Abstract
In Drosophila melanogaster, as in other insects, a waxy layer on the outer surface of the cuticle, composed primarily of hydrocarbon compounds, provides protection against desiccation and other environmental challenges. Several of these cuticular hydrocarbon (CHC) compounds also function as semiochemical signals, and as such mediate pheromonal communications between members of the same species, or in some instances between different species, and influence behavior. Specialized cells referred to as oenocytes are regarded as the primary site for CHC synthesis. However, relatively little is known regarding the involvement of the oenocytes in the regulation of the biosynthetic, transport, and deposition pathways contributing to CHC output. Given the significant role that CHCs play in several aspects of insect biology, including chemical communication, desiccation resistance, and immunity, it is important to gain a greater understanding of the molecular and genetic regulation of CHC production within these specialized cells. The adult oenocytes of D. melanogaster are located within the abdominal integument, and are metamerically arrayed in ribbon-like clusters radiating along the inner cuticular surface of each abdominal segment. In this video article we demonstrate a dissection technique used for the preparation of oenocytes from adult D. melanogaster. Specifically, we provide a detailed step-by-step demonstration of (1) how to fillet prepare an adult Drosophila abdomen, (2) how to identify the oenocytes and discern them from other tissues, and (3) how to remove intact oenocyte clusters from the abdominal integument. A brief experimental illustration of how this preparation can be used to examine the expression of genes involved in hydrocarbon synthesis is included. The dissected preparation demonstrated herein will allow for the detailed molecular and genetic analysis of oenocyte function in the adult fruit fly.
Protocol
Part 1. Fillet preparation of the adult Drosophila abdomen.
The procedure outlined immediate below prepares the abdomen for the subsequent removal of the oenocytes (see Part2 of protocol). The same procedure can also be used for the preparation of the other tissues attached to the inner surface of the cuticle, including the dorsal vessel (i.e. heart) and fat body.
Note that in the video article the oenocyte dissection technique is demonstrated on an adult wild-type (Canton-S) male fly, six days of age. Identical techniques can be used to remove the oenocytes from female flies. The abdomen fillet preparation takes approximately 5 minutes to perform.
Before beginning it is important to have ready several fine dissection pins (see below and Part 2) and a dissection needle (see Part 2). Commercially available dissection pins and needles are often too large for this protocol, so we have taken to fabricating our own in lab. To create the dissection needle, tungsten wire (0.005") is first threaded through the shaft of hypodermic needle (27G, aluminum hub). Thread the wire through the tip of the hypodermic needle so that it emerges from the hub. Crimp the wire protruding from hub; this prevents the wire from being pulled out of the hypodermic needle. Next, cut the wire at the opposite end of the crimp such that several millimeters (~3-4mm) of wire protrude from the tip of the hypodermic needle. Passing the tip of exposed tungsten through a flame will sharpen the wire to a very fine point. A disposable 5ml plastic pipette when wedged into the hub of the hypodermic needle acts as convenient handle for this dissection tool. The fine dissection pins are made in a similar way. After the sharpening step, grasp the very tip of the wire firmly with forceps to prevent it from moving and make a cut approximately 3mm from the tip to release the small dissection pin. Use care to prevent pin from being flung from the grasp of the forceps.
To fillet prepare the adult Drosophila abdomen:
Secure the fly to the dissection plate (Sylgard; Dow-Corning) by placing a fine dissection pin though the thorax.
Remove the legs and wings of the fly. While removing the most posterior set of legs, create an opening in the cuticle at the point just anterior to where the thorax meets the abdomen. This opening is used later as an access point for making an incision along the ventral surface of the abdomen (see Step 5)
Secure the abdomen of the fly in place by placing a second dissection pin through the genital segment.
Cover the fly with chilled Shields and Sang M3 insect medium (Sigma). Remove any trapped air by drawing it off with a Pasteur pipette.
Using forceps open the abdomen by making an incision along the ventral midline from the opening in the cuticle created by removing the legs to the genital segment. Remove the guts and gonads from the abdomen.
Sever the cuticle and tracheae connecting the abdomen to the thorax. The only remaining tissue connecting the abdomen to the thorax should be the heart. This provides the abdominal cuticle with some stability, and prevents it from rotating or twisting during the next step.
Pin flat the anterior most corners of the abdominal cuticle. Remove the thorax. Pulling the cuticle taut, pin flat the posterior corners of the cuticle. The pin in the genital segment may need to be repositioned to make the cuticle lay flat.
