Abstract
Background
The decline of coral reefs globally underscores the need for a spatial assessment of their exposure to multiple environmental stressors to estimate vulnerability and evaluate potential counter-measures.
Methodology/Principal Findings
This study combined global spatial gradients of coral exposure to radiation stress factors (temperature, UV light and doldrums), stress-reinforcing factors (sedimentation and eutrophication), and stress-reducing factors (temperature variability and tidal amplitude) to produce a global map of coral exposure and identify areas where exposure depends on factors that can be locally managed. A systems analytical approach was used to define interactions between radiation stress variables, stress reinforcing variables and stress reducing variables. Fuzzy logic and spatial ordinations were employed to quantify coral exposure to these stressors. Globally, corals are exposed to radiation and reinforcing stress, albeit with high spatial variability within regions. Based on ordination of exposure grades, regions group into two clusters. The first cluster was composed of severely exposed regions with high radiation and low reducing stress scores (South East Asia, Micronesia, Eastern Pacific and the central Indian Ocean) or alternatively high reinforcing stress scores (the Middle East and the Western Australia). The second cluster was composed of moderately to highly exposed regions with moderate to high scores in both radiation and reducing factors (Caribbean, Great Barrier Reef (GBR), Central Pacific, Polynesia and the western Indian Ocean) where the GBR was strongly associated with reinforcing stress.
Conclusions/Significance
Despite radiation stress being the most dominant stressor, the exposure of coral reefs could be reduced by locally managing chronic human impacts that act to reinforce radiation stress. Future research and management efforts should focus on incorporating the factors that mitigate the effect of coral stressors until long-term carbon reductions are achieved through global negotiations.
Introduction
Corals globally are exposed to diverse and often interacting physico-chemical and biological disturbances [1], [2]. The diversity, spatio-temporal heterogeneity, and interactions of these disturbances have complicated the understanding of the response of coral assemblages to multiple stressors [1], and reduced the potential for spatially targeted coral reef management strategies. To counteract species extinctions predicted by many [e.g. 3], [4], [5], corals would have to adapt to temperatures of more than 2°C above normal thresholds by the turn of the century [6], [7], in addition to coping with a suite of other stressors [8]. For example, local stressors such as eutrophication from coastal watersheds exacerbate coral stress by changing the oligotrophic conditions where coral reefs function optimally [9], [10], [11], [12], while overfishing and removal of grazers is accelerating a shift towards algal dominance [9], [13], [14].
Given the bleak view of the status and prognosis for coral reefs globally, timely identification of spatial gradients of their exposure to global and local stressors is needed so that appropriate counter-measures can be formulated and implemented. The management strategies proposed include among others: (i) protecting coral reef locations with biological and environmental conditions that render them less exposed or vulnerable to stress [15], [16], [17], [18]; and (ii) reducing anthropogenic disturbances such as overfishing and pollution, which are likely to reduce the resistance and tolerance of corals to radiation (temperature and ultraviolet light) stress [19], [20], [18]. Understanding of where, when and how global and local stressors affect corals can strengthen the decision support needed for appropriate coral reef management [7], [21], [22], [23], [24]. The two important considerations that have arisen from these multidisciplinary studies are: (i) assessment of the degree of exposure to multiple interacting stressors at different scales; and (ii) understanding how the environment interacts with the coral community structure and coral-algal symbiosis in influencing their sensitivity, vulnerability and adaptability to thermal, radiation and other physiological and biomechanical disturbances. The first of these two metrics are evaluated here as one of the important considerations that underpins the concepts of the resilience and vulnerability of coral reefs more generally [25].
Ecosystem vulnerability, although defined in different ways, is most often conceptualized as a function of the exposure, sensitivity and adaptive capacity of the perturbed organisms or ecosystems [26]. Sensitivity is a property of a system that is difficult to estimate and is dependent on the interaction between the biological and ecological characteristics of a system as well as on the attributes of the environmental stimulus [27]. Unlike sensitivity and adaptive capacity, exposure is an attribute of the relationship between the system and perturbations, rather than of the system itself [26]. These three metrics of vulnerability overlap and the environmental and biological processes that drive them are frequently interdependent [27]. For instance, many of the determinants of coral sensitivity (e.g. acclimatization) are similar to those that influence or constrain a system's adaptive capacity (e.g. genetic and species diversity, dispersal, and connectivity).
In this study, we derive a generic exposure metric and translate it into fuzzy logic mathematical expressions. The modelling of coral exposure, like many reef processes, is often hindered by poor knowledge of the physiology of corals complicated by contradicting theories on coral-environment interactions [18], sparse data, and poor precision [28]. Frequently, important observations are lacking and potentially valuable information may be non quantitative [29], which may limit the usefulness of these models. For example, the ability of corals to adapt or acclimatize to abnormal conditions is not well understood [30]. Fuzzy logic, first introduced by Zadeh [31], offers a methodology for dealing with these problems and provides an alternative approach to modelling complex systems. For example, translating data layers to fuzzy measures results in standardised measures of the possibility of belonging to a given set along a continuous scale from 0 to 1 [32]. This approach is more realistic than a binary set membership rule as is used in Boolean analyses, especially when there is uncertainty inherent in the input data [29].
Stressor interactions, coral response and environmental thresholds
In benthic aquatic habitats, the light and temperature environment is highly dynamic and is primarily a function of hydrodynamics (tidal regime, currents, and stratification), cloud cover, and turbidity among other factors [33], [34], [35]. For instance, extreme tides in turbid waters causes a much greater increase in benthic irradiance than in clear water [34], [36], which has been shown to cause significant coral mortality [34], [37], [38], [39]. Moreover, as wind speed falls, vertical-mixing decreases, resulting in decreased evaporative cooling and transfer of deeper cool water, which increases the likelihood of thermal stress on corals [6], [33], [40]. Based on published hypotheses and conceptual deductions about the likely response of corals to a given stressor (Appendix S1), we use a systems analytical approach to idealize the coral-environment relationships. We considered a series of composite stressors derived from combinations of sea surface temperature (SST), UV irradiance, wind speed, tidal range, and chlorophyll a concentration data. SST, UV, wind magnitude and consistency (together referred here as radiation) are considered to be the primary climatic drivers of coral reef exposure. Tides and SST variability are considered to be stress antagonistic or reducing variables that mitigate the primary climatic stressors. Sedimentation and eutrophication are stress reinforcing or exacerbating interactive stressors because they can undermine the resilience of the coral reef ecosystem through either undermining physiological homeostasis or the recovery processes after disturbance [12], [41]. Coral exposure is a function of derived stressors that interact with radiation having either reinforcing (additive or multiplicative) or reducing affects (antagonistic) [2], [42], [43]. It is this combination of reinforcing and reducing effects that causes the complex and sometimes surprising behavior of composite coral-environmental systems that is not well predicted by simple models that consider one or few coral-environmental variables [44].
