase, deoxynucleotides (dNTPs) and biotinylated PGMY and β-globin primers. PCR reactions were run in Applied Biosystems Gold-plated 96-Well GeneAmp PCR System 9700 at 50 °C for 2 min, 95 °C for 9 min and 40 cycles, at 50% ramp rate, of 95 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min and finally, at 72 °C for 5 min before holding indefinitely at 72 °C. 100 μl of denaturing solution was added to the PCR product before allowing the denatured amplicons to hybridize on to a strip containing specific probes for 37 HPV genotypes and β-globin reference bands. Positive reactions appeared as blue lines on the strip after exposure to Streptavidin-horseradish peroxidase. Two independent investigators, using the HPV reference guide provided, interpreted the genotyping results.
Statistical methods
The Mann-Whitney rank sum test was used to compare medians with associations considered statistically significant at p< 0.05. All analyses were carried out using the StatXact software (Cytel).
Results
Population characteristics
139 endocervical swabs were taken at baseline from HIV-1 infected, HSV-2 seropositive women enrolled in the Basadi study. Detailed demographic characteristics are presented in Table 1. Briefly, the median age of study participants was 32 years with 71% reporting to be single and over 78% reporting the use of condoms. The majority of women had a CD4 <200 cells/μl with the median viral load >100,000 copies/ml. Over half of the participants were educated to a least junior secondary level.
Table 1.
Clinical and behavioral characteristics of 139 HIV-infected women who were genotyped for human papillomavirus before the initiation of antiretroviral therapy.
| Variable | Numerical Value |
|---|---|
| Clinical Variable | Median (Interquartile range) |
| Age, median years | 32 (29–39) |
| HIV RNA levels at baseline, median log10 copies/ml | 5.08 (4.49–5.65) |
| CD4 count, median cells/μl | 157 (105–187) |
| No. of pregnancies, median | 2 (1–3) |
| Behavioral Variable | Number (%) |
| Marital Status | |
| Single | 99 (71.2) |
| Married | 9 (6.5) |
| Co-habitating | 28 (20.1) |
| Widowed | 2 (1.4) |
| Divorced | 1 (0.7) |
| Other | 0 (0) |
| Education | |
| None | 7 (5) |
| Primary | 44 (31.7) |
| Junior Secondary | 64 (46) |
| Senior Secondary | 16 (11.5) |
| Tertiary | 8 (5.8) |
| Contraception | |
| Abstinence | 15 (10.8) |
| Condoms only | 109 (78.4) |
| Oral or injectable hormonal contraceptives (+/− condoms) | 8 (5.8) |
| IUD (+/− condoms) | 2 (1.4) |
| Tubal ligation (+/− condoms) | 4 (2.9) |
| Spermicide alone | 1 (0.7) |
| None | 0 (0) |
HPV genotyping
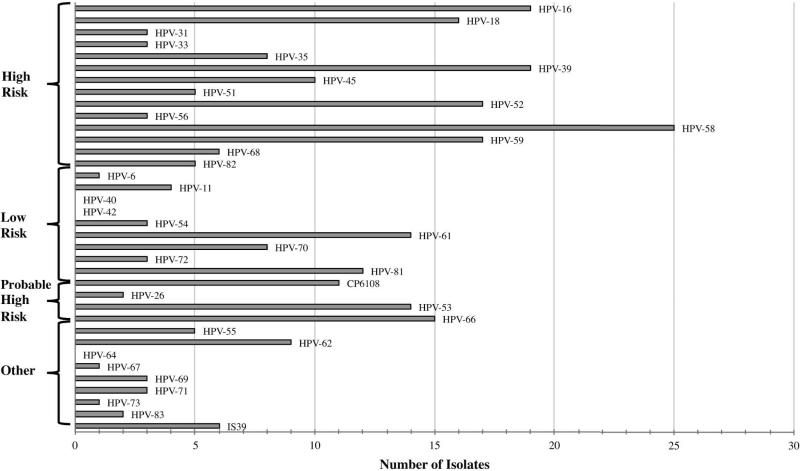
Of 139 endocervical swabs, 95 (68%) tested positive for one or more HPV genotypes. All tests were positive for the β-globin endogenous control. Of those infected with HPV, 61/95 (64%) participants were infected with high-risk (oncogenic) HPV and 56/95 (59%) with low-risk (non-oncogenic) HPV. More than one HPV genotype was identified in 67/95 (71%) participants. The Linear Array Genotyping assay is capable of detecting 37 HPV genotypes of which 33 were prevalent in endocervical swabs take from this cohort (Figure 1). The most prevalent genotypes in HPV-infected women were: HPV 58 (26%), HPV 16 (20%), HPV 39 (20%), HPV 52 (18%), HPV 59 (18%) and HPV 18 (17%). The median number of HPV genotypes was 2 (IQR: 1–4).
