Abstract
Monosomal karyotype (MK), defined as ≥ 2 autosomal monosomies or a single monosomy in the presence of other structural abnormalities, was confirmed by several studies to convey an extremely poor prognosis in patients with acute myeloid leukemia (AML) with a 4-year overall survival after diagnosis of < 4%. A recent investigation by the Southwest Oncology Group found that the only MK+ patients alive and disease free > 6 years from diagnosis received allogeneic hematopoietic cell transplantation (HCT). To expand this observation, we retrospectively analyzed 432 patients treated with HCT at the Fred Hutchinson Cancer Research Center, 14% of whom were MK+. The 4-year overall survival of patients after HCT was 25% for MK+ AML and 56% for MK− AML (adjusted hazard ratio = 2.29, P < .0001). Among the MK+ patients, complex karyotype was associated with a significantly worse outcome than patients with noncomplex karyotype (adjusted hazard ratio = 2.70, P = .03). Thus, although the prognosis of MK+ patients remains worse than that for MK− patients in the transplantation setting, HCT appears to improve the overall outcome of MK+ patients, especially patients without a complex karyotype. However, the 28% of MK+ patients > 60 years had only a 6% 4-year survival rate after HCT, stressing the need for new approaches in these patients.
Introduction
Since the initial description of monosomal karyotype (MK) among patients with acute myeloid leukemia (AML) by Breems et al,1 several groups have confirmed the prognostic significance of this entity.2–5 Defined as ≥ 2 distinct autosomal monosomies or a single autosomal monosomy in the presence of other structural abnormalities, MK identifies a distinct subset of AML with an extremely poor prognosis, with the Southwest Oncology Group (SWOG) finding that < 4% of MK+ patients were projected to be alive 4 years after initial diagnosis.2 In fact, in that study the only MK+ patients found to be alive and disease free > 6 years from diagnosis were 2 that received allogeneic hematopoietic cell transplantation (HCT) while in first complete remission (CR1).2 This observation does not, however, answer the question of the overall utility of allogeneic HCT in the treatment of MK+ AML because the number of patients treated with this procedure in the previous study was unknown. Therefore, we have attempted to address the efficacy of HCT in the treatment of MK+ AML by reviewing our recent experience at the Fred Hutchinson Cancer Research Center (FHCRC).
Methods
Patients
Among consecutive patients with AML who had cytogenetic evaluation performed at the FHCRC/Seattle Cancer Care Alliance (SCCA) between April 2006 and April 2010, 432 received HCT at our institution, accounting for 95% of all patients who received a transplant for AML during the 4-year time period. This formed the starting point for our analysis. Table 1 describes their characteristics. Cytogenetic results at initial diagnosis from outside institutions, whenever available, were reviewed by an FHCRC/SCCA cytogeneticist. The majority (82%) of HCTs were performed in CR. The study was approved by the institutional review office at the FHCRC/SCCA.
Table 1.
Patient characteristics of the transplantation cohort (n = 432)
| Value | |
|---|---|
| Median age, y (range) | 51 (0-74) |
| Age | |
| 0-17 y, n (%) | 35 (8) |
| 18-30 y, n (%) | 56 (13) |
| 31-40 y, n (%) | 49 (11) |
| 41-50 y, n (%) | 75 (17) |
| 51-60 y, n (%) | 126 (29) |
| 61-80 y, n (%) | 91 (21) |
| Sex | |
| Male, n (%) | 229 (53) |
| Female, n (%) | 203 (47) |
| Conditioning | |
| MA, n (%) | 295 (68) |
| NMA, n (%) | 137 (32) |
| Donor | |
| Allo related, n (%) | 154 (36) |
| Allo unrelated, n (%) | 272 (63) |
| Auto, n (%) | 6 (1) |
| Stem cell source | |
| BM, n (%) | 64 (15) |
| PBSC, n (%) | 319 (74) |
| Cord, n (%) | 49 (11) |
| Cytogenetic risk before treatment | |
| Good, n (%) | 30 (7) |
| Intermediate, n (%) | 254 (59) |
| Poor, not MK, n (%) | 88 (20) |
| Poor, MK, n (%) | 60 (14) |
| Status at transplantation | |
| Remission, n (%)* | 353 (82) |
| Relapse, n (%) | 79 (18) |
MA indicates myeloablative; NMA, nonmyeloablative; and PBSC, peripheral blood stem cell.