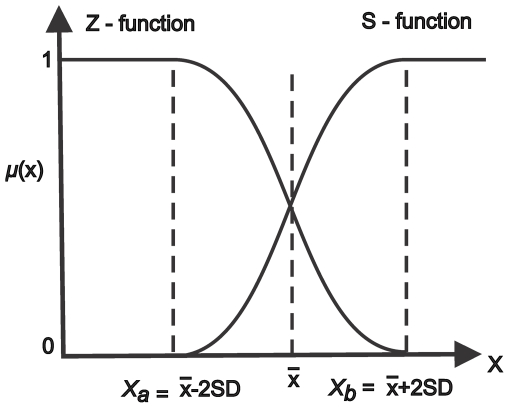
Most methods for estimating thresholds of environmental attributes, such as thermal and sediment levels, above which stress responses such as coral bleaching, diseases and mortality are likely to occur [6], [45], [46] mostly rely on availability of response observations (e.g., [47]). There are limited insights for identifying when thresholds may be crossed, in a setting with interactive, and cumulative impacts of multiple stressors, which often result in spurious and confounding effects [2], [24]. In addition, a system's response to stressors can adopt various linear and non-linear complex behaviour patterns, which for modelling purposes can be represented in many forms of fuzzy logic membership functions including trapezoidal, sinusoidal, logistic, Gaussian etc [2], [48]. In this study, we estimate environmental limits of corals (x a and x b) based on the distribution of global environmental data for locations where corals are found. We assume that geophysical variables in coral reef areas are distributed normally, where x a and xb are two standard deviations from the mean on the lower and upper tail. For simplicity, we assign a normal cumulative function (represented as logistic curve in fuzzy logic membership function) as the response of the interaction between coral and environment, where coral exposure is a function of the environmental variables considered, and initially increases or decreases exponentially along the environmental gradient respectively above or below the user defined minimum threshold (x a), before levelling off at a user defined maximum threshold (xb) [2] (Fig. 1).
Figure 1. Increasing (S curve) and decreasing (Z curve) sigmoidal membership functions, which were used to standardize the environmental data.
x
a and x
b are the control points for the lower and upper bounds along the stressor gradient; SD is the standard deviation, while 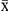 is the mean.
is the mean.
Because coral bleaching and mortality is driven by factors such as temperature and their interactions with other stressors like pollution and sedimentation, it may be possible to prevent some damage by reducing the impact of stressors that are not related to climate change [18], [19]. Additionally, fishing can influence grazers and algae and subsequently influence the overall recovery rates and resilience to climate disturbances [9]. This study aims to identify the global spatial gradients of thermal and eutrophication stressors and of the key factors that reduce these stressors to develop a broad-scale metric of environmental exposure for coral reefs. In addition, we address the questions: (i) which of these stressors are corals most exposed to in their respective locations, (ii) which reef locations are least and most exposed to thermal and UV radiation and sedimentation stress, and (iii) how do these stress and reinforcing and reducing variables interact globally?
Materials and Methods
We used environmental data from satellite observations and model outputs to derive variables that represent temperature and UV light (radiation), reinforcing and reducing stress.
Sea surface temperature
Sea surface temperature derived variables were obtained from the second version of the coral reef temperature anomaly database (CoRTAD) [23]. This database contains global SST and related thermal stress metrics at an approximately 4-km resolution weekly from 1982 through 2008, derived from measurements from the Advanced Very High Resolution Radiometer onboard NOAA suite of polar orbiting satellites. The global accuracy of the retrieval algorithm based on comparisons with in situ buoys indicates values of 0.02–0.5°C [49]. When compared with in situ temperature from data loggers at shallow depth in the western Indian Ocean, RMSE of 0.87°C were reported [16]. The CoRTAD reanalysis database has also been evaluated using in situ observations from different coral reef locations globally and at depths ranging from 0–9 m, which corresponds to depths of most coral reef habitats [23]. This evaluation reported RMSEs ranging from 0.49–0.81°C, and a coefficient of determination (R2) of 0.72–0.96 [23]. Overall, the performance of this data for global coastal applications is adequate, notwithstanding the fact that radiometers measure the temperature at the sea surface while most in situ measurements are based on bulk temperature at shallow depths.
We downloaded time series of weekly SST anomalies (WSSTAs), defined as the weekly averaged temperature in excess of 1°C or more above that week's long term average value; and thermal stress anomalies (TSAs), defined as the temperature excess of 1°C or more above the climatologically (long-term average) warmest week of the year (the warmest week of the 52 climatologically weeks averaged over 27 years) [23], from the National Oceanographic Data Center website (http://www.nodc.noaa.gov/sog/Cortad). Two different cumulative estimates of thermal stress were computed from each of these metrics: TSAs and WSSTAs were summed for each year and averaged over 27 years; and for each year, a maximum duration (in weeks) that WSSTA and TSA were greater than or equal to 1°C were computed and averaged over 27 years. These two metrics, the mean annual cumulative and mean yearly maximum duration, represent the characteristic magnitude and duration of the anomalies at a given location, which are important predictors of coral stress [23], [45]. Mean SST and the coefficient of variation for the 27-year monthly mean time series were also computed.
Chlorophyll and suspended solids
Oceanic satellite observations in the visible and near-infrared bands allow for the measurement of a variety of ocean color information including phytoplankton chlorophyll-a, total suspended matter (TSM), and colored dissolved organic matter (CDOM) [50], [51]. For modeling purposes, ocean waters are commonly described as being of Case I or case II types [52], [53]. The former type are those waters whose optical properties are determined primarily by phytoplankton and related colored dissolved organic matter (CDOM) and detritus degradation products; while the later represents the turbid coastal zones influenced by land drainage or sediment re-suspension, with optical properties mainly influenced by CDOM of terrestrial origin, mineral particles, various suspended sediments, urban discharges and industrial wastes [52].
The application of ocean color data in coral reef areas is limited by the complexity of the water's optical properties in shallow coastal environments where they are found. The standard Case I algorithm for deriving chlorophyll concentration fail in turbid coastal waters resulting in over estimation of chlorophyll along most coastal areas [53], even if due to terrestrial influence considerable enhancements of the algal biomass in these shallow zones is expected. Further, the standard algorithms for both water types were developed on the assumption of optically deep waters. Therefore in clear shallow bottoms that are highly complex or reflective as with the case in coral reefs and atolls, bottom reflection can induce an increase in marine reflectance, which is wrongly interpreted as ocean color constituents [54]. Given these problems, until special algorithms that take into account the complexity in coral reef areas are developed and incorporated in the standard processing chains of the current ocean color satellites, the usefulness of ocean color data for coral reef applications will remain limited [54], [55].
