Summary
In the first volume of Developmental Cell, it was reported that the classic Drosophila neurogenic gene neuralized encodes a ubiquitin ligase that monoubiquitylates the Notch ligand Delta, thus promoting Delta endocytosis. A requirement for ligand internalization by the signal-sending cell, although counterintuitive, remains to date a feature unique to Notch signaling. Ten years and many ubiquitin ligases later, we discuss sequels to these three papers with an eye towards reviewing the development of ideas for how ligand ubiquitylation and endocytosis propel Notch signaling.
Introduction
The Notch pathway is an evolutionarily conserved signaling system deployed over and over again during development to direct the specification of almost every cell type in the metazoan body plan (Fortini, 2009). Given this widespread use of Notch signaling, it is not surprising that both gains and losses in Notch signaling are associated with inherited human disorders and cancer. The identification of Notch as a potential therapeutic target, along with its importance in manipulating embryonic and adult stem cells, underscore the need to understand how Notch ligands activate signaling and how Notch activation is regulated spatially. Ten years ago three papers in Volume 1 of Developmental Cell suggested a potential role for ubiquitylation of ligands as a mechanism to regulate Notch signaling (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001). Here we review what was known then, what is known now, and discuss these findings in the context of what we yet need to know to fully understand the role of ligand ubiquitylation in Notch signaling.
Both the Notch receptors and ligands are cell surface proteins, and while the requirement for direct cell-cell contact in Notch signaling offers a mechanism for cells to communicate and respond to each other, the transmembrane nature of the Notch ligands also appears fundamental to ligand activation of Notch receptors on the surface of neighboring cells (D'Souza et al., 2008; Nichols et al., 2007b). Endocytosis had long been recognized as an important mechanism to down-regulate cell surface receptors following ligand activation and to contribute to signal propagation and intensity. The idea, however, that ligands on the surface of a signal-sending cell must be internalized to activate Notch on the signal-receiving cell suggested a novel role for endocytosis in activation of a signaling pathway. Roles for ligand endocytosis both before and following Notch engagement have been proposed, and models to account for ligand ubiquitylation in activation of Notch signaling in these contexts will be discussed in this review.
Mechanisms that restrict Notch signaling to one of two interacting cells are paramount to the timing and acquisition of the correct cell fate during development (Fortini, 2009). While cells that receive the Notch signal induce developmental programs distinct from those sending the signal, the specific outcome is often context-dependent. In fact, the effects of Notch signaling on tissue patterning and morphogenesis may involve either positive or negative regulation of cellular differentiation, proliferation, survival and apoptosis. In its most classic example, Notch signaling mediates a process of lateral inhibition to restrict cell fates among bipotential progenitors, such that losses in Notch signaling lead to the expansion of one cell fate at the expense of another. Specifically, in the ventral ectoderm of the developing Drosophila embryo, loss-of-function mutations in Notch result in massive expansion of neural cells at the expense of the epidermal fate (Poulson, 1940; Poulson, 1937). This so-called neurogenic phenotype is the most famous of the Notch phenotypes that presents as embryonic lethality. Indeed, it was this embryonic lethal phenotype that allowed the identification of a small group of “neurogenic genes” that both phenocopy and interact genetically with Notch (Lehmann et al., 1983). These genes turned out to function also in numerous developmental events outside the nervous system, reflecting the pleiotropic nature of the Notch signaling pathway. Although the neurogenic genes do indeed encode protein components of the Notch pathway, it has been a challenge to understand the roles of these proteins in the mechanics of Notch signaling.
Neuralized is REALLY an E3 ubiquitin ligase
neuralized (neur) is one of the original zygotic lethal neurogenic genes (Lehmann et al., 1983). However, nearly two decades passed before the biochemical basis of Neur activity in Notch signaling was realized. The initial cloning of the neur gene in the early 1990s revealed a pioneer protein containing putative DNA-binding sequences similar to bacterial repressors, suggesting that Neur might encode a regulator of transcription (Boulianne et al., 1993). Specifically, Neur was found to encode a new type of zinc finger that in 1993 was dubbed the “RING finger” (Lovering et al., 1993), initially identified for the human RING1 gene (really interesting new gene 1). Identification of this RING motif in another 27 putative DNA-binding proteins defined a new family of RING domain proteins (Deshaies and Joazeiro, 2009). In 1996, the RING finger motif was reclassified as a zinc-binding domain distinct from other zinc-fingers, and rather than binding DNA, it was proposed to mediate protein-protein interactions (Deshaies and Joazeiro, 2009). Nevertheless, the actual function of a RING domain remained a mystery until a series of papers published between 1997 and 1999 identified ubiquitin-ligase activity associated with the RING that allowed direct transfer of ubiquitin (Ub) to substrates (Deshaies and Joazeiro, 2009). Together, these studies implied that most, if not all, members of the large RING domain family might be E3 Ub ligases, instantly expanding the number of known E3 ligases. This was an important advance, because if E3s provided substrate specificity as proposed, then large numbers should exist to account for the ubiquitous nature of this post-translational modification. At the time, however, few E3s had been identified. This prediction turned out to be correct and today more than 600 human genes have been identified that encode RING-based E3 Ub ligases (Deshaies and Joazeiro, 2009).
Spurred on by these findings, four papers published in 2001 (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001; Yeh et al., 2001) presented biochemical evidence that the Neur RING domain had in vitro E3 Ub ligase activity. Importantly, genetic studies in this work indicated that the RING domain was necessary for Neur to rescue the neurogenic phenotype of neur mutant embryos.
Neuralized ubiquitylates Notch ligands to target them for endocytosis
Providing a possible clue as to how Neur might regulate Notch signaling, studies from both flies and frogs (Deblandre et al., 2001; Lai et al., 2001; Pavlopoulos et al., 2001; Yeh et al., 2001) identified the Notch ligand Delta as a substrate for the Neur RING domain E3 Ub ligase activity. Consistent with this idea, Neur localized to the plasma membrane, physically interacted with Delta, and promoted Delta ubiquitylation. Surprisingly, the addition of Ub to Delta stimulated its removal from the cell surface via endocytosis and correlated with a loss in Delta protein. Although the role of Ub in proteasome-dependent proteolysis was well established, recent reports had suggested that this modification might serve additional functions (Hicke and Dunn, 2003). In fact, ubiquitylation, and in particular, mono-ubiquitylation was beginning to be appreciated as a signal for endocytosis of transmembrane proteins (Hicke, 2001). In vitro ubiquitylation assays indicated that Neur monoubiquitylated Delta, consistent with Neur ubiquitylation inducing Delta endocytosis. The distinct sites and types of ubiquitylation for endogenous Notch ligands induced by Neur have yet to be established. In this regard, it is important to note that Ub is linked covalently to lysine residues, and two particular lysines in the intracellular domain of Delta (and also in the other Drosophila ligand Serrate, later shown to be a Neur substrate as well) have been since implicated in ligand signaling activity (Glittenberg et al., 2006; Parks et al., 2006). Moreover, analyses of frog and fly embryos and tissues, both live and fixed, indicated that in the absence of Neur activity, Notch ligands accumulate on the cell surface (De Renzis et al., 2006; Le Borgne and Schweisguth, 2003b; Pavlopoulos et al., 2001; Deblandre et al., 2001; Lai et al., 2001), presumably because they lack Ub to direct their internalization. Together these findings suggested that Neur at the plasma membrane interacted with and ubiquitylated Delta, possibly to regulate the level of cell surface ligand available to activate Notch receptors on neighboring cells and establish polarity of signaling during lateral inhibition.