The breakdown was 228 (65%) in first remission, 101 (29%) in second remission, 16 (4.5%) in greater than second remission, and 8 (2.3%) unknown.
As a control group, a subset of patients not receiving a transplant from the SWOG studies2 was used. Specifically, all patients from a transplantation study (S9034) or those who later underwent HCT were excluded. Among the remaining patients not receiving a transplant, we included the 432 patients in CR1 who survived ≥ 6 months from the date of initial registration. This allowed us to account for the fact that the patients who underwent a HCT had to remain in remission for a minimum time before HCT could be performed, whereas the patients not undergoing HCT did not “guarantee time.” The characteristics of patients in this control group are summarized in Table 2.
Table 2.
Patient characteristics of the nontransplantation control cohort (n = 432)
| Value | |
|---|---|
| Median age, y (range) | 51 (17-79) |
| Age | |
| 0-17 y, n (%) | 1 (0.2) |
| 18-30 y, n (%) | 54 (13) |
| 31-40 y, n (%) | 77 (18) |
| 41-50 y, n (%) | 84 (19) |
| 51-60 y, n (%) | 130 (30) |
| 61-80 y, n (%) | 102 (20) |
| Sex | |
| Male, n (%) | 235 (54) |
| Female, n (%) | 197 (46) |
| Cytogenetic risk | |
| Good, n (%) | 83 (19) |
| Intermediate, n (%) | 221 (51) |
| Poor, not MK, n (%) | 68 (156) |
| Poor, MK, n (%) | 22 (5) |
| Unknown, n (%) | 38 (9) |
| Status at reference date* | |
| Remission (in CR1), n (%) | 368 (85) |
| Relapse, n (%) | 64 (15) |
Reference date is 6 months after registration for SWOG studies.
Cytogenetic studies
At diagnosis or evaluation before and after transplantation, samples from BM aspirates were tested for cytogenetic abnormalities with the use of standard culturing and G-banding analysis at SCCA. Karyotype designation was based on the International System for Human Cytogenetic Nomenclature.6 Only clonal abnormalities were considered as positive results. Abnormalities were considered clonal if ≥ 2 metaphases had the same aberration in case of a structural abnormality or an extra chromosome or if ≥ 3 shared the same abnormality in case of a monosomy. The karyotype analysis was based on 20 metaphases for each sample as a routine procedure. Cytogenetic studies of the SWOG patients were described previously.2
Statistical analysis
The χ2 test was used to compare proportions. Overall survival (OS) from the date of transplantation or the date of registration to study (typically around the time of diagnosis) plus 6 months (denoted the reference date) for the nontransplantation cohort was used as the endpoint for survival. The Kaplan-Meier method was used to estimate survival curves. The median follow-up time of surviving patients was 36 months (range, 6-98 months). Cox regression analysis was used to compare survival between groups, adjusting for age at transplantation (0-40 years, 41-60 years, and 61-80 years), conditioning regimen (myeloablative, nonmyeloablative), disease status at transplantation (relapse, remission), and months from diagnosis to transplantation (0-18 months, > 18 months). All P values are 2-sided and reflect the mortality hazard ratio (HR) analysis, not an analysis of survival percentages at a fixed time.
Results
Incidence of cytogenetic abnormalities in patients with AML
Table 3 summarizes cytogenetic findings in the 432 patients who received a transplant. According to conventional cytogenetic risk classification,7–9 good-risk patients (including core binding factor AML and promyelocytic leukemia) accounted for 7% of these 432, intermediate risk (including patients with a normal karyotype) for 59%, and poor risk for 34%. A complex karyotype, defined as ≥ 3 chromosomal abnormalities, was noted in 66 patients (15% of all 432). Of the 148 total poor-risk patients, 60 (41%) had a MK. More specifically, 23 patients had a complex but not MK, 17 had a MK but not complex karyotype, 43 had both a MK and a complex karyotype, and 65 had neither a MK nor a complex karyotype but rather had simple but prognostically unfavorable abnormalities such as monosomy 7. However, 68% of patients with monosomy 7 had a MK. Among patients with a MK the most common monosomies involved chromosomes 7, 17, 5, 18, and 13. Among these, only monosomy 7 was also seen in MK− patients in this cohort.