Figure 1. Distribution of HPV genotypes among HIV-infected women in Botswana.
HPV 58 was the most prevalent HPV genotypes in this cohort of women. HPV 16 and 18 were among the most common high-risk subtypes. The number of individuals infected with HPV 52 may be under-represented due to cross-reactivity within the genotyping assay.
Association between HPV genotypes and cervical cytological abnormalities
A total of 127 women had an interpretable Pap smear and associated HPV prevalence data (Table 2). Abnormal cervical cytology was detected in 87/127 (68.5%) of these HIV-infected women: high-grade cervical squamous intraepithelial lesions (HSIL) in 16.5% (21/127), low-grade SIL (LSIL) in 42.5% (54/127) and atypical squamous cells of undetermined significance (ASCUS) in 9.4% (12/127). The presence of SILs and ASCUS was significantly associated with contemporaneous HPV infection (RR=1.43, 95% CI: 1.05–1.93) (p=0.02). Among HPV-infected women, prevalence of abnormal cytology was significantly higher among those infected with multiple (≥2) HPV genotypes (RR=1.35, 95% CI: 1.07–1.72) (p=0.01). High-risk HPV 18 and 39 were significantly associated with cervical dysplasia (p=0.046) and (p=0.016), respectively, although there was no detectable association with the presence of HPV 16 and subsequent epithelial abnormalities (p=0.84). There was a possible association with the presence of HPV 52 and dysplasia (RR=1.27, 95% CI: 0.97–1.66) (p=0.08) and this may be of interest given that the prevalence of HPV 52 is possibly underestimated due to cross-reactivity within the genotyping assay.
Table 2.
Prevalence of HPV genotypes and association with cervical cytology
| Number with cytological result |
|||||||
|---|---|---|---|---|---|---|---|
| HPV status | n = (% of 95) | HSIL (n=21) | LSIL (n=54) | ASCUS (n=12) | Normal (n=40) | Risk Ratio (95% CI) | p |
| Any HPV | 95 (100) | 15 | 40 | 9 | 20 | 1.43 (1.05–1.93) | 0.022 |
| Multiple | 67 (70.5) | 12 | 29 | 6 | 12 | 1.35 (1.07–1.72) | 0.012 |
| High-Risk HPV |
|||||||
| 16 | 19 (20) | 5 | 7 | 0 | 5 | 1.04 (0.74–1.44) | 0.84 |
| 18 | 16 (16.8) | 5 | 5 | 2 | 2 | 1.29 (1.00–1.66) | 0.046 |
| 31 | 3 (3.2) | 0 | 2 | 1 | 0 | 1.29 (0.88–1.91) | 0.19 |
| 33 | 3 (3.2) | 1 | 0 | 1 | 1 | 0.97 (0.43–2.18) | 0.95 |