Counterintuitively, Neuralized functions in signaling cells
Seemingly at odds with the idea that Neur promotes Delta endocytosis, the neurogenic phenotype resulting from loss of neur was thought to result from the failure of Notch signaling, implying that Neur is a positive effector of Notch signaling. How could removal of ligand from the cell surface and its subsequent degradation promote Notch signaling? As Neur had been reported to function cell autonomously in Notch signaling (Lai and Rubin, 2001a, b; Yeh et al., 2000) the Neur-induced degradation of Delta was proposed in two of the original Developmental Cell papers (Deblandre et al., 2001; Lai et al., 2001) to relieve the inhibitory effects produced by ligand binding to Notch in the same cell, a phenomenon called receptor cis-inhibition. According to this model, Neur-mediated ubiquitylation of Delta targets ligand for endoctyosis and subsequent degradation, thus decreasing the amount of ligand available for binding to Notch in cis. Removal of cis-inhibiting ligand would allow Notch to bind ligand in trans on adjacent cells, leading to receptor activation. By contrast, in the absence of Neur, Delta would not be ubiquitylated or degraded, and thus accumulate to high levels to effectively cis-inhibit Notch receptors and block Notch activation cell autonomously. Although a logical rationale for how Neur-mediated Delta endocytosis could promote Notch receptor activation (and an idea not without merit – we discuss below how Neur might have a subsidiary role in cis-inhibition), this model, nonetheless, turned out to be wrong.
We now know, as proposed in one of the original Developmental Cell papers (Pavlopoulos et al., 2001), and later reinforced by additional genetic mosaic experiments (del Alamo and Mlodzik, 2006; Le Borgne and Schweisguth, 2003b; Li and Baker, 2004; Overstreet et al., 2004), that Neur function is necessary in the signaling cells in flies, and is highly expressed and localized to cells that send rather than receive Notch signals. Although Neur is not likely to function in Notch signal reception, enhanced Neur expression can indeed antagonize ligand cis-inhibition of receptor in a cell autonomous manner as proposed initially (Glittenberg et al., 2006). In the Drosophila wing and eye, receptor cis-inhibition by ligand is an essential mechanism for determination of cell fate (reviewed in del Alamo et al., 2011). In both contexts, ligand ubiquitylation by Neur is not necessary for cis-inhibition of receptor, as Neur deficient cells cannot activate Notch in adjacent cells, but can cis-inhibit receptor (Glittenberg et al., 2006; Miller et al., 2009). However, Neur overexpression represses cis-inhibition, possibly by clearing ligand from the plasma membrane, leaving open the possibility that Neur may regulate Notch activation in receiving cells by modulating the amount of ligand available to cis-inhibit receptor (Glittenberg et al., 2006).
Nonetheless, the requirement for Neur in signaling cells presents an obvious paradox. How could removal of ligand from the cell surface induced by Neur ubiquitylation enhance ligand signaling potential? And how might we explain the even more bizarre observation that cell surface ligand accumulates in the absence of Neur but fails to activate Notch signaling? Further complicating our understanding of Neur’s role in Notch signaling, not all Notch-dependent developmental events in flies appeared to require Neur. Could it be that Neur is a regulator rather than a core component of the Notch signaling pathway? Alternatively, if Neur ubiquitylation is required absolutely to generate ligand-signaling activity, additional E3 Ub ligases must exist that function in Neur-independent processes that require Notch signaling.
Enter Mind bomb, another Notch ligand E3 Ub ligase
The original zebrafish mutagenesis screens published in 1996 described several mutants with Notch loss-of-function phenotypes that identified components of the Notch signaling pathway, in particular Notch ligands and receptors. Despite the strong Notch-like neurogenic phenotype described for the mind bomb (mib) mutant, genetic linkage analysis failed to identify any of the usual suspects. A study published in Developmental Cell in 2003, identified mib using a positional cloning approach (Itoh et al., 2003). Surprisingly, mib was found to encode three RING domains, raising the interesting possibility that Mib, although structurally distinct from Neur, was also a E3 Ub ligase that functioned in Notch signaling (Chen and Corliss, 2004; Itoh et al., 2003). Indeed, the mouse homolog of Mib, called DIP-1 (for DAPK-interacting protein-1), had been reported to bind and polyubiquitylate the death-associated protein kinase (DAPK) to signal proteasome degradation of this important apoptosis regulator (Jin et al., 2002).
As found for DIP-1, the Mib RING domains had E3 Ub ligase activity and satisfyingly, Mib bound and ubiquitylated Xenopus Delta and zebrafish DeltaD (Chen and Corliss, 2004; Itoh et al., 2003). The identification of Notch ligands as additional Mib substrates likely accounted for the many different loss-of-Notch phenotypes described for zebrafish mib mutants. In fact, as found for Neur, Mib ubiquitylation promoted ligand endocytosis, although this did not appear to promote ligand degradation as previously reported for Neur (Deblandre et al., 2001; Lai et al., 2001). Importantly, elegant cell transplantation analysis suggested that Mib was required by the ligand signal-sending cell to activate Notch signaling during lateral inhibition (Itoh et al., 2003). Furthermore, the neurogenic phenotype produced by a form of Delta lacking intracellular domain (ICD) sequences was rescued by the addition of sequences encoding Ub, suggesting the presence of Ub on the ligand ICD (presumably attached to lysine residues by Mib normally) is required for ligand to activate Notch. Additionally, as found for Neur, ligands accumulated on the cell surface in Mib mutant cells but were defective in activating Notch signaling. Together, these findings were more consistent with a role for Mib ubiquitylation in generating ligand signaling potential through promoting ligand endocytosis rather than regulating levels of ligand for Notch activation.
The identification of zebrafish mib revealed the existence in fly, mouse and human genomes of two mib homologs, mib1 and mib2, suggesting conservation of this E3 Ub ligase throughout metazoans. It turns out that neither of the mib-related genes are expressed in the neurogenic region of developing embryos, accounting for their omission as classic neurogenic genes (Lai et al., 2005; Le Borgne et al., 2005; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005). It has also been suggested that differential expression of Drosophila Neur and Mib1 accounts for the requirements for these genes in distinct developmental events. In fact, we now know that Neur-independent Notch activation is due to the presence of Mib1, and in situations where both E3 ligases are expressed, simultaneous depletion of both Mib1 and Neur are required to produce loss-of-Notch phenotypes (Lai et al., 2005; Le Borgne et al., 2005; Pitsouli and Delidakis, 2005; Wang and Struhl, 2005). The finding that Neur and Mib1 function similarly in Drosophila provides strong support for ligand ubiquitylation as an absolute requirement for ligand signaling activity, rather than as a regulator of ligand levels or activity in limited developmental contexts. Consistent with this notion, both Neur and Mib1 bind and ubiquitylate both Delta and Serrate to induce endocytosis that correlates with ligand signaling potential (Lai et al., 2001; Lai et al., 2005; Le Borgne et al., 2005; Pitsouli and Delidakis, 2005).
Despite numerous examples of functional redundancy, Mib1 was unable to rescue the neurogenic phenotype produced by loss of Neur, suggesting that Neur may have additional functions not supplied by Mib1 in this particular context (Le Borgne et al., 2005). In fact, the identification and characterization of a phosphoinositide-binding domain at the N-terminus of Neur has suggested that Neur might participate in the mechanics of endocytosis in some way in addition to ubiquitylating ligand (Skwarek et al., 2007). Consistent with potential functional differences for these structurally distinct E3 ligases, Drosophila Mib2 functions to maintain muscle integrity and survival (Carrasco-Rando and Ruiz-Gomez, 2008; Nguyen et al., 2007), and neither Mib1 nor Neur rescue the Mib2 muscle defects, identifying a unique requirement for Mib2 in this context. Although, earlier myoblast fusion events in fly myogenesis require the Mib2 RING domains, implicating the Ub ligase activity, the later Mib2-dependent events in differentiating muscle do not require the RING domains and do not involve Notch signaling. That the role proposed for Mib2 in muscle attachment and stability requires neither E3 catalytic activity nor Notch signaling, while muscle fusion events require both, lends further support to the idea that ligand ubiquitylation is a critical event in Notch activation.