To derive chlorophyll estimates taking into account these problems we carried out a series of analyses with ocean color observations from the Sea-viewing Wide Field-of-view Sensor (SeaWiFS), Moderate Resolution Imaging Spectro-radiometer (MODIS), and Medium Resolution Imaging Spectrometer Instrument (MERIS) sensors (Appendix S2). The GlobColour processor at the European Space Agency's GlobColour project (http://hermes.acri.fr/GlobColour) was used to process Level 2 data from the three sensors to derive monthly level-3 binned products, including case I and case II chlorophyll concentrations with their respective flags, at a resolution 4.63 km at the equator (http://www.GlobColour.info/products_description.html). Data from all the three sensors were merged to derive case I Chlorophyll, while MERIS Case II algorithm was used to retrieve case II chlorophyll [56]. These Level 3 outputs do not spatially differentiate the regions where each of the water types are relevant; therefore further analysis using turbidity flags is required to discern and merge regions with the different water types into a homogenous continuous layer [53]. To achieve this, we used turbidity and depth flags (<30 m) derived from the processing of level 2 products, in a logical expression designed to merge respective case I and case II regions in a given month, and further to exclude shallow water (<30 m) pixels. Having masked shallower depths using the depth flags, we assumed similar water column properties in masked areas to those found in adjacent deeper (>30 m) water pixels, and extrapolated the deeper water pixels to these areas. To achieve this for each layer, we applied 3×3 spatial interpolator, which calculates the median value of 8 pixels adjacent to the pixel being considered. In effect, pixels adjacent to the missing value maintained their original values while the missing pixel was assigned the resulting value from the interpolator [16]. These monthly mean layers were then temporally aggregated for the long-term average.
Doldrums
Global sea surface wind speed (m s−1) estimates for 10 m above sea level at a 28-km resolution are available from the National Climatic Data Center (NCDC, ftp://eclipse.ncdc.noaa.gov/raid1b/seawinds). NCDC wind data is based on the blended observations from multiple sensors, with reduced spatial and temporal gaps of individual satellite samplings, and reduced sub-sampling aliases and random errors [57]. Despite the coastal application of this data by the Coral Reef Watch, inter-comparisons with other products have not been performed because sparse in-situ measurements over the vast ocean surface make errors difficult to quantify [57]. Nonetheless, measurements from each sensor are passed through quality control prior to blending and gridding. Additionally, the blending of cross-calibrated multiple satellite observations is known to increase accuracy and resolution [57], [58].
Daily averaged wind speeds (2000–2009) and the averaged 10-year mean monthly wind speeds (1995–2004) were downloaded. The National Oceanic and Atmospheric Administration (NOAA) coral reef watch defines doldrums as wind conditions with a daily mean of less than 3 m s−1. To estimate the magnitude and consistency of wind regimes in a given location, a doldrums metric was computed by taking the annual average maximum number of days that wind speeds were greater than 3 m s−1 over 10 years (2000–2009) and multiplying this by the 10-year mean monthly average.
Tidal model
Over the last decade, the tidal research group of Le Provost and collaborators have produced a series of finite element solution (FES) tidal atlases; FES-2004 is the latest release. Data are computed from the tidal hydrodynamic equations and tide gauges and altimeter data assimilation [59]. When cross-validated with other tidal products, the FES-2004 atlas was found to be the most accurate, with improved performance in shelf and coastal areas and moderately deeper areas [59], [60]. The accuracy of the 15 tidal components used in the model ranges from 2–12 cm and varies by region [60]. Therefore, local applications would require calibration with tidal observations at the same scale.
The digital FES-2004 tidal model and the associated extraction software were downloaded from the Laboratoire d'Etudes en Géophysique et Océanographie Spatiales website (http://www.legos.obs-mip.fr/en/soa) [59], [60]. The software in C++ was modified to enable gridding of the tidal predictions for a user defined spatial and temporal extents. To minimize the computer processing time, the model's temporal resolution was degraded from hourly to 6-hr interval. These predictions were then aggregated for average, minimum, and maximum heights over seven day intervals and gridded at the model's spatial resolution of roughly 14-km. To capture the long-term conditions and variability, the model was run for 8 years from 1987 with a three-year interval, including 1987, 1990, 1993, 1996, 1999, 2002, 2005, and 2008. Tidal ranges were computed as the long term averaged difference between the weekly maximum and minimum simulated tidal heights.
Ultraviolet radiation
Daily global maps of UV-erythemal (biologically damaging) irradiance at the Earth's surface (for the spectral range 290 to 400 nm and in the units of milli-watts m-2) in a 1 by 1.25 degree grid were retrieved for 1996 to 2001 from the NASA website (http://toms.gsfc.nasa.gov) [61], [62]. This data is derived from the total ozone mapping spectrometer (TOMS) on-board Earth Probe-TOMS satellite. Erythemal radiation is a weighted average of UVA (315–400 nm) and UVB (280 to 315 nm) used as a measure of skin irritation caused by exposure sunlight [63]. Errors associated with this data have not been ascertained for many parts of the world, however evaluations in Canada using a ground-based spectrometer reported absolute accuracy of 6% under normal conditions and 12% under conditions of UV absorbing aerosol plumes [61]. These uncertainties are mostly influenced by the amount of ozone, clouds and aerosols, and terrain height. In the ocean, depth attenuation and the optical properties of the seawater influence the amount of radiation below water surface [61], [64]. Radiative transfer modeling that includes the ocean system has been performed to estimate in-water radiation field [60], [65]. Here we use Erythermal UV with no correction for the seawater optical properties. Previous reports have shown a good correlation of this data with coral bleaching where observations were made at varying depth [16].
The current online values of UV irradiance and Erythemal exposure from EP-TOMS have errors after 2001, and therefore can not be used for UV changes as these are more prone to time-dependent errors from cloud cover and aerosols. The application of this data here is limited to global mean, where the overall error is expected to be relatively small, as the mainly negative cloud-height errors and other positive errors usually partly cancel, leading to an overall smaller error [66]. Consequently, UV average from 1997 to the end of 2001 was computed to represent local conditions in each grid square.
Coral exposure
Environmental variables were grouped into three categories based on the role that they play as coral stressors: (1) radiation variables, consisting of variables derived from temperature (mean SST, TSA and WSSTA magnitude and duration), UV-erythermal and wind speed data (doldrums index); (2) stress reinforcing variable (TSM and chlorophyll-a), representing sedimentation and eutrophication; and (3) stress reducing variables, consisting of SST variability and tidal range. Values of each variable that correspond with the approximately 4000 reef locations were extracted, and examined for normality and log10-transformations applied where necessary (Appendix S3). For each variable, a membership function with similar behavior pattern to a normal cumulative distribution function was used to characterize the relationship between coral exposure and a stress variable. Membership functions capture the degree to which the variable x is a member of a fuzzy set A using a suitably chosen function μ(x) [48]. Here we used spline-based logistic functions:
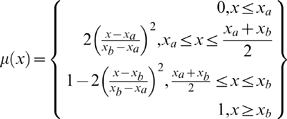 |
(1) |
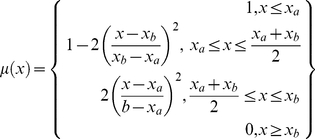 |
(2) |
where xa and xb are control values and correspond to the lower and upper bound of a stressor values, respectively (Table 1). These were calculated for each variable as the mean value of minus or plus two standard deviations, respectively. Radiation and reinforcing variables were normalized using an increasing curve (Eq. 1) and stress reducing variables were normalized using a decreasing curve (Eq. 2) (Fig. 1).