| 35 | 8 (8.4) | 3 | 3 | 0 | 2 | 1.10 (0.73–1.67) | 0.65 |
| 39 | 19 (20) | 5 | 8 | 1 | 3 | 1.24 (0.96–1.60) | 0.10 |
| 45 | 10 (10.5) | 3 | 5 | 1 | 1 | 1.35 (1.06–1.72) | 0.016 |
| 51 | 5 (5.3) | 0 | 4 | 0 | 1 | 1.18 (0.75–1.85) | 0.49 |
| 52 | 17 (17.9) | 5 | 5 | 1 | 2 | 1.27 (0.97–1.66) | 0.079 |
| 56 | 3 (3.2) | 0 | 1 | 0 | 1 | 0.76 (0.19–3.04) | 0.69 |
| 58 | 25 (26.3) | 5 | 10 | 3 | 5 | 1.18 (0.92–1.52) | 0.2 |
| 59 | 17 (17.9) | 3 | 7 | 0 | 6 | 0.9 (0.60–1.34) | 0.61 |
| 68 | 6 (6.3) | 3 | 1 | 0 | 2 | 0.97 (0.55–1.73) | 0.92 |
| 73 | 1 (1.1) | 1 | 0 | 0 | 0 | 1.10 (0.49–2.47) | 0.82 |
| 82 | 5 (5.3) | 1 | 3 | 0 | 0 | 1.37 (0.97–1.83) | 0.073 |
| Low-Risk HPV |
|||||||
| 06 | 1 (1.1) | 0 | 2 | 0 | 0 | 1.23 (0.73–2.07) | 0.44 |
| 11 | 4 (4.2) | 0 | 1 | 2 | 0 | 1.29 (0.88–1.91) | 0.19 |
| 54 | 3 (3.2) | 0 | 2 | 0 | 1 | 0.97 (0.43–2.18) | 0.95 |
| 55 | 5 (5.3) | 1 | 3 | 0 | 0 | 1.34 (0.97–1.83) | 0.073 |
| 61 | 14 (14.7) | 1 | 5 | 2 | 5 | 0.89 (0.57–1.39) | 0.6 |
| 70 | 8 (8.4) | 2 | 6 | 0 | 0 | 1.43 (1.16–1.75) | 0.0006 |
| 72 | 3 (3.2) | 0 | 2 | 0 | 1 | 0.97 (0.43–2.18) | 0.95 |
| 81 | 12 (12.6) | 1 | 6 | 2 | 2 | 1.22 (0.90–1.65) | 0.21 |
| CP6108 | 11 (11.6) | 2 | 6 | 2 | 1 | 1.07 (0.73–1.57) | 0.74 |
| Probable High-Risk |
|||||||
| 26 | 2 (2.1) | 0 | 2 | 0 | 0 | 1.23 (0.73–2.07) | 0.44 |
| 53 | 14 (14.7) | 1 | 6 | 0 | 5 | 0.84 (0.51–1.37) | 0.48 |
| 66 | 15 (15.8) | 2 | 10 | 1 | 0 | 1.49 (1.26–1.76) | <0.0001 |
| Unknown Risk |
|||||||
| 62 | 9 (9.5) | 2 | 5 | 2 | 0 | 1.44 (1.19–1.75) | 0.0002 |
| 67 | 1 (1.1) | 1 | 0 | 0 | 0 | 1.10 (0.49–2.47) | 0.82 |
| 69 | 3 (3.2) | 0 | 2 | 0 | 1 | 0.97 (0.43–2.18) | 0.95 |
| 71 | 3 (3.2) | 0 | 2 | 0 | 1 | 0.97 (0.43–2.18) | 0.95 |
| 83 | 2 (2.1) | 0 | 3 | 0 | 1 | 1.10 (0.62–1.96) | 0.32 |
| 84 | 2 (2.1) | 1 | 1 | 0 | 0 | 1.23 (0.73–2.07) | 0.77 |
| IS39 | 6 (6.3) | 1 | 3 | 0 | 1 | 1.18 (0.75–1.85) | 0.7 |
NOTE: Data for the presence of a genotype in each cytological result may be less than the total number of isolates for that genotype as cervical cytology was not available for all women. ASCUS: atypical squamous cells of undetermined significance; HSIL: high-grade squamous intraepithelial lesions; LSIL: low-grade squamous intraepithelial lesions.
