Abstract
This video describes the establishment of liver metastases in a mouse model that can be subsequently analysed by bioluminescent imaging. Tumour cells are administered specifically to the liver to induce a localised liver tumour, via mobilisation of the spleen and splitting into two, leaving intact the vascular pedicle for each half of the spleen. Lewis lung carcinoma cells that constitutively express the firefly luciferase gene (luc1) are inoculated into one hemi-spleen which is then resected 10 minutes later. The other hemi-spleen is left intact and returned to the abdomen. Liver tumour growth can be monitored by bioluminescence imaging using the IVIS whole body imaging system. Quantitative imaging of tumour growth using IVIS provides precise quantitation of viable tumour cells. Tumour cell death and necrosis due to drug treatment is indicated early by a reduction in the bioluminescent signal. This mouse model allows for investigating the mechanisms underlying metastatic tumour-cell survival and growth and can be used for the evaluation of therapeutics of liver metastasis.
Protocol
Introduction
The liver is often the first site of metastatic disease and may be the only site of spread in as many as 30 40% of patients with advanced disease. However options for treating metastatic liver tumours are few with a minority of patients suitable for resection and only 23% major responders to chemotherapeutics. Preclinical research in this field has been limited by the absence of a suitable mouse model to study isolated liver disease. We describe a split-spleen approach that can be used to reliably establish uniform, diffuse liver metastases. Resection of this half of the spleen avoids the consequences of splenic tumour growth and the other half of the spleen is returned with an intact portal circulation to maintain splenic immune protective properties. The model we describe is quite simple to perform and can be done quickly through a single incision. The bioluminescent properties of the tumour cell line allows accurate quantification of treatment response without sacrifice of animals.
Protocol
I. Cell Preparation
Lewis lung carcinoma cells are grown Dulbecco's Modified Essential Medium supplemented with 10% fetal calf serum at 37°C, 5 % CO2 and harvested by trypsinisation when sub-confluent.
Cells were counted using a Nucleocounter (Chemo-metec, Denmark) and a single cell suspension of 1 X 106 cells in 200 μl was prepared in serum free media.
II. Mouse Preparation
Note: All procedures were approved by the ethics committee of University College Cork. In our laboratory we use injectable anaesthetics to anesthetize the mouse; alternatively, one can use inhaled anaesthetics to achieve the same effect. The mouse strain we used is the hairless MF1 nu/nu strain obtained from Harlan Laboratories (Oxfordshire, England). Female MF1 nu/nu mice weighing 16-22 g and of 6-8 weeks of age were included in this experiment. Other strains such as the C57 and Balb/c may also be used with appropriate tumour cell lines.
Anaesthesia was delivered by intraperitoneal injection of 100ul of 1.5 mg ketamine and 300 ug xylazine.
The depth of anaesthesia is assessed using toe pinch. There should be no withdrawal reflex with toe pinch.
The anesthetized mouse is properly positioned and taped over a heating pad.
The abdomen is prepped with a 10% betadine solution.
The abdomen and surgical site are draped in a sterile fashion.
III. Abdominal Incision
A small subcostal incision is made in the skin.
The incision is extended to 2-3 cm.
The abdominal wall musculature were divided and peritoneal cavity entered.
IV. Exposure and Division of Spleen
The spleen is mobilised and gently exteriorized on a pre-cut sterile gauze.
Two 4-0 vicryl ligatures are tied around the mid body of the spleen.
Spleen is divided between the two ligatures creating two hemi-spleens.
V. Injection of Cells into the Hemi-spleen
A 27 G is used to inject a 100 μL volume of cells into one hemi-spleen.
Tumour cells are allowed to drain to the liver for ten minutes providing sufficient time for the majority of the injected cells to reach the liver.
VI. Excision of Hemi-spleen, Abdominal closure and Recovery
The injected hemi-spleen is excised.
The other hemi-spleen is returned to the abdominal cavity.
The muscle layer is closed with 4-0 PDS suture using a simple running stitch.
The abdominal skin is closed with 4-0 Prolene using interrupted stitches.
Fluid bolus is administered and post-operative analgesia, Carpofen (5 mg/kg) is administered by IP injection for 24 h post surgery.
The mouse is allowed to recover from anaesthesia
VII. IVIS Whole Body Bioluminescent Imaging
Mouse is anaesthetised using injectable anaesthetic.