Table 1. Summary statistics for the variables used in the analyses based on (a) coral reef location points, and (b) all pixels within the image spatial boundaries (35N&S, 180E&W); (c) is control values xa & xb used in standardizing the variable layers.
| Radiation | Reducing | Reinforcing | ||||||||||
| Mean SST | Mean sum SSTA | Mean sum TSA | Mean SSTA duration | Mean TSA duration | UV | Doldrum index | Tidal range | SST coeff. of var. | Chlorophyll | TSM | ||
| (a) | Coral reef areas | |||||||||||
| N | 3822 | 3901 | 3901 | 3901 | 3901 | 3958 | 3963 | 3914 | 3822 | 3274 | 3325 | |
| Average | 26.9 | 16.0 | 3.9 | 2.0 | 0.8 | 250.0 | 20.1 | 0.7 | 5.6 | 0.7 | 0.8 | |
| Std dev | 1.3 | 1.2 | 1.4 | 1.4 | 1.6 | 22.9 | 1.5 | 2.3 | 1.6 | 1.2 | 1.5 | |
| Min | 20.8 | 11.0 | 1.0 | 0.7 | 0.1 | 136.6 | 1.0 | 0.1 | 1.5 | 0.0 | 0.1 | |
| Max | 29.6 | 52.0 | 24.3 | 10.6 | 5.7 | 322.4 | 111.5 | 3.3 | 21.9 | 13.8 | 44.9 | |
| (b) | Global values | |||||||||||
| Average | 22.1 | 21.0 | 13.9 | 5.7 | 4.0 | 244.3 | 21.5 | 0.7 | 20.9 | 0.2 | 0.8 | |
| Std dev | 4.4 | 1.5 | 2.6 | 2.6 | 2.6 | 45.6 | 1.7 | 2.2 | 2.2 | 0.6 | 0.8 | |
| Min | 14.5 | 0.9 | 0.0 | 0.0 | 0.0 | 124.5 | 0.0 | 0.0 | 1.3 | 0.0 | 0.1 | |
| Max | 29.7 | 90.3 | 84.7 | 16.5 | 12.9 | 419.6 | 134.8 | 4.9 | 51.8 | 27.2 | 45.2 | |
| (c) | Control values | |||||||||||
| Xa | 24.3 | 10.8 | 1.9 | 1.0 | 0.3 | 204.2 | 9.0 | 0.1 | 2.2 | 0.1 | 0.2 | |
| Xb | 29.6 | 23.7 | 8.2 | 4.1 | 2.1 | 295.8 | 45.1 | 3.6 | 14.4 | 2.4 | 2.0 | |
Mean SST and UV were not log transformed.
Spatial Principal Component Analyses (SPCA) was used to combine the standardized variables within each category. Principal Component Analysis transforms each variable into a linear combination of orthogonal common components (output layers), or latent variables with decreasing variation. The linear transformation assumes the components will explain all of the variance in each variable. Hence, for each output the latent component layer carries different information, which is uncorrelated with other components. This enables a reduction of output maps because the last transformed map(s) may be discarded as they have little or no variation left and may be virtually constant. The component weightings were calculated using coefficients of linear correlation to weigh the contribution of factors in spatial principal component analysis [67]. SPCA was performed to synthesize the standardized variables within radiation, stress reducing, and stress reinforcing categories. A final composite map from each of these three groups was computed by summing PC's with contribution ratio >1, weighted by their respective contribution ratio (Equation 3; [68], [16]).
| (3) |
where Yi is the ith principal component, while αi is its corresponding contribution ratio.
The output maps were standardized between zero and one, representing low and high exposure respectively. To combine the stress reducing and radiation variables, SPCA procedure described above was repeated with standardized radiation and reducing variables as the input variables. The output PC's were synthesized using a weighted sum equation (Eq. 3) to yield a layer with estimates of exposure to radiation taking into account the contribution from reducing variables. Fuzzy-integration-based approach was used to integrate the output from this procedure with the reinforcing variables into a single composite layer. [69] lists five fuzzy operators that are most useful for combining fuzzy data (AND, OR, sum, product and gamma). Given two fuzzy sets (standardized layers) A and B, the fuzzy sum operator produces a layer whose values are equal to or greater than each of the input layers A and B and results in an increased effect [69]. We therefore used fuzzy sum operator to reflect the reinforcing behaviour of sediment and eutrophication to radiation stress:
| (4) |
where 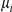 .is the membership value for i-th map, and i = A, B, n maps.
.is the membership value for i-th map, and i = A, B, n maps.
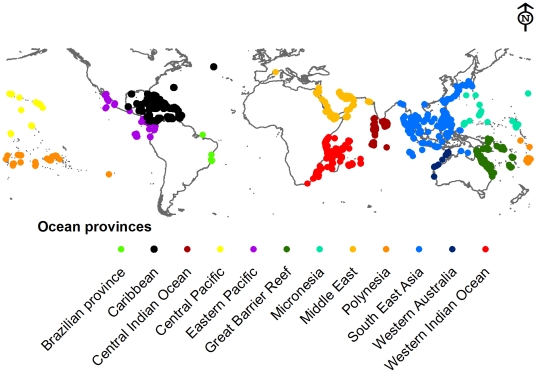
Coral reef location data was obtained from the Reef Base website (http://reefgis.reefbase.org/) and the Wildlife Conservation Society monitoring sites in the western Indian Ocean [70]. The location data were grouped into eleven oceanic provinces [9] (Fig. 2). For the respective locations, exposure metrics as described above were extracted for the corresponding locations. Box plots of exposure metrics by stressors against the coral reef provinces were plotted.
Figure 2. Coral reef locations grouped into eleven oceanic provinces after Donner (2009).
Coral reef locations were obtained from Reefbase (http://reefgis.reefbase.org/), WCS coral monitoring sites in the western Indian Ocean, and from Ateweberhan & McClanahan (2010).
Exposure gradation
Exposure gradation, also termed “defuzzification,” is a process where fuzzy application outputs are converted into a crisp output to facilitate their interpretation [48]. We used an iso-cluster (clustering) approach to partition exposure membership grades map into 4 user-defined clusters of statistically homogenous classes (i.e. low, moderate, high and severe).