Table 3.
Characteristics of patients with AML with and without MK who received a transplant and associated 4-year OS
| No MK |
MK |
|||
|---|---|---|---|---|
| n (%) | 4-y OS, % | n (%) | 4-y OS, % | |
| All patients | 372 (86) | 56 | 60 (14) | 25 |
| Age | ||||
| 0-40 y | 128 (91) | 63 | 12 (9) | 56 |
| 41-60 y | 170 (85) | 57 | 31 (15) | 28 |
| 61-80 y | 74 (81) | 44 | 17 (19) | 6 |
| Sex | ||||
| Male | 198 (86) | 53 | 31 (14) | 30 |
| Female | 174 (86) | 61 | 29 (14) | 18 |
| Cytogenetic risk before treatment | ||||
| Good | 30 (100) | 48 | 0 | |
| Intermediate | 254 (100) | 59 | 0 | |
| Poor | 88 (59) | 52 | 60 (41) | 25 |
| Monosomy | ||||
| −7 | 16 (32) | 41 | 34 (68) | 26 |
| −17 | 0 | 19 | 13 | |
| −18 | 0 | 17 | 20 | |
| − 5 | 0 | 15 | 0 | |
| −13 | 1 | 13 | 39 | |
| Complex | ||||
| No | 349 (95) | 57 | 17 (5) | 46 |
| Yes | 23 (35) | 51 | 43 (65) | 17 |
| Remission | ||||
| First remission | 193 (45) | 65 | 35 (8) | 30 |
| Second remission | 97 (22) | 54 | 4 (1) | 25 |
| All others | 82 (19) | 39 | 21 (5) | 16 |
The proportion of patients with MK+ AML increased with age. Only 9% of patients younger than age 40 years had MK+ disease, but the percentage increased to 19% for patients older than age 60 years (Table 3; P = .07). No sex distribution differences were noted in the proportion of patients with MK (P = .82).
Prognostic effect of MK in relation to age, type of monosomy, and complex karyotype
The 4-year OS from the date of transplantation was 48% for patients with good risk cytogenetics, 59% for those with intermediate risk cytogenetics, and 52% for those with poor risk disease without MK (Table 3). The presence of MK among the poor-risk group decreased the 4-year OS to 25%. Increasing age was associated with poorer survival, and this effect was more pronounced among patients with MK. Among patients aged 41- 60 years, 4-year OS was 57% for those without MK and 28% for those with MK (adjusted HR = 3.15, P < .0001). Among patients aged 61-80 years, 4-year OS was 44% for those without MK and only 6% for those with MK (adjusted HR = 2.23, P = .009). The mortality was largely because of disease recurrence, accounting for 63% in MK− patients and 68% in MK+ patients. For MK+ patients older than age 60 years, relapse-related mortality accounted for 75%. The remaining mortality was considered transplantation associated. Among the 17 MK+ patients older than age 60 years, 65% used nonmyeloablative conditioning regimen, including the single patient alive at 3 years after transplantation.
Among the MK+ patients, specific monosomies did not appear to differ in the prognosis, except for monosomy 5. Fourteen of the 15 MK+ patients with monosomy 5 did not survive to 18 months after transplantation. The estimated overall 4-year survival was 0% for this group (Table 3).
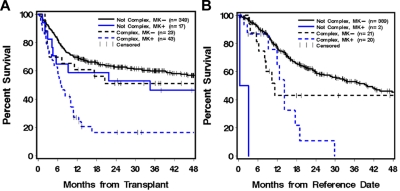
Because of the association in our study between a complex karyotype and a MK and because a previous study showed that the prognostic effect of a complex karyotype largely reflected this association in patients who generally did not receive a HC transplant,1 we analyzed the prognostic significance of MK in relation to complex karyotype among our patients who received a transplant. MK did not appear to significantly worsen the prognosis of patients without a complex karyotype (adjusted HR = 1.40, P = .31), but it appeared to decrease the survival of patients with a complex karyotype (adjusted HR = 2.00, P = .11; Table 3; Figure 1A).complex karyotype decreased the 4-year OS from 46% to 17% (adjusted HR = 2.70, P = .03) even though other characteristics of the patients were similar (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Among MK− patients, the association of complex karyotype with survival was less pronounced and not statistically significant (HR = 1.50, P = .20; Figure 1A).