HPV 70 was present only in women with SIL (RR=1.43; 95% CI: 1.16–1.75) (p<0.001) and HPV-62 was also absent from women with normal cervical cytology (RR=1.44; 95% CI: 1.19–1.75) (p<0.001). The probable oncogenic type HPV 66 had the strongest association as 13.8% of women with abnormal cytology had detectable HPV-66 when compared to none of the 40 women with normal cytology. (RR=1.49; 95% CIL1.26–1.76) (p<0.0001).
Risk factors for HPV infection and abnormal cytology
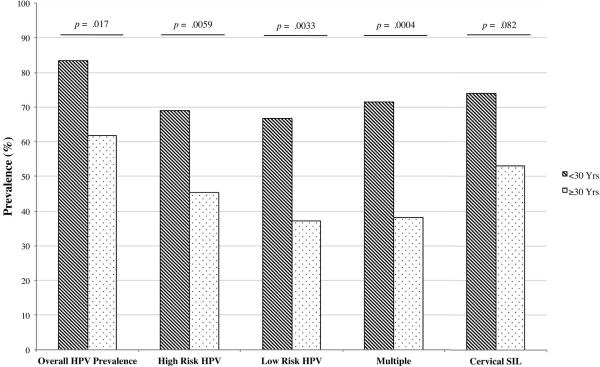
HPV prevalence decreased with increasing age as participants under the age of 30 years had a significantly higher overall prevalence (RR=1.35; 95% CI: 1.1–1.7) – of both high-risk (RR=1.59; 95% CI: 1.2–2.2) and low-risk (RR=1.70; 95% CI: 1.2–2.4) genotypes – as well as multiple HPV infections (RR=2.69; 95% CI: 1.5–4.8) (Figure 2). Those with an HIV viral load >100,000 copies/ml were more likely to be infected with high-risk HPV but not low-risk or multiple subtypes (RR=1.43; 95% CI: 1.04–1.97) (p=0.028). Women who previously had one or more pregnancies had a higher HPV prevalence (58.2% vs. 33.3%) although the small number of women in this study who had never been pregnant (n=9) precluded the determination of any significant association between parity and high-risk HPV infection (p=0.24).
Figure 2. Association of age with HPV prevalence and cervical cytology.
Women under the age of 30 years (n=42) had a significantly higher HPV prevalence in general, as well as with high-risk and low-risk subtypes, when compared to women over 30 years of age (n=97). This age group was also more likely to be infected with multiple HPV genotypes as well as trend towards a higher rate of cervical squamous intraepithelial lesions (SIL).
Lower CD4 counts (<200 cells/μl) were associated with an increased prevalence of HSIL and LSIL although not to a statistically significant level (RR=2.19; 95% CI: 0.92–4.80) (p=0.077) with a trend for women <30 years to have abnormal cervical cytology (p=0.082) (Figure 2).
Discussion
This is the first report of human papillomavirus prevalence and cervical cytology among HIV-infected women in Botswana. The high prevalence (24.8% in adults) and incidence (1.56%) of HIV in Botswana places the burden of cervical cancer within such a demographic and they are likely to represent the majority of prevalent HPV genotypes [NACA, 2008]. Although these data may not be indicative of the general population, the associations attributed to specific HPV subtypes and SIL presented here provide critical information to inform future vaccine policy within the country. The effectiveness of vaccination efforts will ultimately rely on an epidemiological evaluation of the relationship between SIL cases and high-risk HPV types.