Data for the three stress categories and for the final model were extracted for the sample reef locations. Correspondence analyses [71] were performed to detect the structural relationships among the oceanic provinces based on the three stress groups and on the exposure classes. The results of correspondence analysis were presented on a bi-plot that represents the configurations of points in projection planes formed by the first two principal axes [71]. To determine the distribution of sampled locations by region on the basis of their respective partial exposure scores, exposure space bi-plots of reinforcing against radiation and reducing stresses were generated. Contours were also drawn on these exposure space bi-plots based on the break points of final model exposure classes.
Results
Global patterns
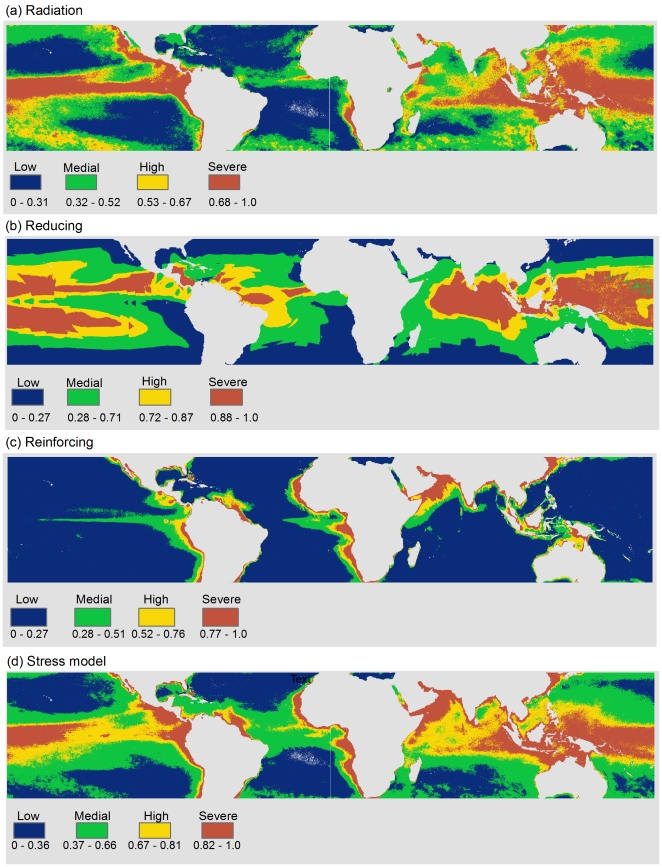
Analyses of the partial and overall exposure from the three stress groups indicate that corals at locations in all the 12 oceanic provinces were evaluated as highly exposed to radiation and reinforcing stress, albeit with spatial variability within the regions (Figs. 3, 4, 5). Ordination of the oceanic provinces by their respective exposure scores in each of the three stress groups in a correspondence analyses showed that 90% of the variation was captured by the first principal axis (c1) (Fig. 3a). The marginal variances explained by the stress categories and their relative position on the correspondence bi-plot indicates that reinforcing variables were most influential (negative in c1), and in descending order radiation and reducing (lack of); radiation stress was neutral among all regions; and the reducing stress had the lowest influence on the first axis (Fig. 3a).
Figure 3. Correspondence bi-plots of the oceanic provinces based on the three stress groups (radiation, reducing, and reinforcing) and based on exposure severity class.
(a & b respectively.
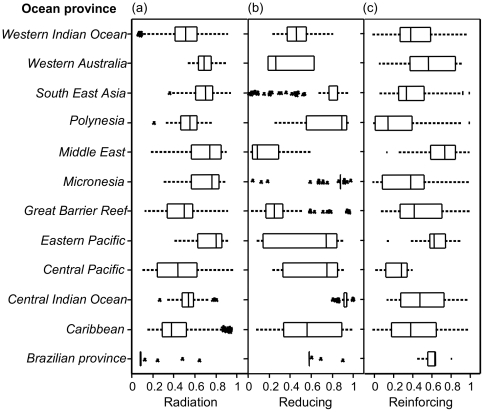
Figure 4. Box plots of distribution of, radiation, reducing, and reinforcing stress.
(a, b, & c respectively).
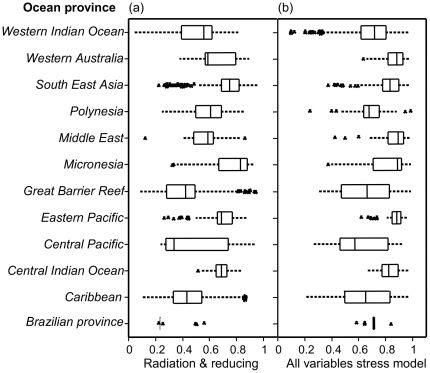
Figure 5. Box plots of distribution of combined radiation stress and stress reducing variables, and the overall exposure model.
(a & b respectively).
When the regions were grouped based on their assigned exposure grades (Fig. 3b), a pattern emerged where regions clustered around two exposure extremes as follows: South East Asia, Eastern Pacific, Micronesia, and the central Indian ocean grouped on the severe exposure extreme, primarily due to low reducing (high reducing scores) and high radiation stress scores (Fig. 3a, b), the Middle East and Western Australia were also in this group primarily due to high scores from reinforcing stress (Fig. 3a). The second cluster of regions strongly associated with moderate-high exposure included the Caribbean, Great Barrier Reef (GBR), Central Pacific, Polynesia and the western Indian Ocean, all with moderate-high scores from radiation and GBR strongly associated with reinforcing stress; while the Brazilian province with low exposure did not conform well to any of these groups (Table 2, Fig. 3a, b). Partial exposure scores from the three stress groups indicate that the Caribbean, GBR, South East Asia, and the western Indian Ocean were highly variable as depicted by the outliers in the lower and higher extremes of the whiskers (Fig. 4) and by the distribution of sample points in the exposure space bi-plots (Fig. 6b, f, j, l).