Figure 1.
OS of MK and complex karyotype. OS of MK in relation to complex karyotype among patients with AML undergoing HCT (A). Complex karyotype with MK+ patients showed worse survival than other groups (adjusted P = .03). Results from the nontransplantation control group are shown (B).
To account for the fact that the patients who underwent HCT had to remain in remission for a minimum time before HCT could be performed and the patients not undergoing HCT did not “guarantee time,” we included only patients who survived for > 6 months from the date of registration to SWOG studies in the nontransplantation control group; 85% were still in CR1 at this reference date. For this control group, the overall 4-year OS was 0% for MK+ patients (n = 22) compared with 42% for MK− patients (n = 410) and with 25% for MK patients who underwent HCT. Figure 1B shows outcomes according to MK status and presence of complex karyotype. Among patients with a complex karyotype, MK+ decreased the 4-year OS from 42% to 0%, but the difference was not significant by multivariate analysis, given the small sample size (adjusted HR = 1.83, P = .13; Figure 1B).
Discussion
The newly defined subgroup of cytogenetic abnormalities, MK, has been shown to convey an unfavorable prognosis in AML, particularly among older patients.1,2 However, because it was unclear how many of these patients had undergone HCT and because of a suggestion that HCT might be beneficial in MK,2 we examined the effect of MK on 4-year survival among patients with AML undergoing HCT.
MK was identified in 14% of patients and was more frequent with increased age at transplantation, present in only 9% of patients age 40 years or younger but in 19% of patients older than 60 years. Consistent with previous reports,1,2 MK was only seen in patients with poor-risk cytogenetics, accounting for 41% of this group (Table 3). Similar to the experience with chemotherapy, MK status in the context of allogeneic transplantation was able to effectively divide the poor-risk cytogenetic group into 2 subgroups, patients without MK who had a better outcome and patients with MK who did much worse. However, after transplantation the outcomes appeared much improved, with a 4-year survival of 52% in patients who were MK− and 25% in patients who were MK+, outcomes that appear to be improved from the 14% and 3% 4-year survival rates, respectively, seen in the SWOG study in which therapies were largely chemotherapy based.2 The differences in the above-mentioned numbers are not entirely because of the different treatments. It is undoubtedly the case, for example, that patients who were able to survive to transplantation had a better inherent prognosis than patients who did not receive a transplant. Answering the question of the true effect of allogeneic HCT on MK+ AML would require identifying patients at diagnosis and then allocating them to transplantation versus nontransplantation strategy at a time when both options were possible. Despite the issues of selection bias, similar to that occurring in single-arm phase 2 studies, we believe such bias is unlikely to account for all of the difference between 25% and 3% in 4-year survival. To partially address this issue, we used, as a control group, a subset of the patients from SWOG studies who achieved CR1 and survived ≥ 6 months without later undergoing HCT. The nontransplantation cohort had similar demographic and cytogenetic characteristics to the HCT cohort (Tables 1–2), although obviously there are many other factors that determine which patients are referred for and undergo HCT. The 4-year OS for the MK+ patients in this control group was 0% compared with 25% in the HCT cohort (Figure 1). Although not allowing for a firm conclusion and by no means a substitute for a randomized comparative study, these data support the efficacy of allogeneic HCT for MK+ AML.
Compared with a recent study of 212 patients with AML that reported a 3-year OS rate of 40% for MK+ patients who received a transplant during CR1 and 17% for MK+ patients who received a transplant during CR2,10 the 4-year OS from our MK+ cohort was 30% for patients who received a transplant in CR1 and 25% for patients who received a transplant during CR2 (Table 3). However, it is hard to directly compare the results because of other potential differences in the study population. For instance, our patients were 5 to ∼ 6 years older in median age than the other study. We further addressed the effects of age and complex karyotype on the outcome of patients who underwent HCT in relation to MK, which has not been defined previously.