Luciferin substrate is given by intra-peritoneal injection ten minutes before imaging
Mice are placed on the stage and imaged for 3 min using IVIS Whole body imaging system
Luminescence from the liver can be measured using a region of interest tool
VIII. Exposure of mouse liver
Mouse is anaesthetised using injectable anaesthetic.
A rooftop abdominal incision allows visualisation of diffuse liver metastasis in vivo.
The mouse is euthanized by cervical dislocation and the liver may be excised for further analysis.
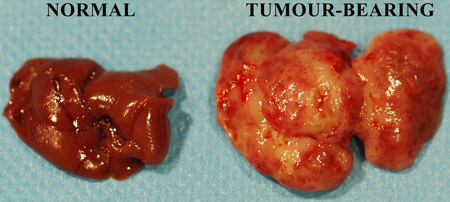 Figure 1. Freshly excised murine untreated and tumour-infiltrated livers.
Figure 1. Freshly excised murine untreated and tumour-infiltrated livers.
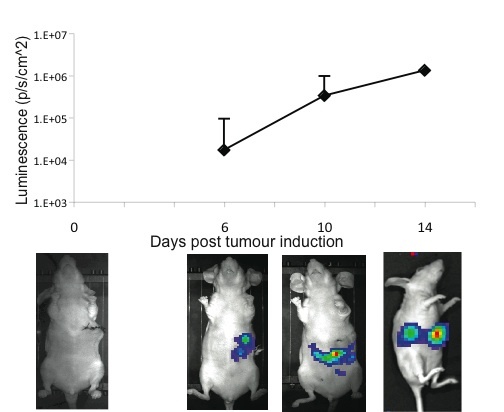 Figure 2. Kinetics of hepatic tumour growth as evidenced by IVIS whole body imaging.
Figure 2. Kinetics of hepatic tumour growth as evidenced by IVIS whole body imaging.
Discussion
The development of reliable in vivo models for the study of liver metastasis is becoming increasing important with the advent of new therapeutic strategies. For the accurate study of new strategies, animal models should be relatively easy to perform, form a significant number of metastasis, involve all the steps of the metastatic cascade and have a reporter system that allows detection and counting of a limited number of metastatic cells. The split-spleen approach as described involves a single operation and is a relatively easy technique to learn to establish uniform diffuse liver metastasis with a very low risk of carcinomatosis and portal vein thrombosis1. The division of the spleen into two hemi-spleens allow the injection of tumour cells into one hemi-spleen. Tumour cells enter the portal circulation and travel to the liver, mimicking the first step of the metastatic cascade. Excision of the injected hemi-spleen avoids the growth of a splenic tumour and the return of the other hemi-spleen ensures the retention of splenic immunological properties which the animal had prior to the procedure.. This model may be utilised for a variety of tumour types. The Lewis lung luciferase cell line allows the bioluminescent quantification of liver disease over time. The enhanced sensitivity of the bioluminescence system allows the detection and quantification of a small number of metastatic cells. This allows accurate timing for the initiation of treatment protocols and enables quantification of liver disease without sacrifice of the animal. The simplicity, durability and reproducibility make this model suitable for the study of various treatment strategies including novel chemotherapeutics2, gene therapy3 and immunotherapy1,4.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was funded through the Cork South Infirmary Victoria University Hospital Breast fund, the Irish Cancer Society (CRI07TAN) and the Cork Cancer Research Centre. Lewis lung cell line stably expressing luciferase was a kind gift from Dr. Karin Jooss, Cell Genesys, Inc., San Francisco, USA.
References
- Kasuya H. Mouse models of subcutaneous spleen reservoir for multiple portal venous injections to treat liver malignancies. Cancer Res. 2005;65:3823–3827. doi: 10.1158/0008-5472.CAN-04-2631. [DOI] [PubMed] [Google Scholar]
- Liu QZ, Tuo CW, Wang B, Wu BQ, Zhang YH. Liver metastasis models of human colorectal carcinoma established in nude mice by orthotopic transplantation and their biologic characteristic. World J Gastroenterol. 1998;4:409–411. doi: 10.3748/wjg.v4.i5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Ni S, Yang X, Kiviat N, Lieber A. Xenograft models for liver metastasis: Relationship between tumor morphology and adenovirus vector transduction. Mol Ther. 2004;9:650–657. doi: 10.1016/j.ymthe.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Cashman JP. Immune gene therapy as a neoadjuvant to surgical excision to control metastatic cancers. Cancer Lett. 2008 doi: 10.1016/j.canlet.2007.11.042. [DOI] [PubMed] [Google Scholar]