Table 2. Regional statistics for all three-stress groups, for radiation and reducing composite, and for the stress model.
| Ocean province | Radiation | Reducing | Reinforcing | Radiation & Reducing | Stress model | ||||||
| N | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Western Indian Ocean | 407 | 0.5 | 0.0 | 0.5 | 0.0 | 0.4 | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 |
| Western Australia | 26 | 0.7 | 0.0 | 0.4 | 0.0 | 0.6 | 0.1 | 0.7 | 0.0 | 0.9 | 0.0 |
| South East Asia | 412 | 0.7 | 0.0 | 0.8 | 0.0 | 0.4 | 0.0 | 0.7 | 0.0 | 0.8 | 0.0 |
| Polynesia | 123 | 0.5 | 0.0 | 0.8 | 0.0 | 0.3 | 0.0 | 0.6 | 0.0 | 0.7 | 0.0 |
| Middle East | 96 | 0.7 | 0.0 | 0.2 | 0.0 | 0.7 | 0.0 | 0.6 | 0.0 | 0.9 | 0.0 |
| Micronesia | 62 | 0.7 | 0.0 | 0.8 | 0.0 | 0.4 | 0.0 | 0.8 | 0.0 | 0.8 | 0.0 |
| Great Barrier Reef | 1530 | 0.5 | 0.0 | 0.3 | 0.0 | 0.5 | 0.0 | 0.4 | 0.0 | 0.7 | 0.0 |
| Eastern Pacific | 103 | 0.7 | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 | 0.7 | 0.0 | 0.9 | 0.0 |
| Central Pacific | 17 | 0.5 | 0.1 | 0.7 | 0.1 | 0.3 | 0.0 | 0.5 | 0.1 | 0.6 | 0.1 |
| Central Indian Ocean | 169 | 0.5 | 0.0 | 0.9 | 0.0 | 0.5 | 0.0 | 0.7 | 0.0 | 0.8 | 0.0 |
| Caribbean | 1035 | 0.4 | 0.0 | 0.6 | 0.0 | 0.4 | 0.0 | 0.4 | 0.0 | 0.7 | 0.0 |
| Brazilian province | 18 | 0.1 | 0.0 | 0.6 | 0.0 | 0.6 | 0.0 | 0.3 | 0.0 | 0.7 | 0.0 |
Figure 6. Exposure space bi-plots of reinforcing against combined radiation and reducing variables, with contours showing exposure grades (i.e. low, moderate, high, severe) based on the final exposure model.
The GBR, Middle East and Western Australia were, in relative terms, exposed to high stress reducing effect (thus low exposure scores) from tidal movement and high temperature variability, while the central Indian Ocean, Central and Eastern Pacific, Polynesia, and South East Asia were relatively exposed to low reducing effect as shown by the high partial exposure scores attributed to low stress reducing conditions (Fig. 4b). Western Indian Ocean and the Caribbean reefs were moderately exposed to reducing conditions with the later province being highly variable (Table 2, Fig. 3a; Fig. 4). In the Middle East, high reinforcing stress was mainly in the Persian Gulf (Bahrain and Iran) and the Gulf of Oman, Southern Red Sea and Gulf of Aden, and Southern Asia on the Gulf of Kutch on the Northerly Gujarat coast among other locations.
Regional patterns
The Central Pacific, Micronesia and Polynesia oceanic provinces were weakly exposed to reinforcing variables, and the overall exposure was largely due to high exposure to radiation stress (top and bottom right distribution of sample points in the exposure space bi-plots of Fig. 6 d,g,i). These regions were also exposed to relatively low stress-reducing effects alongside the central Indian Ocean and a more variable eastern pacific (Figs. 4, 5, 6). In South East Asia, all locations with low to moderate overall exposure grades (on the bottom left of the exposure space bi-plots) were in the Far East in the coral reefs of Japan, while the rest of the region was mostly high to severely exposed primarily from radiation stress and a low stress reducing effect (Fig. 6-j). The reinforcing effect was generally low to moderate, but some locations were highly exposed to reinforcing effect including Kagoshima Bay and Western Shikoku in Japan, Polillo Islands and Bolinao in the Philippines, Pari Island, East Kalimantan, and Tanjong Berakit in Indonesia, and several locations in Thailand, Cambodia and Malaysia (Fig. 7, See Appendix S4). Reefs in South East Asia, including the Islands of Peghu, and Peru in Taiwan and Indonesia respectively, Honcau and Holong Bay in Vietnam, and Shikoku in Japan are overall severely exposed, although they have low to moderate exposure to radiation they have high to severe exposure to reinforcing stress (Table 2, Fig. 7, and See Appendix S4).
Figure 7. Composite layers for radiation, reducing, reinforcing stress categories, and the overall stress model.
(a, b, c & d respectively).
Western Australia is exposed to severe conditions due to reinforcing stress. Despite the high exposure to doldrums, the tidal variability was high and likely to mitigate radiation stress, for example in Tantabiddi, mangrove islands, and Onslow reef, all had low to moderate radiation stress. Ningaloo and Abrolhos Islands have low radiation but were severely exposed to reinforcing stress alongside Dampier Archipelago, and in the South of Conzine and Withnell Bays. The reefs in the offshore islands off NW Australia in Seringapatam Reef, Hibernia, Timor Sea Reefs, Scott Reef, and Rowley Shoals were not exposed to high reinforcing stress, but were highly exposed to radiation stress due to high doldrums and low tide variability.
The Central Indian Ocean had many reefs with high exposure primarily due to radiation stress (Fig. 6c). These reefs are generally exposed to relatively low reinforcing stress, with the exception of reefs in Sri Lanka and India (Fig. 6c, Fig. 7). The GBR was moderately exposed to radiation and highly exposed to reinforcing stress, and to relatively high stress reducing effects of winds and tides. However, reefs in Kimbe Bay in Papua New Guinea and Solomon Islands in South-west Pacific were highly exposed to radiation stress.
The western Indian Ocean reefs were mostly ranked high to severe in overall exposure, except for some reefs mainly in South Africa, Mauritius, Reunion, Rodrigues, and Torres Reef in Mozambique, which were least exposed (Fig. 6-l, see Appendix S4). Although highly variable, the main stress contributor in this region was radiation and reinforcing stress. In most coral reef locations where exposure grade was severe, radiation and reinforcing stress were both high (Fig. 6-l). These reefs included Malindi and Kiunga in Kenya, reefs in southern Tanzania, most of western Madagascar, and Berreira and Vamizi reefs in Mozambique among others. Reefs at Grand River South East in Mauritius, Lagoa Pinnacle and Coral Gardens in Mozambique had low exposure to radiation stress but were severely exposed to reinforcing stress (Fig. 6-l, See Appendix S4).
In the Eastern Pacific, reef locations in the south were mostly severely exposed to radiation stress and included Inguana, Saboga, Uraba, Taboga, Contadora Islands, and the Gulf of Chiriqui in Panama, several reefs in Gorgona Island in Colombia, Culera Bay in Costa Rica, and the Galapagos Archipelago in Ecuador (Fig. 6-e, 7, and See Appendix S4). The northern part of the Eastern Pacific, including along the Gulf of California, had high reducing and reinforcing effects.
Overall the Caribbean region was moderate to highly exposed but also with high spatial heterogeneity in the exposure variables (Figs. 4, 5, 6, 7). The overall exposure of Caribbean reefs to radiation stress was moderate but several locations were outliers and highly exposed to radiation stress. Reef locations in high and severe exposure grades, with reinforcing as the major stress contributors, included reef locations in eastern Panama, Belize, the Bahamas, Cuba, eastern Mexico, and the Florida Keys (Fig. 7; See Appendix S4).