In vertebrates, Mind bomb, but not Neuralized, is required for Notch signaling
Studies in flies have demonstrated clearly a critical role for Neur in Notch signaling, which involves ligand ubiquitylation and endocytosis in the generation of a productive signal. It was therefore surprising when two groups reported in 2001 that mice lacking the mammalian neur homolog (neur1) do not display any obvious Notch-like developmental phenotypes (Ruan et al., 2001; Vollrath et al., 2001). Moreover, these mice are viable and fertile, which is in strong contrast to the mid-gestation lethality reported for gene knockouts of core components of the Notch signaling pathway. At the time of these findings, it was assumed that additional E3 Ub ligases must exist to account for the general lack of Notch-specific phenotypes in the neur1 mutant mice. Indeed, the subsequent identification and characterization of neur2 supported this view (Song et al., 2006). Specifically, Neur2 contains a RING domain that is necessary and sufficient for E3 ligase activity. Moreover, like Neur1, Neur2 binds to Xenopus and mouse Delta homologs, and when coexpressed with Delta in cells, ubiquitylates it. Although these findings left open the possibility that neur2 expression accounts for the lack of Notch signaling defects in neur1 knockout mice, this turned out not to be the case.
As found for neur1 knockout mice, gene targeting to produce neur2 null homozygotes resulted in viable offspring lacking obvious Notch signaling defects (Koo et al., 2007), again possibly due to compensation by Neur1. To determine definitively whether or not Neur activity is required for Notch signaling, mice homozygous for deletion of both neur1 and neur2 were generated, and again viable offspring were produced with no obvious Notch-like abnormalities (Koo et al., 2007). This surprising finding indicates that neither Neur1 nor Neur2 are required for normal mammalian development, viability, or survival. Given the potent neurogenic phenotypes associated with neur mutation in flies, it is especially significant that analysis of brains isolated from neur1 neur2 double knockout mice failed to show any obvious morphological defects. Moreover, animals defective in neur1, neur2 and mib2 gene expression also lack obvious Notch-dependent morphological phenotypes (Koo et al., 2007), suggesting that mib1 may be the only E3 ligase gene essential in the embryo for Notch signaling. Indeed, disruption of the mib1 gene alone produces the known constellation of Notch-like mutant phenotypes in developing mouse embryos (Barsi et al., 2005; Koo et al., 2005a).
Studies of Mib function in mammalian cell culture corroborate the role of Mib in Notch signaling. Cells expressing Notch ligands and lacking Mib1 do not activate Notch reporters or target gene expression in mammalian co-culture assays (Hansson et al., 2010; Yamamoto et al., 2010). Interestingly, the Mib2 homolog, skeletrophin, is aberrantly expressed in multiple myeloma cells where it binds and ubiquitylates the Notch ligand Jagged2 to activate Notch signaling in bone marrow stroma cells, which may promote and maintain the malignant state (Takeuchi et al., 2005). Importantly, ubiquitylated Jagged2 is not degraded in myeloma cells, which is in strong contrast to the effects of Jagged1 ubiquitylation by Neur1. Neur1-dependent ubiquitylation targets Jagged1 for degradation, and not surprisingly, this results in loss of Jagged1-induced Notch signaling in co-culture assays (Koutelou et al., 2008). Based on similar ectopic expression, Neur1 has been proposed to function as a tumor suppressor through degrading Jagged1 and preventing expression of Notch target genes required for the development of medulloblastoma (Teider et al., 2010). The down-regulation of Jagged1 by Neur1 is reminiscent of the initial studies in flies and frogs that placed a strong emphasis on Neur-induced ubiquitylation in targeting Notch ligands for degradation (Deblandre et al., 2001; Lai et al., 2001). Thus, overexpressed Neur ubiquitylates ligand, thereby stimulating ligand degradation and downregulation of Notch signaling, yet genetic studies suggest strongly that the effects of Neur overexpression do not reflect the normal functions of mammalian Neur ligases. Interestingly, studies in zebrafish have identified a structurally unrelated E3 ligase that targets DeltaA for Ub-based degradation, which is proposed to repress Notch signaling during lateral inhibition (Diks et al., 2006; Diks et al., 2008; Sartori da Silva et al., 2010).
There are studies in mammalian cells suggesting that Mib and Neur ubiquitylation have distinct effects on Notch ligands. For example, it has been proposed that Mib1 ubiquitylation stimulates Delta endocytosis, while ubiquitylation by Neur2 is needed for subsequent trafficking of Delta through an endosomal pathway (Song et al., 2006). Another study suggested that Neur2 stimulates transcytosis of Delta from the basolateral to apical plasma membrane in polarized mammalian epithelial cells (Benhra et al., 2010). Although the latter findings are similar to those described for Neur in the regulation of Delta signaling activity during Drosophila sensory organ development (see below), the Neur-induced Delta transcytosis observed in mammalian cells has yet to be linked to Notch signaling. Additionally, whether Delta transcytosis is unique to Neur and not mediated by Mib has not been reported. Finally, over expression of zebrafish Mib in mammalian cells, in contrast to that reported for Neur in flies (Glittenberg et al., 2006), cannot relieve the cis-inhibitory activity of ligands (Itoh et al., 2003). Therefore, despite clear roles for both Neur and Mib in numerous Notch-dependent events in flies, the current evidence in mammalian cells is most consistent with the idea that ubiquitylation of Notch ligands by Mib, but not Neur, potentiates their ability to activate Notch.
What about Neur in zebrafish? Mutants in neur1 or neur2 have not yet been analyzed in zebrafish, however, neither neur1 nor neur2 rescue the zebrafish mib1 neurogenic phenotype (Song et al., 2006). Assuming that appropriate levels of Neur1 and Neur2 were achieved in these rescue experiments, these findings argue against zebrafish neur and mib being functionally redundant. In contrast, rescue experiments in zebrafish indicate that Mib1 and Mib2 are functionally redundant (Koo et al., 2005b; Zhang et al., 2007b), even though they have overlapping and distinct substrate specificities for different Notch ligands (Zhang et al., 2007a). Similar rescue experiments for Mib1 and Mib2 in mice have not yet been reported.
Together, the data indicate that while Neur and Mib1 perform similar roles in Notch signaling in flies, the vertebrate Neur and Mib proteins are not functionally equivalent. So far, only Mib has been shown to play a role in Notch signaling in both flies and vertebrates. Although it is the rule rather than the exception for obvious homologs to maintain functional conservation, examples do exist where homologous proteins possess different biochemical properties and cellular activities in different organisms (see Dickinson et al Forum in this issue).
Why is Notch ligand endocytosis essential for signaling?
Loss of either Neur or Mib leads to accumulation of ligands on the cell surface, and paradoxically, the failure of the ligands to signal. The requirement for ligand endocytosis so far appears unique to the Notch signaling system. Nevertheless, why ligands need to be removed from the cell surface to activate the Notch receptor in adjacent cells has remained controversial for several years.
Before the discovery that neur encodes a Ub ligase, there was genetic evidence that endocytosis played a uniquely important role in Notch signaling in Drosophila. Genetic mosaic studies with the Drosophila shibire mutant published in 1997 showed that dynamin (encoded by the shibire gene) is required in both Notch signaling and receiving cells for sense organ development (Seugnet et al., 1997). As the GTPase dynamin is best known for its role in pinching-off endocytic vesicles from the plasma membrane, this finding presaged the critical role of ligand endocytosis in signaling cells. (The role of endocytosis in the receiving cells is another source of controversy (Fortini, 2009) that will not be discussed here.) A requirement for ubiquitylation of Delta in promoting Notch signaling through ligand endocytosis was foreshadowed also by the observation that Delta normally accumulates in endosomes in Drosophila tissues associated with active Notch signaling (Parks et al., 2000; Parks et al., 1995). In addition, Delta proteins that lack the intracellular domain, or with specific intracellular domain mutations, fail to signal and instead of accumulating in intracellular puncta, accumulate at the plasma membrane presumably because they are unable to undergo endocytosis (Chitnis et al., 1995; Dorsky et al., 1997; Henrique et al., 1997; Nichols et al., 2007a; Parks et al., 2006; Sun and Artavanis-Tsakonas, 1996). It was discovered later, however, that endosomal ligand is also present in some mutant cells that do not signal (Glittenberg et al., 2006; Overstreet et al., 2004; Wang and Struhl, 2004, 2005). This likely represents constitutive ligand endocytosis that regulates cell surface ligand levels, which is distinct from ligand endocytosis that activates Notch. Neur and/or Mib are required for signaling-specific internalization, and at least sometimes also for constitutive endocytosis of Notch ligands (Wang and Struhl, 2005). Therefore, not all ligand endocytosis can be considered a direct consequence of active Notch signaling, a fact that often complicates interpretation of in vivo studies where Notch is ubiquitous (Matsuda and Chitnis, 2009). In fact, the majority of Jagged1 endocytosis in mammalian cells appears to be Mib1 independent, but Mib is required absolutely for ligand to activate Notch signaling (Yamamoto et al., 2010).