HPV was present in 68% of the women in our study with 70% of those being infected with one or more high-risk genotypes, similar to prevalence rates seen in neighboring sub-Saharan countries [Sahasrabuddhe et al., 2007; Smith-McCune et al., 2010]. HPV 16 was not the predominate genotype in this cohort, a difference between HIV-uninfected and –infected women that has been previously reported [Luque et al., 2006; Moodley et al., 2009]. Variations in the geographical distribution of HPV genotypes have been reported in sub-Saharan Africa and in our study we found that HPV 58 to be the most prevalent genotype in HPV-infected women. At a prevalence of >25% this is a similar distribution to those HPV types found in bordering Zambia and Zimbabwe [Averbach et al., 2010; Sahasrabuddhe et al., 2007]. Over the last two decades there has been a significant upwards trend worldwide in the prevalence of HPV 52 and HPV 58 in ICC [Li et al., 2010]. Attention should be drawn to the prevalence of HPV 52 in this study, which may be underestimated at 18%. The probe for HPV 52 is known to be cross-reactive for HPV 33, 35 and 58 so our analysis excluded the presence of HPV 52 if HPV 33, 35 or 38 were present. A more liberal interpretation of the results would make HPV 52 the most prevalent subtype at 40% (38/95) [Averbach et al., 2010].
One possible explanation for the lower-than-expected HPV prevalence in this group is that the success of sexual health interventions in Botswana may have led to a decrease in the incidence of new HPV infections in HIV-infected women. Furthermore, in Uganda, prevalence rates of HPV in HIV-infected women have been reported at ~75% [Banura et al., 2008; Banura et al., 2010], whereas in Zambia a study found >90% of HIV-infected women to harbor one or more HPV subtypes [Sahasrabuddhe et al., 2007]. Neighboring Zimbabwe has an HPV rate of ~65% in dually infected women with HIV, which is very similar to the 68% we found in Botswana [Averbach et al., 2010].
Viral clearance by CD4 T cells is important in resolving HPV infection and there are clear associations between low CD4 counts and cervical dysplasia [Schuman et al., 2003]. The high rates of cervical cytological abnormalities observed here can probably be attributed largely to this study's focus on severely immunosuppressed women initiating ART who had a median CD4 count of 157 cells/μl. The trend for women under the age of 30 years to have abnormal cervical cytology may be attributable to the higher rates of individual and multiple HPV infections within younger women, with lower age being an established predictor of HPV infection [del Amo et al., 2005; Luchters et al., 2010]. Similar to what has been reported in Kenya we found no relationship between parity and high-risk HPV acquisition, despite it being a potential predictor of infection, although differences in our cohort may be as a result of the low number of women who had never been pregnant [Dames et al., 2009; Luchters et al., 2010; Munoz et al., 2002].
While we found no association with HPV 16 and SIL, high-risk HPV 18 and 45 were detected in a significant number of women presenting with SIL. The role of HPV 18 in cervical dysplasia and subsequent ICC has been well documented in developed countries but given the high prevalence of other high-risk types in Botswana, its role in the development of cervical cancer in this region has yet to be ascertained. HPV 45 is responsible for ~10% of ICC in Africa, an observation that may extend to Botswana given that these data here demonstrate a strong association between cervical dysplasia and HPV 45 [Moodley et al., 2009]. This genotype may be an important tipping point in selecting an appropriate HPV vaccine strategy for Botswana as phylogenetic similarities between HPV 45 and 18 may potentially lead to the development of cross-reactive antibodies by the HPV 16/18 vaccine, Cervarix [Paavonen et al., 2009; Romanowski, 2010].
These data demonstrate a significant association between the presence of HPV 70 and abnormal cervical cytology. The risk classification of this HPV type may be somewhat contentious as it is considered phylogentically high-risk due to its similarities with HPV 16, but epidemiologically it is thought to be low-risk [Schiffman et al., 2009]. There are limited data as to the oncogenic potential of HPV 70, which may exhibit altered histopathological features in immunocompromised HIV-infected women that are not normally found in HIV-uninfected groups. The most statistically significant association between an HPV type and SIL was with HPV 66, a probable oncogenic genotype. A high prevalence of HPV 66 and associated HSIL has been reported among HIV-infected woman with low CD4 counts in South Africa, which emphasizes that the geographical disparity in HPV genotype prevalence between sub-Saharan Africa and North America necessitates important consideration for the development of future therapeutic interventions [Firnhaber et al., 2010].