Discussion
Coral reefs globally are highly dependent on radiation, but are also exposed to radiation stress when values exceed normal seasonal and inter-annual ranges [72]. Stress, as used here, is the environmental exposure and does not distinguish the physiological acclimatization or genetic adaptation that determines the corals and other organisms' sensitivity to these forces. The degree of sensitivity will determine how organisms counter these stresses and therefore our metric is only a comparative baseline of the forces that are exogenous to the reef organisms. This exposure measure alone will not have predictive power in determining responses to the environment, which requires the sensitivity and adaptive capacity of the organisms, but does provide a basis for understanding the forces that these organisms face.
The results suggest considerable spatial heterogeneity globally but also some clear groupings based on our metrics of radiation stress and reinforcing and reducing variables. The spatial heterogeneity of coral stressors and their influence on coral physiology provide a basis to tailor management strategies that can address locally relevant threats [20], [73]. Determining the specific spatial locations with lower or higher cumulative stress and with significant non-climate change related stressors can assist this prioritization process. Despite the difficulties of discriminating among stressors [74], the results of this study demonstrate the utility of disaggregating stress into various components to emphasize management strategies and to effectively reduce the degradation of coral reefs [75]. The implications of this variability are discussed below in terms of the classification of reefs based on these variables and potential management recommendations.
There is increasing concern globally that enhanced runoff from human land uses is leading to the degradation of coral reefs [41]. It has been argued from studies on the inshore reefs of GBR that poor water quality lowers the radiation tolerance of scleractinian corals [12]. It has also been shown that the bioerosion, growth, and recovery rates of coral reefs are often slowed by high nutrient concentrations [41], [76]. Low water quality can reduce the stress of light and its interaction with temperature to increase bleaching response [72], [77]. However, corals stressed by sedimentation and eutrophication may have a lower capacity to tolerate the effects of other stressors and recover slower, making these factors as overall reinforcing variables [41], [78]. Consequently, if these studies are relevant globally, sedimentation and eutrophication reinforce coral reef stress and improved water quality will increase regional-scale resilience to global climate change.
Our results indicate that sedimentation and eutrophication (reinforcing stresses) are common in all regions, but differ in their intensity and co-occurrence with radiation and reducing stressors.
In the western Indian Ocean, coral locations exposed to high reinforcing stress correspond to those areas with high river runoff and sedimentation [79]–[82] (Fig. 6-l, See Appendix S4). These locations are exposed to moderate radiation stress but overall are severely exposed to high reinforcing effect of water quality from highland runoff. Local management of the coastal watershed in these areas is expected to shift the overall exposure towards lower severity grades. On the GBR, eutrophication is increasing principally due to land use in the adjacent coastal catchment area [83]–[86]. From our 1520 sample points in GBR, there is great variability but the majority of coral locations are moderately to highly exposed to water quality reinforcing stress (Fig. 4, 7). Given that the exposure of GBR reefs to radiation stresses are relatively moderate ((Fig. 6-f), a management strategy that improves water quality is predicted to increase reef resiliency [13], [78].
The central Indian Ocean lies within a different domain of exposure, where corals are exposed to high radiation stress but have little reinforcing stress, except in Sri Lanka and off India. Despite most of this region having small direct human impacts, synergistic effect of increased temperature and UV is the dominant stressor and has led to current significant coral declines associated with climatic anomalies [87]. In the most remote areas of the Chagos Islands, there is also evidence for fast reef recovery after these disturbances, which may arise from the low reinforcing stresses [7], [88]. Our results indicate that this region has low stress reducing effect from temperature variability and tidal amplitude, making it one of the most exposed to climate change alongside Micronesia and South East Asia.
In the Middle East, there was moderate to high radiation stress, with recent reports indicating exposure to high thermal anomalies [23] and similar conditions for the future [7]. Corals in the Middle East are also exposed to high levels of natural eutrophication, along with Western Australia, Eastern Pacific, and the GBR. Despite their exposure to extreme environments that are close to the limits of their thermal distribution [19], less frequent bleaching disturbances have been predicted in the future [7]. As a result, managing the highly eutrophic conditions and the chronic human impacts in these regions could possibly reduce coral decline.
In the Caribbean, coastal development—among other disturbances such as diseases and bleaching—has been associated with mortality of corals and the increase in macroalgae [74], [89]. Our study shows that coral reefs in the south western and the western boundary of the Caribbean, including Belize, reefs off Panama, Costa Rica, Colombia and Venezuela, are severely exposed to stresses, primarily due to reinforcing stress and moderate radiation stress and compounded by a low reducing effect. In Belize for example, there has been reports of high coral decline due to nutrification, bleaching, and diseases among other factors [74], [90], in agreement to our results indicating a high-severe exposure primarily due to reinforcing and radiation stresses (Fig. 2, See Appendix S4). Declines continue despite the integrated adaptive approach to marine protected area management currently in place since the late 1990's. This scenario provides an example of the difficulties of managing for both large-scale climate disturbances and the regulation of land-based sources of pollution and siltation in areas where the main sources of pollution are far away from the reefs [91], [92].
While these results are largely expected to correspond to the observed degree or extent to which coral reefs are subject to the perturbations, including the proximity to river discharges, coastal cities and agricultural areas, they may not necessarily correlate with the current reef status and observed changes in the respective regions or specific coral reef locations. Internal elements of biological and ecological adaptive capacity (i.e. genetic and species diversity, dispersal and connectivity) and sensitivity (e.g. acclimatization, overall health) that are critical to such predictions are not considered here and may explain mismatches between exposure and vulnerability. Recent model predictions are indicating that adaptations of corals through physiological and genetic changes of corals and zooxanthellae will not match the rate of temperature increase from climate change under the business-as-usual scenarios [30]. Environmental factors that counter the effects of radiation stressors or reduction of the reinforcing stress factors may play a greater role in the maintenance of the health of coral reefs.
Management implications
The global variability in coral exposure to stresses, as evidenced by the distribution of coral locations by region in the exposure space (Fig. 6) portrays the degree to which various management strategies are locally relevant. For example, the variability of exposure among coral reef locations in the Caribbean, GBR, South East Asia, and western Indian Ocean indicate the potential for a high within-region dynamics (Fig. 6). This offers an opportunity for spatially targeted management strategies to possibly reverse the well-documented significant decline of coral reefs in these regions (e.g. [45]). While management can act to reduce the exposure to anthropogenic pressures, few if any practical large-scale options exist for reducing climate related stress. Under this framework, effective local management needs to target moving reef locations, especially those that are moderately exposed to climate related stress, towards low reinforcing conditions through improved water quality.