The discovery that the endocytic adaptor protein epsin is essential specifically in Notch signal-sending cells in Drosophila, C. elegans, and in mice linked the critical requirements for ligand ubiquitylation and endocytosis (Chen et al., 2009; Overstreet et al., 2003; Overstreet et al., 2004; Tian et al., 2004; Wang and Struhl, 2004). Epsin binds ubiquitylated cargo, and promotes both clathrin-dependent and clathrin-independent endocytosis (Chen and De Camilli, 2005; Chen et al., 1998; Sigismund et al., 2005).
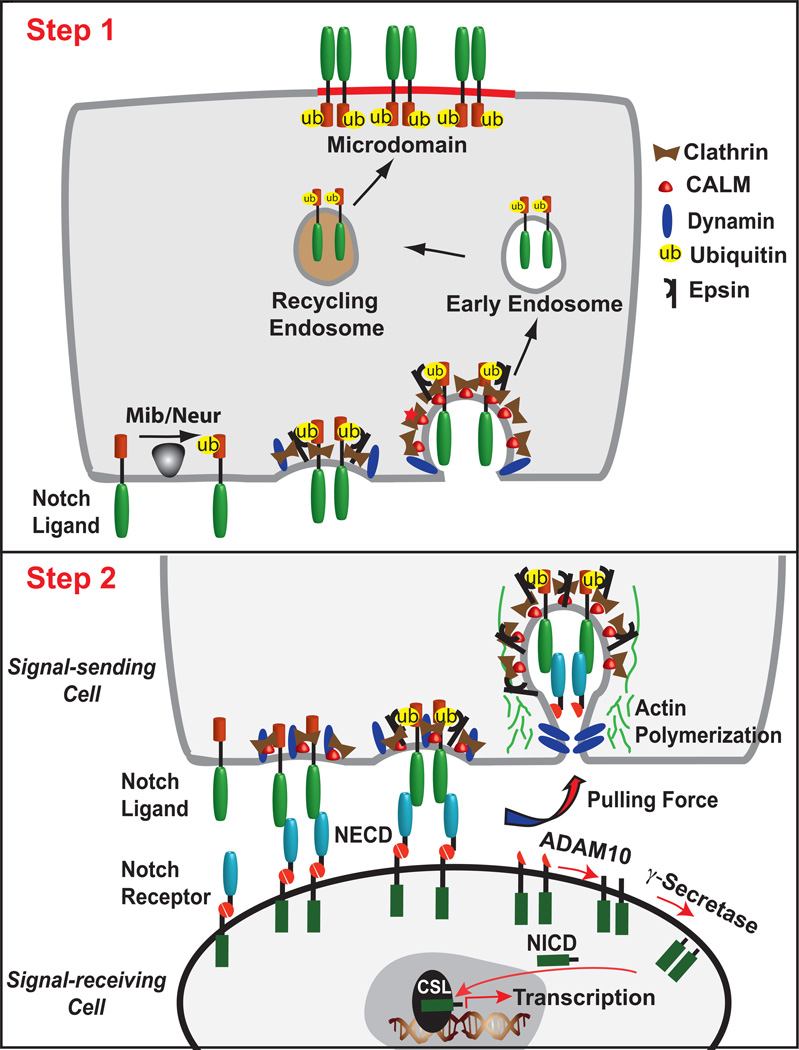
We will discuss below how studies in flies and mammalian cells have suggested that ligands may undergo two distinct endocytic events to activate Notch. The first ligand endocytic event would occur prior to engagement with Notch to facilitate recycling in the generation of an active ligand (Figure, Step 1). Following interactions with Notch on adjacent cells, a second ligand endocytic event generates a pulling force to allow activating Notch proteolysis (Figure, Step 2). Although there is evidence to support aspects of both these models, it is yet unclear whether the first, second, or both endocytic events are necessary for ligands to activate Notch signaling. Moreover, how ligand ubiquitylation would function in either ligand recycling or the generation of a pulling force remains to be determined.
Figure. Schematic of proposed routes and outcomes for Neur/Mib-dependent ligand endocytosis.
Step 1: Ligand delivered to the cell surface is ubiquitylated by either Mib or Neur, which facilitates interactions with the endocytic adaptor epsin to promote ligand endocytosis. Following internalization, ligand delivered to the early endosome enters the recycling endosome from which it is returned to the plasma membrane. In this scenario, Neur-mediated ubiquitylation serves as a signal for ligand transcytosis to a specific micro-domain conducive to signaling. Step 2: The critical role of ligand endocytosis is to exert a pulling force on the Notch receptor in adjacent cells. To overcome resistance to endocytosis of ligand bound to Notch on an adjacent cell, ligand is proposed to harness mechanical force inherent to endocytosis to dissociate the Notch extracellular domain (NECD) from the intact Notch heterodimer. Internalization of ligand-bound NECD exposes the remaining membrane-associated Notch to activating proteolysis, first by ADAM followed by γ-secretase to release the Notch intracellular domain (NICD) that moves to the nucleus to directly participate in transcription of Notch target genes.
Ubiquitylation to promote ligand recycling
The recycling model assumes that newly synthesized ligand delivered to the cell surface cannot activate Notch and requires endocytosis, trafficking and recycling back to the cell surface to gain signaling activity (Figure Step 1). Ligand recycling was proposed initially as one of several creative explanations to account for the requirement of ligand endocytosis in Notch signaling. In its original formulation, the idea was that ligand would be “activated” by packaging into exosomes (Le Borgne and Schweisguth, 2003a). Later, Wang and Struhl (2004) proposed a different recycling model, based on two observations about epsin function in wing imaginal discs. First, they observed that epsin is not required for constitutive endocytosis of Delta, but is needed absolutely for ligand endocytosis associated with Notch signaling. Second, they observed that if the intracellular domain of Delta is replaced with known internalization and recycling signals, the recombinant Delta signals in an epsin-independent manner. Taken together, these observations suggested that epsin may target ubiquitylated Delta to a specific endosomal pathway, in which it is activated and recycled back to the plasma membrane in an “active” form. The authors showed further that a presumed proteolytically processed form of Delta present in wild-type cells is absent in cells lacking epsin. However, it is notable that to date no one has identified an “activated” form of ligand, and a more recent study of ligand recycling failed to obtain evidence for Delta processing (Rajan et al., 2009).
Nevertheless, the recycling idea was reinforced by two papers proposing that in sensory organ development, the recycling proteins Sec15 and Rab11 may work in signal-sending cells to promote Delta recycling and thereby signaling (Emery et al., 2005; Jafar-Nejad et al., 2005). However, in neither paper was it shown directly that Sec15 or Rab11 are required in the signal-sending cell to obtain an active ligand. In addition, two more recent papers proposed that Neur-dependent Delta trafficking from the basolateral membrane to an apical actin-rich structure (transcytosis) juxtaposes Delta with Notch on adjacent cells and thus enables it to signal (Benhra et al., 2010; Rajan et al., 2009). As no activated form of Delta has been identified, this role for recycling seems most plausible. In fact, recycling is well known to function in the spatial positioning of signaling receptors and effectors to ensure specific cellular responses. Additionally, recycling could serve to regulate the level of cell surface ligand available to activate Notch, and thereby account for the signaling defects reported for non-polarized Delta1-OP9 cells defective in Rab11 recycling (Emery et al., 2005).