Part 2. Oenocyte dissection
The abdomen fillet preparation exposes the internal surface of the abdominal cuticle and attached tissues including the heart, fat body, tracheae, body wall muscles, epidermis, and the oenocytes. The heart lies along the dorsal midline. Fat body (opaque tissue) covers most of the internal cuticular surface. Tracheae (white tubular structures) extend from the lateral spiracle openings, and make extensive branches which infiltrate these tissues. Lying beneath these tissues, the pigmented oenocytes (amber-colored cells) radiate from the midline to the lateral edge of the cuticle in the posterior region of each segment. The oenocytes lie directly beneath the darkly tanned regions of each tergite (dorsal cuticular plates) and can be difficult to identify without prior knowledge of their location. Another population of ventrally located oenocytes is associated with the sternites (ventral cuticular plates); although it is possible to isolate these cells, this video protocol will only demonstrate the dissection of the dorsal oenocytes. In each abdominal segment, a prominent leaf of fat body tissue lies anterior to the oenocytes; a smaller less obvious leaf of fat body lies posterior to the oenocytes. The prominent leaf of fat body tissue often covers the oenocytes of the segment preceding it. The dorsal vessel and fat body must be removed first in order to expose the oenocytes.
It is important to note that the amber pigmentation of the oenocytes, from which the their name derives (oeno- from Greek oînos, 'wine')1, clearly distinguishes these cells from the surrounding tissues; the intensity of the pigmentation increases with the age of the fly. Moreover, a basal lamina ensheathes the oenocytes2,3, providing support to the cell clusters and preventing the cells from becoming dissociated during the dissection. The basal lamina also aids in the removal of extraneously adhered tissues, such as fat body tissue, by creating a weak point where these adhesions can be easily disrupted.
The procedure outlined below provides detailed instructions for the removal of the oenocytes from the abdominal integument. The oenocytes can be easily removed from abdominal segments 2-5 (male), and 2-6 (female). The oenocytes from the first and last abdominal segments (segments 1, and 6 in males or 7 in females) are often disrupted by the dissection pins holding the corners of the cuticle and are therefore avoided. The removal of the oenocytes takes approximately 10 minutes to perform.
To isolate the adult oenocytes:
Sever the tracheal trunks emanating from the spiracles using a dissection needle.
Remove the fat body and heart by severing the connections to the inner surface of the cuticle with a dissection needle. To do so, position the point of the dissection needle between the fat body and the oenocytes, and drag it from the lateral edge of the cuticle, through to the midline, moving underneath the heart, and continuing to opposite side. Progressing from the posterior to anterior, remove the fat body tissue and the heart from the cuticle of each successive segment. If performed carefully the oenocytes remain attached to the cuticle, and the fat body and heart can be removed whole.
Body wall muscles strap the oenocytes to the inner surface of the cuticle. Using the dissection needle sever the longitudinal body wall muscles of each segment. To do so, drag the dissection needle along the posterior side of the oenocyte strand, gently lifting them from the surface of the cuticle. The ribbon-like oenocyte strands should come away from the cuticle and can be laid to the side while the remaining oenocytes are removed.
Once removed from the cuticle and lying on the dissection plate it may be necessary to clean off any adhered to fat body tissue from the oenocytes. This is done by simply placing the dissection needle between the fat body tissue and the oenocytes, and severing the connection by pushing the needle down and against the dissection plate.
Gather the oenocyte strands by simply poking them with the end of the dissection needle. Once stuck to the end of the dissection needle the oenocytes can be pulled slowly across the surface of the culture medium. The dissected oenocytes can be placed directly into cell lysis buffer appropriate for either RNA or protein extraction. Note that the oenocytes (abdominal segments 2-5) from a single male fly typically yields 15-20ng of total RNA.
Figure Legend:
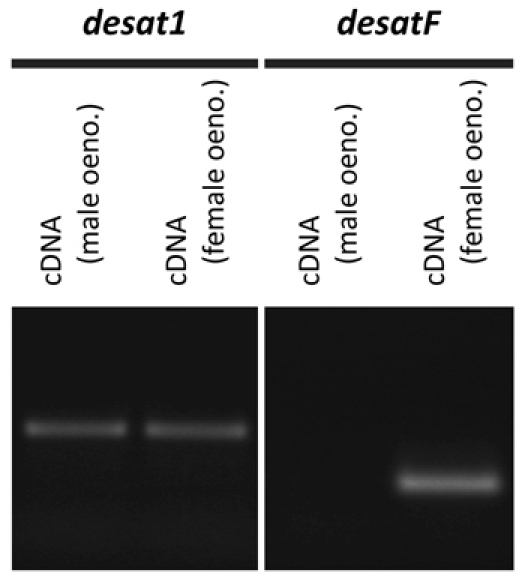 Figure 1. Expression of the desaturase genes, desat1 and desatF, in male and female oenocytes of D. melanogaster. Reverse transcription-PCR amplification of desat1 and desatF from cDNA derived from adult male and female oenocytes. The desat1 gene is expressed in both male and female oenocytes; desatF expression is sexually dimorphic, being expressed exclusively in female oenocytes. Corresponding to these differences in gene expression, desat1 is required for the production of hydrocarbon compounds common to both males and females9, and desatF is involved in the production of female-specific hydrocarbon compounds10,11. desatF has been shown to be specifically expressed in female oenocytes by in situ hybridization12. Note that on average 15-20ng of total RNA is obtained from the oenocytes dissected from an individual fly. RNA was isolated using the RNeasy micro kit (Qiagen); cDNA was produced using the qScript cDND synthesis kit (Quanta BioSciences)