Limitations to our study among HIV-infected women immediately prior to ART initiation precluded identifying HPV genotypes present in HIV-uninfected women and men as well as those types present in HIV-infected women with CD4 >200 cells/μl. The lack of subsequent Pap smears at later time-points prevented a more detailed association between HPV types and the development of invasive cervical cancer. Nevertheless, this study utilised a highly sensitive method to give a detailed analysis of specific HPV genotypes in the population most at-risk for cervical neoplasia in Botswana.
The importance of high-risk HPV types other than 16 and 18 in high-grade dysplasia and ICC among women in sub-Saharan Africa has not been conclusively established. Furthermore, the control of HPV 16 and 18 through vaccination programs may lead to the establishment of other oncogenic HPV types that are prevalent in this region. A quadrivalent vaccine may appear initially as the most attractive public health option given that it offers specific protection against four subtypes (6, 11, 16 and 18) but these may be at the expense of inducing potentially cross-reactive antibodies that are found in a bivalent vaccine. With the potential to provide protection against HPV 31 and 45, a cross-reactive bivalent vaccine may prove to be the optimal choice for a public health initiative to combat invasive cervical cancer in this region [Dames et al., 2009].
Acknowledgments
The project described was supported by Award Number K23HD049292 to C. Wester from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health.
Footnotes
None of the authors have any conflicts or potential conflicts of interest.
References
- Auvert B, Lissouba P, Cutler E, Zarca K, Puren A, Taljaard D. Association of oncogenic and nononcogenic human papillomavirus with HIV incidence. J Acquir Immune Defic Syndr. 2010;53(1):111–116. doi: 10.1097/QAI.0b013e3181b327e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbach SH, Gravitt PE, Nowak RG, Celentano DD, Dunbar MS, Morrison CS, Grimes B, Padian NS. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24(7):1035–1042. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banura C, Franceschi S, Doorn LJ, Arslan A, Wabwire-Mangen F, Mbidde EK, Quint W, Weiderpass E. Infection with human papillomavirus and HIV among young women in Kampala, Uganda. J Infect Dis. 2008;197(4):555–562. doi: 10.1086/526792. [DOI] [PubMed] [Google Scholar]
- Banura C, Sandin S, van Doorn LJ, Quint W, Kleter B, Wabwire-Mangen F, Mbidde EK, Weiderpass E. Type-specific incidence, clearance and predictors of cervical human papillomavirus infections (HPV) among young women: a prospective study in Uganda. Infect Agent Cancer. 2010;5:7. doi: 10.1186/1750-9378-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman N, Birley HD, Renton AM, Hanna NF, Ryait BK, Byrne M, Taylor-Robinson D, Stanley MA. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102(6):768–774. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- Dames DN, Ragin C, Griffith-Bowe A, Gomez P, Butler R. The prevalence of cervical cytology abnormalities and human papillomavirus in women infected with the human immunodeficiency virus. Infect Agent Cancer. 2009;4(Suppl 1):S8. doi: 10.1186/1750-9378-4-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Amo J, Gonzalez C, Losana J, Clavo P, Munoz L, Ballesteros J, Garcia-Saiz A, Belza MJ, Ortiz M, Menendez B, del Romero J, Bolumar F. Influence of age and geographical origin in the prevalence of high risk human papillomavirus in migrant female sex workers in Spain. Sex Transm Infect. 2005;81(1):79–84. doi: 10.1136/sti.2003.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firnhaber C, Van Le H, Pettifor A, Schulze D, Michelow P, Sanne IM, Lewis DA, Williamson AL, Allan B, Williams S, Rinas A, Levin S, Smith JS. Association between cervical dysplasia and human papillomavirus in HIV seropositive women from Johannesburg South Africa. Cancer Causes Control. 