The requirement for ligand recycling in Drosophila may actually be restricted to polarized cells as reported for sensory organ precursors (SOPs). First, Rab11 is not required for all Notch signaling events. Particularly, loss of Rab11 activity does not perturb Delta signaling in the germline or in the developing eye (Banks et al., 2011; Windler and Bilder, 2010). Second, Rab5 is also not required for signaling in the germline and probably not in the eye either (Banks et al., 2011; Windler and Bilder, 2010); if recycling is required to generate an active ligand, then Rab5 GTPase activity, which is prerequisite for entry into the Rab11 recycling pathway, should also be essential. Finally, experiments with Drosophila auxilin mutants also suggest that recycling is not the primary role of ligand endocytosis in Notch signaling. Auxilin is an endocytic protein that stimulates uncoating of newly endocytosed clathrin-coated vesicles. Auxilin is required for ligand endocytosis and signaling in all developmental contexts tested except for the germline (Banks et al., 2011; Eun et al., 2008; Eun et al., 2006; Hagedorn et al., 2006; Kandachar et al., 2008). It has been observed that overexpression of clathrin and/or epsin bypasses most of the requirement for auxilin in Notch signaling (Banks et al., 2011; Eun et al., 2008). This means that the function of auxilin in signaling cells is to maintain the pool of free clathrin and possibly also epsin, rather than to uncoat ligand-containing vesicles efficiently for passage through a recycling pathway. Assuming that in the absence of auxilin, vesicle uncoating remains inefficient even when clathrin and/or epsin are overexpressed, this result implies that passage of ligand through an endosomal pathway is not essential for ligand to activate receptor. Taken together, these three observations argue that ligand recycling is not a general, core feature of Notch activation. Nonetheless, recycling to relocalize ligand appears to be a feature of Notch signaling specific to polarized cells, and this has provided the key support for the recycling model.
Ligand ubiquitylation recruits epsin to produce a pulling force
Notch signals through a remarkable mechanism that involves a series of proteolytic events that result ultimately in the release of the Notch intracellular domain (NICD) allowing it to move to the nucleus where it directly activates expression of Notch target genes (Fortini, 2009). In Drosophila imaginal discs, Parks et al. (2000) were the first to show that the Notch extracellular domain (NECD) colocalizes with Delta in endosomes in cells known to activate Notch signaling in neighboring cells. In a leap of prescient imagination, the authors proposed that endocytosis of Delta would impart a molecular strain on bound Notch to separate the NECD from intact Notch and result in activating proteolysis for downstream signaling. In support of this model, NECD is localized to intracellular vesicles in Delta cells following coculture with mammalian cells expressing Notch (Hansson et al., 2010; Heuss et al., 2008; Nichols et al., 2007a). Importantly, losses in ligand endocytosis prevent NECD transendocytosis by ligand cells and this correlates with Notch signaling defects in flies and mammalian cells (Nichols et al., 2007a; Parks et al., 2000).
Structural studies suggest that Notch receptors are locked-down in a protease-resistant conformation and that major structural changes are required to expose Notch to activating proteolysis (Gordon et al., 2008). Binding between ligand and Notch cells would present a resistance to endocytosis of Notch by the ligand cell, which may stimulate recruitment of specific cellular factors to form an endocytic structure that could overcome resistance to endocytosis of ligand bound to Notch. In particular, ligands could employ mechanical force intrinsic to endocytosis (Liu et al., 2010; McMahon and Gallop, 2005) to pull on bound Notch and dissociate the pre-formed Notch heterodimer. Specifically, force exerted on ligand-bound Notch would unfold or destabilize the heterodimeric interactions allowing physical or enzymatic removal of the Notch extracellular domain (NECD). Uptake of NECD by the ligand cell would expose the remaining membrane-bound Notch to activating proteolysis for the generation of NICD and activation of downstream signaling.
It is clear that epsin is required for ligand signaling activity, and it is possible that epsin may function downstream of ligand binding to Notch to participate in the generation of mechanical force during the endocytic process (D'Souza et al., 2008; Nichols et al., 2007b). Notch binding to ligand has been reported to induce ubiquitylation (Hansson et al., 2010) and additional ligand clustering could amass multiple Ub-binding sites for epsin. The assembly of multiple low affinity mono-Ub interactions between the epsin Ub-interaction motifs and Ub attached to ligands would strengthen these interactions (Barriere et al., 2006; Hawryluk et al., 2006), and allow ligand to overcome resistance to internalization when bound to cell surface Notch (Figure Step 2). In fact, replacement of the Delta intracellular domain with a single Ub motif that can undergo polyubiquitylation promotes internalization and signaling activity in zebrafish (Itoh et al., 2003). A non-extendable Ub, however, signals only weakly even though it promotes endocytosis (Wang and Struhl, 2004), supporting the idea that multiple Ub interaction sites are required for ligands to activate Notch, possibly through providing stable associations with epsin-containing endocytic vesicles.
Epsin, dynamin, clathrin and the actin cytoskeleton have all been implicated in generating mechanical force to invaginate the plasma membrane during formation of endocytic vesicles (Liu et al., 2010; McMahon and Gallop, 2005). Importantly, studies in flies, worms and mammalian cells have indicated that these same components are required for ligand cells to activate signaling in Notch cells. It is therefore tempting to speculate that ligand cells require epsin to orchestrate the formation of a molecularly distinct endocytic structure specialized in force generation. In addition to membrane bending, epsin has also been reported to regulate the actin cytoskeleton during endocytosis (Horvath et al., 2007; Maldonado-Baez and Wendland, 2006), which together could endow cells with sufficient pulling force to induce conformational changes in ligand-bound Notch and initiate activating proteolysis. Additionally, Mib that binds and ubiquitylates ligand colocalizes with and physically interacts with sorting nexin 5 (Yoo et al., 2006) that contains a BAR (bin/amphiphysin/rvs) domain associated with membrane curvature (Itoh and De Camilli, 2006). Therefore, ligand targeted for endocytosis following interactions with Notch is likely to be associated with multiple force-generating factors. Accordingly, ligand-signaling activity most probably relies on endocytic events involving mechanical force to bend the membrane, form and release endocytic vesicles, rather than later steps required for vesicle trafficking. This idea is consistent with the observations that neither Rab5 nor Rab11 GTPases, nor efficient vesicle uncoating by auxilin, are required by signal-sending cells in several Notch-dependent developmental contexts (Banks et al., 2011).
In summary, the data to date are most consistent with the idea that Neur/Mib-dependent ligand endocytosis exerts a pulling force on the receptor that activates signaling. In polarized cells, ligand transcytosis could regulate additional regulatory events prior to Notch-induced ligand endocytosis required to activate signaling.
Regulation of Neur and Mib to control Notch signaling
A family of eight proteins called the Bearded (Brd) family, encoded by genes in two distinct complexes, regulates Neur activity in Drosophila by competing with ligands for Neur binding. Overexpression of a single Brd family protein results in Notch-like phenotypes (Bardin and Schweisguth, 2006; Chanet et al., 2009; De Renzis et al., 2006; Fontana and Posakony, 2009). These genes have largely redundant functions; while deletion of all eight Brd family genes results in embryonic lethality, deletion of all of the Brd family genes in either complex has at most a weak effect (Chanet et al., 2009). There is solid evidence in support of the idea that Brd proteins repress Neur activity by binding to Neur, and thereby preventing Neur from accessing and ubiquitinating Delta. When a Brd family protein is overexpressed, the Delta/NECD endosomes that accumulate normally in embryonic mesoderm cells are absent, and instead Delta accumulates at the plasma membrane as in neur mutants (De Renzis et al., 2006). Similarly, Brd family protein overexpression inhibits Delta endocytosis in SOPs, and blocks Delta/Neur binding (Bardin and Schweisguth, 2006). The Brd family/Neur interactions have been studied in detail. All Brd proteins have at least one so-called NxxN motif that binds to the Neuralized homology repeats (NHRs) present in Neur (Bardin and Schweisguth, 2006; Commisso and Boulianne, 2007; Fontana and Posakony, 2009; He et al., 2009). The Drosophila ligands Delta and Serrate each contain a NxxN motif, and Delta’s has been shown to be required for Neur binding and Neur-dependent endocytosis (Fontana and Posakony, 2009). Importantly, the Notch-like mutant phenotypes generated by overexpression of Brd family proteins depend on the NxxN motifs (Bardin and Schweisguth, 2006; De Renzis et al., 2006; Fontana and Posakony, 2009; Chanet et al., 2009).