Figure 1. Expression of the desaturase genes, desat1 and desatF, in male and female oenocytes of D. melanogaster. Reverse transcription-PCR amplification of desat1 and desatF from cDNA derived from adult male and female oenocytes. The desat1 gene is expressed in both male and female oenocytes; desatF expression is sexually dimorphic, being expressed exclusively in female oenocytes. Corresponding to these differences in gene expression, desat1 is required for the production of hydrocarbon compounds common to both males and females9, and desatF is involved in the production of female-specific hydrocarbon compounds10,11. desatF has been shown to be specifically expressed in female oenocytes by in situ hybridization12. Note that on average 15-20ng of total RNA is obtained from the oenocytes dissected from an individual fly. RNA was isolated using the RNeasy micro kit (Qiagen); cDNA was produced using the qScript cDND synthesis kit (Quanta BioSciences)
Discussion
In this video article we present a detailed dissection protocol for the preparation of the oenocytes from adult D. melanogaster in a manner suitable for molecular analysis. An abbreviated text-based account of the dissection method has been described elsewhere2, and the resulting oenocyte preparation has been demonstrated to be appropriate for the extraction of both RNA and protein2. Using this preparation it may also be possible to develop methods for the culturing of explanted oenocytes. A protocol for an oenocyte explant culture would aid investigations aimed at determining the autonomous metabolic capacity of these cells to synthesize and secrete the chemical constituents of cuticular hydrocarbons. These techniques in combination with a recently developed oenocyte-Gal4 line4, which permits the targeted genetic manipulation of the oenocytes, will allow for the detailed molecular genetic analysis of oenocyte function in the adult fruit fly.
Oenocyte cells are found both in the larva and the adult fly. While these cells share many morphological and ultrastructural similarities, it is important to note that the oenocytes of the adult fly are developmentally distinct from those that differentiate during embryonic development and exist during larval stages5. The adult oenocytes have been reported to be derived from the histoblasts6, forming de novo during pupation and continuing to increase in number during the first week of adult life7. While the oenocytes of the adult fruit fly are required for the production of cuticular hydrocarbons4, it remains to be determined if larval oenocytes support a similar function. Likewise, larval oenocytes maintain hepatocyte-like functions with regard to lipid metabolism8; a similar role has not yet been shown for the oenocytes of the adult. The preparation demonstrated here will aid investigations that are aimed at determining the degree to which the larval and adult oenocytes are functionally similar.
Disclosures
No conflicts of interest declared.
Acknowledgments
We would like to thank Amsale Belay for her assistance in filming of this video article. This work was funded by CIHR, NSERC and CRC grants to JDL.
References
- Wielowiejski H. Ueber das blutgewebe der insekten. Zeit. Wiss. Zool. 43:512–536. [Google Scholar]
- Krupp JJ, Kent C, Billeter JC, Azanchi R, So AK, Schonfeld JA, Smith BP, Lucas C, Levine JD. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- Locke M. Surface membranes, Golgi complexes, and vacuolar systems. Annu. Rev. Entomol. 2003;48:1–27. doi: 10.1146/annurev.ento.48.091801.112543. [DOI] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- Gould AP, Elstob PR, Brodu V. Insect oenocytes: a model system for studying cell-fate specification by Hox genes. J. Anat. 2001;199:25–33. doi: 10.1046/j.1469-7580.2001.19910025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Johnston P. Cell lineage of the Drosophila abdomen: the epidermis, oenocytes and ventral muscles. J. Embryol. Exp. Morphol. 1982;72:197–208. [PubMed] [Google Scholar]
- Johnson MB, Butterworth FM. Maturation and aging of adult fat body and oenocytes in Drosophila as revealed by light microscopic morphometry. J. Morphol. 1985;184:51–59. doi: 10.1002/jmor.1051840106. [DOI] [PubMed] [Google Scholar]
- Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–280. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- Marcillac F, Bousquet F, Alabouvette J, Savarit F, Ferveur JF. A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics. 2005;171:1617–1628. doi: 10.1534/genetics.104.033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertemps T, Duportets L, Labeur C, Ueyama M, Wicker-Thomas C. A female-specific desaturase gene responsible for diene hydrocarbon biosynthesis and courtship behaviour in Drosophila melanogaster. Insect Mol. Biol. 2006;15:465–473. doi: 10.1111/j.1365-2583.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- Legendre A, Miao XX, Da Lage JL, Wicker-Thomas C. Evolution of a desaturase involved in female pheromonal cuticular hydrocarbon biosynthesis and courtship behavior in Drosophila. Insect. Biochem. Mol. Biol. 2008;38:244–255. doi: 10.1016/j.ibmb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Shirangi TR, Dufour HD, Williams TM, Carroll SB. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 2009;7:e1000168–e1000168. doi: 10.1371/journal.pbio.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]