Age remains an important independent prognostic factor in patients with AML undergoing HCT, because the negative effect of MK in younger patients (< 40 years) was much weaker. In older patients, our data showed a 4-year OS of 6% among MK patients older than age 60 years, not substantially higher than the SWOG data of < 1% among patients 41 years and older.
As shown previously, the type of autosomal monosomy present did not lead to differences in outcome in general.1 However, our data showed a particularly dismal prognosis for monosomy 5, with a 0% 4-year OS compared with 13%-39% of 4-year OS associated with other common monosomies, such as −7, −13, and −17 in our cohort (Table 3).
Of particular interest is the effect of complex karyotype in relation to MK on the outcome of patients with AML who received a transplant. Unlike in the nontransplantation setting in which the effect of complex karyotype is lost when MK is taken into consideration, MK patients with a complex karyotype who received a transplant have a much worse outcome than MK patients without a complex karyotype. In the transplantation setting, complex karyotype may have less prognostic value among non-MK patients, but the number of such patients in our cohort was small and limited our ability to evaluate this subgroup. Future studies with larger patient cohorts are warranted.
Overall, survival after HCT appears mostly affected by disease relapse rate rather than transplantation-associated mortality because our data showed approximately two-thirds of patients, both MK− and MK+, died of relapse. Among MK+ patients older than 60 years, the relapse-associated mortality was 75%. Therefore, intensifying the regimen may be considered to further improve outcome.
In summary, our analysis of patients with AML treated with HCT suggests that transplantation may partially overcome the poor prognostic effect of MK, particularly in patients older than 61 years. In older patients with MK+ AML both current HCT and non-HCT approaches are quite unsuccessful, stressing the need for new approaches for such patients.
Supplementary Material
Acknowledgments
B.M.S. was supported by the National Institutes of Health (CA78902 and CA018029).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.F., B.S., E.E., and F.R.A. reviewed and analyzed the data and wrote the manuscript; L.Z., M.F., and B.M.S. collected the data; and M.O. analyzed the SWOG data. All authors contributed to writing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Min Fang, Fred Hutchinson Cancer Research Center, 825 Eastlake Ave E, G7-500, Seattle, WA 98109-1023; e-mail: mfang@fhcrc.org.
References
- 1.Breems DA, Van Putten WL, De Greef GE, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26(29):4791–4797. doi: 10.1200/JCO.2008.16.0259. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of monosomal karyotype in young adult and elderly acute myeloid leukemia: the Southwest Oncology Group (SWOG) experience. Blood. 2010;116(13):2224–2228. doi: 10.1182/blood-2010-02-270330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrot A, Luquet I, Pigneux A, et al. Prognostic value of the ‘monosomal karyotype’ entity in elderly patients with AML: a GOELAMS study of 186 patients with unfavourable cytogenetic abnormalities [abstract]. Blood. 2009;114(22) doi: 10.1182/blood-2010-09-307264. Abstract 1577. [DOI] [PubMed] [Google Scholar]
- 4.Granada I, Brunet S, Hoyos M, et al. Prognostic value of monosomal karyotype in patients with primary acute myeloid leukemia on behalf of Spanish CETLAM Group [abstract]. Blood. 2009;114(22) Abstract 1003. [Google Scholar]
- 5.Ahn HK, Kim D, Park S, et al. Monosomy karyotype in acute myeloid leukemia predicts adverse treatment outcome with low complete remission rate and worse event-free and overall survival, and associates with high functional activity of multidrug resistance (MDR) [abstract]. Blood. 2009;114(22) Abstract 3112. [Google Scholar]
- 6.Shaffer LG, Slovak ML, Campbell LJ. An International System for Human Cytogenetic Nomeclature. Basel, Switzerland: S. Karger Publishers Inc; 2009. [Google Scholar]
- 7.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 8.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92(7):2322–2333. [PubMed] [Google Scholar]
- 9.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96(13):4075–4083. [PubMed] [Google Scholar]
- 10.Oran B, Dolan M, Cao Q, Brunstein C, Warlick E, Weisdorf D. Monosomal karyotype provides better prognostic prediction after allogeneic stem cell transplantation in AML patients. Biol Blood Marrow Transplant. 2011;17(3):356–364. doi: 10.1016/j.bbmt.2010.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.