Regulation by Brd proteins is limited. First, a clear requirement for Brd proteins has been shown so far in only one developmental context in Drosophila - sharpening the ectoderm/mesectoderm boundary at the embryonic dorsal/ventral axis (Bardin and Schweisguth, 2006; Chanet et al., 2009; De Renzis et al., 2006). In addition, regulation by Brd proteins is specific to Neur; Mib proteins do not have NHRs, Brd family proteins do not bind Mib1, and Brd family protein overexpression interferes with Neur-dependent, but not Mib-dependent Notch signaling (Bardin and Schweisguth, 2006). Moreover, consistent with the observation that Neur does not regulate Notch signaling in vertebrates, Brd family genes appear to be specific to insects (Fontana and Posakony, 2009). There are however, regulators of vertebrate Mib that function in an entirely different manner.
In contrast to the competitive-inhibition that regulates Neur ubiquitylation of Notch ligands in flies, two independent mechanisms have been reported to regulate Mib1 protein levels and thereby influence vertebrate Notch signaling. A recent study identified vertebrate Mib1 as a substrate for the cell polarity protein PAR-1 kinase, in which Mib phosphorylation results in auto-ubiquitylation that targets Mib for degradation by the proteasome (Ossipova et al., 2009). Losses in Mib1 protein result in less Delta ubiquitylation and a consequential loss of Notch signaling, leading to an expansion of progenitors that differentiate as neurons. It will be interesting to learn how Mib1 attaches Ub chains to itself to target proteasomal degradation, yet mono-ubiquitylates Notch ligands to activate Notch signaling. In addition to regulating the numbers of neurons produced during development, Notch signaling can regulate the morphology of neuronal processes in post-mitotic neurons. In this regard, Mib1 is enriched in the postsynaptic density isolated from mouse brains and can inhibit neurite outgrowth and branching of neurons (Choe et al., 2007). The cyclin-dependent kinase 5 (CDK5) enriched in neurons phosphorylates Mib1 and suppresses the inhibitory effects of Mib1 on neurite morphology. Similar to the mechanism of PAR-1 regulation of Mib in neurogenesis, CDK5 phosphorylation is proposed to enhance Mib1 ligase activity leading to destabilization (Choe et al., 2007). Although the loss of Mib protein depends on CDK5 kinase activity, the CDK5 effects on neurite morphology were not linked directly to a Notch signaling failure as reported for PAR-1. Nonetheless, these examples suggest the existence of mechanisms to control the strength of Notch signaling by regulating Mib1 levels. As PAR-1 has been connected to Notch signaling in Drosophila embryos, this mode of Mib1 regulation may well occur in Drosophila as well (Bayraktar et al., 2006).
What we still need to know…
Analyses in worms will provide a missing link to the surprising finding that Neur1 and Neur2 do not function in vertebrate Notch signaling, as Neur most certainly does in flies. The C. elegans database indicates the presence of a neur gene (F10D7.5), which is consistent with the requirement for epsin, a Ub-binding endocytic adaptor, by the C. elegans ligand LAG-2 to signal to the Notch-related GLP-1 in germline cells (Tian et al., 2004). Experiments however, directly addressing a role for C. elegans neur in development or Notch signaling, specifically as an E3 ligase for Notch-like ligands, have yet to be reported. Moreover, it is still unclear whether C. elegans has a true mib ortholog, despite the large number of RING domain encoding genes identified in database searches.
The antimorphic phenotypes associated with some zebrafish Mib mutants are consistent with Mib/Mib2 heterodimer interactions (Zhang et al., 2007a) similar to those described for other E3 RING domain ligases (Deshaies and Joazeiro, 2009); however, it will be important to determine how such interactions regulate Mib ubiquitylation of Notch ligands. Moreover, structure-function analysis has identified zebrafish Mib and Mib2 N-terminal sequences required for interactions with ligands (Chen and Corliss, 2004; Itoh et al., 2003; Zhang et al., 2007a), but further work is needed to define the physiologically relevant ligand-binding domains. It will also be important to determine if Notch ligands in flies and mammalian cells have distinct specificities for Mib1 and Mib2 as reported for the zebrafish Delta ligands (Zhang et al., 2007a), and establish whether ligands are always mono-ubiquitylated, as multiple mono-ubiquitylation and different Ub chain linkages may have distinct cellular functions (Haglund and Dikic, 2005). In this regard, it is important to note that while both gain-of-function and loss-of-function approaches have been used to support a role for ligand ubiquitylation in Notch signaling, evidence of Ub on endogenous ligands and their interactions with either Neur or Mib have yet to be reported. Finally, whether Notch ligands always rely on ubiquitylation for internalization or if this modification specifically enables ligands to induce endocytic force to activate Notch remains to be determined. Also, are there two separate ligand ubiquitylation events – one for recycling (Step 1), and another for mechanical force generation (Step 2)? And if so, are the ubiquitylated lysines or the character of the ubiquitylation events distinct?
An obvious deficiency in our knowledge regarding the role of ligand ubiquitylation is the identity and function of deubiquitinating enzymes in regulating ligand-signaling activity. Furthermore, are ubiquitylation and recycling required for Notch ligands to access a specific microdomain to signal as previously proposed (Heuss et al., 2008)? If so, how does this relate to enhanced expression of Delta (Itoh et al., 2003) or specific glycosphingolipids (Hamel et al., 2010) ameliorating the defects in ligand endocytosis caused by decreased Mib activity? Moreover, do epsins function in ligand signaling simply as endocytic adaptors for ubiquitylated ligands, or do epsins bound to ubiquitylated ligands participate directly in some special version of ligand endocytosis that activates Notch? Although an absolute requirement for ligand recycling in Notch signaling seems unlikely, biophysical evidence that Notch ligands harness the mechanical force inherent to endocytosis to activate Notch signaling is needed to support the pulling force model. Together these and other questions will continue to challenge our understanding of how ligand ubiquitylation relates to ligand signaling potential.