2010;21(3):433–443. doi: 10.1007/s10552-009-9475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2010 doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- Luchters SM, Vanden Broeck D, Chersich MF, Nel A, Delva W, Mandaliya K, Depuydt CE, Claeys P, Bogers JP, Temmerman M. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infect Dis. 2010;10:18. doi: 10.1186/1471-2334-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque AE, Jabeen M, Messing S, Lane CA, Demeter LM, Rose RC, Reichman RC. Prevalence of human papillomavirus genotypes and related abnormalities of cervical cytological results among HIV-1-infected women in Rochester, New York. J Infect Dis. 2006;194(4):428–434. doi: 10.1086/505876. [DOI] [PubMed] [Google Scholar]
- Moodley JR, Constant D, Hoffman M, Salimo A, Allan B, Rybicki E, Hitzeroth I, Williamson AL. Human papillomavirus prevalence, viral load and pre-cancerous lesions of the cervix in women initiating highly active antiretroviral therapy in South Africa: a cross-sectional study. BMC Cancer. 2009;9:275. doi: 10.1186/1471-2407-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Munoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS, Shah KV, Meijer CJ, Bosch FX. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359(9312):1093–1101. doi: 10.1016/S0140-6736(02)08151-5. [DOI] [PubMed] [Google Scholar]
- NACA . Botswana AIDS Impact Survey III: Statistical Report. National AIDS Coordinating Agency; Gaborone: 2008. [Google Scholar]
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, Hedrick J, Jaisamrarn U, Limson G, Garland S, Szarewski A, Romanowski B, Aoki FY, Schwarz TF, Poppe WA, Bosch FX, Jenkins D, Hardt K, Zahaf T, Descamps D, Struyf F, Lehtinen M, Dubin G, Greenacre M. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- Romanowski B. Efficacy of the HPV-16/18 adjuvanted vaccine against non-vaccine oncogenic HPV types: End-of-study results. 26th International Papillomavirus Congress (IPC); Montreal, Canada. 2010. [Google Scholar]
- Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, Huh WK, Lyon MD, Stringer JS, Parham GP. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96(9):1480–1483. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman P, Ohmit SE, Klein RS, Duerr A, Cu-Uvin S, Jamieson DJ, Anderson J, Shah KV. Longitudinal study of cervical squamous intraepithelial lesions in human immunodeficiency virus (HIV)-seropositive and at-risk HIV-seronegative women. J Infect Dis. 2003;188(1):128–136. doi: 10.1086/375783. [DOI] [PubMed] [Google Scholar]
- Smith JS, Moses S, Hudgens MG, Parker CB, Agot K, Maclean I, Ndinya-Achola JO, Snijders PJ, Meijer CJ, Bailey RC. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010;201(11):1677–1685. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-McCune KK, Shiboski S, Chirenje MZ, Magure T, Tuveson J, Ma Y, Da Costa M, Moscicki AB, Palefsky JM, Makunike-Mutasa R, Chipato T, van der Straten A, Sawaya GF. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5(4):e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol. 2008;109(2 Suppl):S15–21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Strickler HD, Burk RD, Fazzari M, Anastos K, Minkoff H, Massad LS, Hall C, Bacon M, Levine AM, Watts DH, Silverberg MJ, Xue X, Schlecht NF, Melnick S, Palefsky JM. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577–586. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen NJ, Vyankandondera J, van de Wijgert JH. HIV acquisition is associated with prior high-risk human papillomavirus infection among high-risk women in Rwanda. AIDS. 2010;24(14):2289–2292. doi: 10.1097/QAD.0b013e32833cbb71. [DOI] [PubMed] [Google Scholar]
- WHO/ICO . HPV Information Centre. Human Papillomavirus and Related Cancers Summary Report Update; Botswana: 2010. [Google Scholar]
- Woo YL, Damay I, Stanley M, Crawford R, Sterling J. The use of HPV Linear Array Assay for multiple HPV typing on archival frozen tissue and DNA specimens. J Virol Methods. 2007;142(1–2):226–230. doi: 10.1016/j.jviromet.2007.01.029. [DOI] [PubMed] [Google Scholar]