Acknowledgements
We thank Abdiwahab A. Musse for generating the figure and apologize for the omission of works not discussed or cited due to space limitations. We are grateful to our colleagues who answered a plethora of emailed questions. Research in the G. W. laboratory is supported by University of California Cancer Research Coordinating Committee, Canadian Institute of Health Research, Jonsson Cancer Center Foundation, and the National Institutes of Health (R37 NS031885-17, R01 GM085032-01) and J. A. F. laboratory by NICHD grant number R01-HD30680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks SM, Cho B, Eun SH, Lee JH, Windler SL, Xie X, Bilder D, Fischer JA. The functions of auxilin and Rab11 in Drosophila suggest that the fundamental role of ligand endocytosis in notch signaling cells is not recycling. PLoS ONE. 2011;6:e18259. doi: 10.1371/journal.pone.0018259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin AJ, Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev Cell. 2006;10:245–255. doi: 10.1016/j.devcel.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, Lukacs GL. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in Mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- Barsi JC, Rajendra R, Wu JI, Artzt K. Mind bomb1 is a ubiquitin ligase essential for mouse embryonic development and Notch signaling. Mech Dev. 2005;122:1106–1117. doi: 10.1016/j.mod.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bayraktar J, Zygmunt D, Carthew RW. Par-1 kinase establishes cell polarity and functions in Notch signaling in the Drosophila embryo. J Cell Sci. 2006;119:711–721. doi: 10.1242/jcs.02789. [DOI] [PubMed] [Google Scholar]
- Benhra N, Vignaux F, Dussert A, Schweisguth F, Le Borgne R. Neuralized promotes basal to apical transcytosis of delta in epithelial cells. Mol Biol Cell. 2010;21:2078–2086. doi: 10.1091/mbc.E09-11-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulianne GL, de la Concha A, Campos-Ortega JA, Jan LY, Jan YN. The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. Embo J. 1993;12:2586. doi: 10.1002/j.1460-2075.1993.tb05914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Rando M, Ruiz-Gomez M. Mind bomb 2, a founder myoblast-specific protein, regulates myoblast fusion and muscle stability. Development. 2008;135:849–857. doi: 10.1242/dev.015529. [DOI] [PubMed] [Google Scholar]
- Chanet S, Vodovar N, Mayau V, Schweisguth F. Genome engineering-based analysis of Bearded family genes reveals both functional redundancy and a nonessential function in lateral inhibition in Drosophila. Genetics. 2009;182:1101–1108. doi: 10.1534/genetics.109.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, De Camilli P. The association of epsin with ubiquitylated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci U S A. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci U S A. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Corliss DCC. Three modules of zebrafish Mind bomb work cooperatively to promote Delta ubiquitylation and endocytosis. Dev Biol. 2004;267:361–373. doi: 10.1016/j.ydbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta [see comments] Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Choe EA, Liao L, Zhou JY, Cheng D, Duong DM, Jin P, Tsai LH, Peng J. Neuronal morphogenesis is regulated by the interplay between cyclin-dependent kinase 5 and the ubiquitin ligase mind bomb 1. J Neurosci. 2007;27:9503–9512. doi: 10.1523/JNEUROSCI.1408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C, Boulianne GL. The NHR1 domain of Neuralized binds Delta and mediates Delta trafficking and Notch signaling. Mol Biol Cell. 2007;18:1–13. doi: 10.1091/mbc.E06-08-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzis S, Yu J, Zinzen R, Wieschaus E. Dorsal-ventral pattern of Delta trafficking is established by a Snail-Tom-Neuralized pathway. Dev Cell. 2006;10:257–264. doi: 10.1016/j.devcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates Notch signaling. Dev Cell. 2001;1:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Dev Cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- Diks SH, Bink RJ, van de Water S, Joore J, van Rooijen C, Verbeek FJ, den Hertog J, Peppelenbosch MP, Zivkovic D. The novel gene asb11: a regulator of the size of the neural progenitor compartment. J Cell Biol. 2006;174:581–592. doi: 10.1083/jcb.200601081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks SH, Sartori da Silva MA, Hillebrands JL, Bink RJ, Versteeg HH, van Rooijen C, Brouwers A, Chitnis AB, Peppelenbosch MP, Zivkovic D. d-Asb11 is an essential mediator of canonical Delta-Notch signalling. Nat Cell Biol. 2008;10:1190–1198. doi: 10.1038/ncb1779. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Eun SH, Banks SM, Fischer JA. Auxilin is essential for Delta signaling. Development. 2008;135:1089–1095. doi: 10.1242/dev.009530. [DOI] [PubMed] [Google Scholar]
- Eun SH, Lea K, Overstreet E, Stevens S, Lee JH, Fischer JA. Identification of genes that interact with Drosophila liquid facets. Genetics. 2006;175:1163–1174. doi: 10.1534/genetics.106.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana JR, Posakony JW. Both inhibition and activation of Notch signaling rely on a conserved Neuralized-binding motif in Bearded proteins and the Notch ligand Delta. Dev Biol. 2009;333:373–385. doi: 10.1016/j.ydbio.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Glittenberg M, Pitsouli C, Garvey C, Delidakis C, Bray S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. Embo J. 2006;25:4697–4706. doi: 10.1038/sj.emboj.7601337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Arnett KL, Blacklow SC. The molecular logic of Notch signaling--a structural and biochemical perspective. J Cell Sci. 2008;121:3109–3119. doi: 10.1242/jcs.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, Chang HC. Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J Cell Biol. 2006;173:443–452. doi: 10.1083/jcb.200602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Dikic I. Ubiquitylation and cell signaling. Embo J. 2005;24:3353–3359. doi: 10.1038/sj.emboj.7600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel S, Fantini J, Schweisguth F. Notch ligand activity is modulated by glycosphingolipid membrane composition in Drosophila melanogaster. J Cell Biol. 2010;188:581–594. doi: 10.1083/jcb.200907116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson EM, Lanner F, Das D, Mutvei A, Marklund U, Ericson J, Farnebo F, Stumm G, Stenmark H, Andersson ER, et al. Control of Notch-ligand endocytosis by ligand-receptor interaction. J Cell Sci. 2010;123:2931–2942. doi: 10.1242/jcs.073239. [DOI] [PubMed] [Google Scholar]
- Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- He F, Saito K, Kobayashi N, Harada T, Watanabe S, Kigawa T, Guntert P, Ohara O, Tanaka A, Unzai S, et al. Structural and functional characterization of the NHR1 domain of the Drosophila neuralized E3 ligase in the notch signaling pathway. J Mol Biol. 2009;393:478–495. doi: 10.1016/j.jmb.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, Lewis J. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- Heuss SF, Ndiaye-Lobry D, Six EM, Israel A, Logeat F. The intracellular region of Notch ligands Dll1 and Dll3 regulates their trafficking and signaling activity. Proc Natl Acad Sci U S A. 2008;105:11212–11217. doi: 10.1073/pnas.0800695105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Horvath CA, Vanden Broeck D, Boulet GA, Bogers J, De Wolf MJ. Epsin: inducing membrane curvature. Int J Biochem Cell Biol. 2007;39:1765–1770. doi: 10.1016/j.biocel.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Jin Y, Blue EK, Dixon S, Shao Z, Gallagher PJ. A death-associated protein kinase (DAPK)-interacting protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor necrosis factor-induced apoptosis and regulates the cellular levels of DAPK. J Biol Chem. 2002;277:46980–46986. doi: 10.1074/jbc.M208585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandachar V, Bai T, Chang HC. The clathrin-binding motif and the J-domain of Drosophila Auxilin are essential for facilitating Notch ligand endocytosis. BMC Dev Biol. 2008;8:50. doi: 10.1186/1471-213X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005a;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Koo BK, Yoon KJ, Yoo KW, Lim HS, Song R, So JH, Kim CH, Kong YY. Mind bomb-2 is an E3 ligase for Notch ligand. J Biol Chem. 2005b;280:22335–22342. doi: 10.1074/jbc.M501631200. [DOI] [PubMed] [Google Scholar]
- Koo BK, Yoon MJ, Yoon KJ, Im SK, Kim YY, Kim CH, Suh PG, Jan YN, Kong YY. An obligatory role of mind bomb-1 in notch signaling of Mammalian development. PLoS ONE. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutelou E, Sato S, Tomomori-Sato C, Florens L, Swanson SK, Washburn MP, Kokkinaki M, Conaway RC, Conaway JW, Moschonas NK. Neuralized-like 1 (Neurl1) targeted to the plasma membrane by N-myristoylation regulates the Notch ligand Jagged1. J Biol Chem. 2008;283:3846–3853. doi: 10.1074/jbc.M706974200. [DOI] [PubMed] [Google Scholar]
- Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- Lai EC, Roegiers F, Qin X, Jan YN, Rubin GM. The ubiquitin ligase Drosophila Mind bomb promotes Notch signaling by regulating the localization and activity of Serrate and Delta. Development. 2005;132:2319–2332. doi: 10.1242/dev.01825. [DOI] [PubMed] [Google Scholar]
- Lai EC, Rubin GM. neuralized functions cell-autonomously to regulate a subset of notch- dependent processes during adult Drosophila development. Dev Biol. 2001a;231:217–233. doi: 10.1006/dbio.2000.0124. [DOI] [PubMed] [Google Scholar]
- Lai EC, Rubin GM. Neuralized is essential for a subset of Notch pathway-dependent cell fate decisions during Drosophila eye development. Proc Natl Acad Sci U S A. 2001b;98:5637–5642. doi: 10.1073/pnas.101135498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol. 2005;3:e96. doi: 10.1371/journal.pbio.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Notch signaling: endocytosis makes delta signal better. Curr Biol. 2003a;13:R273–R275. doi: 10.1016/s0960-9822(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev Cell. 2003b;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Lehmann R, Jimenez F, Dietrich U, Campos-Ortega JA. On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Devel Biol. 1983;192:62–74. doi: 10.1007/BF00848482. [DOI] [PubMed] [Google Scholar]
- Li Y, Baker NE. The roles of cis-inactivation by Notch ligands and of neuralized during eye and bristle patterning in Drosophila. BMC Dev Biol. 2004;4:5. doi: 10.1186/1471-213X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun Y, Oster GF, Drubin DG. Mechanochemical crosstalk during endocytic vesicle formation. Curr Opin Cell Biol. 2010;22:36–43. doi: 10.1016/j.ceb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R, Hanson IM, Borden KL, Martin S, O'Reilly NJ, Evan GI, Rahman D, Pappin DJ, Trowsdale J, Freemont PS. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993;90:2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Baez L, Wendland B. Endocytic adaptors: recruiters, coordinators and regulators. Trends Cell Biol. 2006;16:505–513. doi: 10.1016/j.tcb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Chitnis AB. Interaction with Notch determines endocytosis of specific Delta ligands in zebrafish neural tissue. Development. 2009;136:197–206. doi: 10.1242/dev.027938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Miller AC, Lyons EL, Herman TG. cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. Curr Biol. 2009;19:1378–1383. doi: 10.1016/j.cub.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Voza F, Ezzeddine N, Frasch M. Drosophila mind bomb2 is required for maintaining muscle integrity and survival. J Cell Biol. 2007;179:219–227. doi: 10.1083/jcb.200708135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JT, Miyamoto A, Olsen SL, D'Souza B, Yao C, Weinmaster G. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007a;176:445–458. doi: 10.1083/jcb.200609014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JT, Miyamoto A, Weinmaster G. Notch signaling--constantly on the move. Traffic. 2007b;8:959–969. doi: 10.1111/j.1600-0854.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Ezan J, Sokol SY. PAR-1 phosphorylates Mind bomb to promote vertebrate neurogenesis. Dev Cell. 2009;17:222–233. doi: 10.1016/j.devcel.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet E, Chen X, Wendland B, Fischer JA. Either part of a Drosophila epsin protein, divided after the ENTH domain, functions in endocytosis of delta in the developing eye. Curr Biol. 2003;13:854–860. doi: 10.1016/s0960-9822(03)00326-9. [DOI] [PubMed] [Google Scholar]
- Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Parks AL, Stout JR, Shepard SB, Klueg KM, Dos Santos AA, Parody TR, Vaskova M, Muskavitch MA. Structure-function analysis of delta trafficking, receptor binding and signaling in Drosophila. Genetics. 2006;174:1947–1961. doi: 10.1534/genetics.106.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Turner FR, Muskavitch MA. Relationships between complex Delta expression and the specification of retinal cell fates during Drosophila eye development. Mech Dev. 1995;50:201–216. doi: 10.1016/0925-4773(94)00336-l. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch MA, Moschonas NK, Delidakis C. neuralized Encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev Cell. 2001;1:807–816. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- Pitsouli C, Delidakis C. The interplay between DSL proteins and ubiquitin ligases in Notch signaling. Development. 2005;132:4041–4050. doi: 10.1242/dev.01979. [DOI] [PubMed] [Google Scholar]
- Poulson D. The effects of certain X-chromosome deficiencies on the embryonic development of Drosophila melanogaster. J Exp Zool. 1940;83:271–325. [Google Scholar]
- Poulson DF. Chromosomal deficiencies and the embryonic development of Drosophila melanogaster. Proc Natl Acad Sci USA. 1937;23:133–137. doi: 10.1073/pnas.23.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan A, Tien AC, Haueter CM, Schulze KL, Bellen HJ. The Arp2/3 complex and WASp are required for apical trafficking of Delta into microvilli during cell fate specification of sensory organ precursors. Nat Cell Biol. 2009;11:815–824. doi: 10.1038/ncb1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Tecott L, Jiang MM, Jan LY, Jan YN. Ethanol hypersensitivity and olfactory discrimination defect in mice lacking a homolog of Drosophila neuralized. Proc Natl Acad Sci U S A. 2001;98:9907–9912. doi: 10.1073/pnas.171321098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori da Silva MA, Tee JM, Paridaen J, Brouwers A, Runtuwene V, Zivkovic D, Diks SH, Guardavaccaro D, Peppelenbosch MP. Essential role for the d-Asb11 cul5 Box domain for proper notch signaling and neural cell fate decisions in vivo. PLoS ONE. 2010;5:e14023. doi: 10.1371/journal.pone.0014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitylated cargos. Proc Natl Acad Sci U S A. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarek LC, Garroni MK, Commisso C, Boulianne GL. Neuralized contains a phosphoinositide-binding motif required downstream of ubiquitylation for delta endocytosis and notch signaling. Dev Cell. 2007;13:783–795. doi: 10.1016/j.devcel.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Song R, Koo BK, Yoon KJ, Yoon MJ, Yoo KW, Kim HT, Oh HJ, Kim YY, Han JK, Kim CH, et al. Neuralized-2 regulates a Notch ligand in cooperation with Mind bomb-1. J Biol Chem. 2006;281:36391–36400. doi: 10.1074/jbc.M606601200. [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S. The intracellular deletions of Delta and Serrate define dominant negative forms of the Drosophila Notch ligands. Development. 1996;122:2465–2474. doi: 10.1242/dev.122.8.2465. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Ohtsuki Y. Skeletrophin, a novel ubiquitin ligase to the intracellular region of Jagged-2, is aberrantly expressed in multiple myeloma. Am J Pathol. 2005;166:1817–1826. doi: 10.1016/S0002-9440(10)62491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teider N, Scott DK, Neiss A, Weeraratne SD, Amani VM, Wang Y, Marquez VE, Cho YJ, Pomeroy SL. Neuralized1 causes apoptosis and downregulates Notch target genes in medulloblastoma. Neuro Oncol. 2010;12:1244–1256. doi: 10.1093/neuonc/noq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans. Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- Vollrath B, Pudney J, Asa S, Leder P, Fitzgerald K. Isolation of a murine homologue of the Drosophila neuralized gene, a gene required for axonemal integrity in spermatozoa and terminal maturation of the mammary gland. Mol Cell Biol. 2001;21:7481–7494. doi: 10.1128/MCB.21.21.7481-7494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- Windler SL, Bilder D. Endocytic Internalization Routes Required for Delta/Notch Signaling. Curr Biol. 2010;20:538–543. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, Chitnis AB, Matsumoto K, Crump JG, Hozumi K, et al. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development. 2010;137:2527–2537. doi: 10.1242/dev.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Dermer M, Commisso C, Zhou L, McGlade CJ, Boulianne GL. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr Biol. 2001;11:1675–1679. doi: 10.1016/s0960-9822(01)00527-9. [DOI] [PubMed] [Google Scholar]
- Yeh E, Zhou L, Rudzik N, Boulianne GL. Neuralized functions cell autonomously to regulate Drosophila sense organ development. Embo J. 2000;19:4827–4837. doi: 10.1093/emboj/19.17.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo KW, Kim EH, Jung SH, Rhee M, Koo BK, Yoon KJ, Kong YY, Kim CH. Snx5, as a Mind bomb-binding protein, is expressed in hematopoietic and endothelial precursor cells in zebrafish. FEBS Lett. 2006;580:4409–4416. doi: 10.1016/j.febslet.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Q, Jiang YJ. Zebrafish Mib and Mib2 are mutual E3 ubiquitin ligases with common and specific delta substrates. J Mol Biol. 2007a;366:1115–1128. doi: 10.1016/j.jmb.2006.11.096. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Q, Lim CH, Qiu X, Jiang YJ. The characterization of zebrafish antimorphic mib alleles reveals that Mib and Mind bomb-2 (Mib2) function redundantly. Dev Biol. 2007b;305:14–27. doi: 10.1016/j.ydbio.2007.01.034. [DOI] [PubMed] [Google Scholar]