Model limitations
The outputs of this study are constrained, among others, by the uncertainty conferred on the results of the membership functions and standardization algorithms. Insufficient or contradictory knowledge on the response of corals to environmental stimuli in the field and the local adaptation and species-specific responses to stress is the main limitation to creating predictive models. In addition, the use of proxies as a substitute for unavailable environment data, may limit the validity of the assumptions because of potential weak causation associated with correlation-based studies. For example, sedimentation and eutrophication proxy is used as a reinforcing variable and defined using a monotonically increasing sigmoid function, as suggested by some field studies [78]. This however contradicts other findings that suggest increased turbidity, which may result from increased chlorophyll, reduces the depth penetration of harmful UVB [36], thereby protecting corals. Similarly, high nutrients and heterotrophy associated with rich plankton and high chlorophyll may prevent the severity and impact of coral bleaching [93], [94]. In addition, the results of localized studies may not necessarily scale to an entire region [95]. The multiple interactive roles of turbidity is an example of the complex nature of multiple stressors, where even a single variable can be viewed mechanistically as multiple stressors with impacts of varying scales [1].
Our model assumes a negative linear relationship between thermal stress and SST variability whereas the relationship may be more complex [70]. Other studies evaluating large variability areas have indicated large thermal stress values in regions with the largest SST variability [9], [70]. This could result in uncertainties in areas of high SST variability in the Arabian Sea, Arabian Gulf, Eastern Pacific, Western Australia and the coast of Brazil. Further, the boundaries of our study preclude several other factors that affect coral health and an ideal systems analysis with unlimited global data for multiple threats would consider. These include: ocean acidification; fishery exploitation; hydrodynamic disturbances; abundance of bio-eroders and corallivores; and coral community structure, among others.
While the low-moderate resolution remote sensing data used in this study demonstrates sufficient variability for explaining large-scale biological processes [16], [23], a coarse grid ignores significant sub-grid details, and very often introduces approximations and uncertainties into model results [96]. The spatial and temporal aggregation, interpolation and integration of data from different spatial and temporal scales contribute to the errors from mismatch in spatial and temporal correlation structure [48].
Conclusions
Despite the limitations described above, these results can be applied to specific reefs if they are downscaled to incorporate indicators of resilience at reef scale [21], [97]. Through the framework presented, integrating many sources of spatially explicit data and scientific knowledge has identified global spatial gradients of radiation, sedimentation and eutrophication stressors and of the key factors that reduce these stresses. This provides a better understand how coral reefs might be managed better under conditions of environmental uncertainty and complexity.
There is high spatial variability of the relative exposures of corals to radiation and reinforcing stressors. Despite radiation stress being dominant, most reef locations identified as severely exposed due to radiation and reinforcing stress are expected to have a lower severity grade if the reinforcing effect from sedimentation and eutrophication were managed. Future studies should focus on incorporating additional coral threats such as acidification, the removal of grazers, and multiple interacting stresses. Enhancement of the knowledge base of the physiological response of corals to environmental stimulus can help improve future models.
Supporting Information
A summary of conceptual deductions of reef coral responses to environmental variables (adopted from [16] ).
(DOC)
A conceptual framework adopted for the analysis of ocean color data.
(TIF)
Normal cumulative density functions fitted on respective environmental parameters (log transformed except for SST and UV).
(TIF)
A table of coral exposure indices i.e. radiation, reducing, reinforcing, each set of coordinates represents coral reef location within respective ocean provinces.
(DOC)
Acknowledgments
Dr. Kenneth Casey and Tess Brandon of NOAA National Oceanographic Data Center (NODC) provided access to the CoRTAD database. Mr. Willem Niewenhuis of the International Institute of Geo-Information Science and Earth Observation helped with FES2004 modifications. The merged satellites ocean color products were processed by ACRI-ST GlobColour service of MyOcean & ESA GlobColour Projects. Phillippe Ganerson and Julien Demaria of ACRI-ST, France, Dr. Roland Doerffer of GKSS research centre, Germany, and Dr. Valborg Byfield of National Oceanographic centre provided useful insights on ocean colour analysis for coastal areas. Anonymous reviewers provided useful comments on the manuscript. All are appreciated.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Macquarie University's Higher Degree Research program, the Australian Research Council, and the Wildlife Conservation Society marine programs through financial support from the John D and Catherine T MacArthur Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hughes TP, Connell JH. Multiple Stressors on Coral Reefs: A Long-Term Perspective. Limnology and Oceanography. 1999;44:932–940. [Google Scholar]
- 2.Halpern SA, McLeod KL, Rosenberg AA, Crowder LB. Managing for cumulative impacts in ecosystem-based management through ocean zoning. Ocean & Coastal Management. 2008;51:203–211. [Google Scholar]
- 3.Sheppard CRC. Predicted recurrences of mass coral mortality in the Indian Ocean. Nature. 2003;425:294–297. doi: 10.1038/nature01987. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- 5.Veron JEN, Hoegh-Guldberg O, Lenton TM, Lough JM, Obura DO, et al. The coral reef crisis: The critical importance of 350 ppm CO2. Marine Pollution Bulletin. 2009;58:1428–1436. doi: 10.1016/j.marpolbul.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world's coral reefs. Marine and Freshwater Research. 1999;50:839–866. [Google Scholar]
- 7.Donner SD. Coping with Commitment: Projected Thermal Stress on Coral Reefs under Different Future Scenarios. PLoS ONE. 2009;4:e5712. doi: 10.1371/journal.pone.0005712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClanahan TR. The near future of coral reefs. Environmental Conservation. 2002;29:460–483. [Google Scholar]
- 9.Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton N, Jackson JBC. Shifting Baselines, Local Impacts, and Global Change on Coral Reefs. PLoS ONE. 2008;6:e54. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carilli JE, Norris RD, Black BA, Walsh SM, McField M. Local Stressors Reduce Coral Resilience to Bleaching. PLoS ONE. 2009;4:e6324. doi: 10.1371/journal.pone.0006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wooldridge SA. Water quality and coral bleaching thresholds: Formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Marine Pollution Bulletin. 2009;58:745–751. doi: 10.1016/j.marpolbul.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Bellwood DR, Hughes TP, Folke C, Nystrom N. Confronting the Coral Reef Crisis. Nature. 2004;429:827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 14.Mumby PJ, Hastings A, Edwards HJ. Thresholds and resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 15.Game ET, McDonald-Madden E, Puotinen MJ. Should We Protect the Strong or the Weak? Risk, Resilience, and the Selection of Marine Protected Areas. Conservation Biology. 2008;22:1619–1629. doi: 10.1111/j.1523-1739.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 16.Maina J, Venus V, McClanahan TR, Ateweberhan M. Modelling susceptibility of coral reefs to environmental stress using remote sensing data and GIS models in the western Indian Ocean. Ecological Modelling. 2008;212:180–199. [Google Scholar]
- 17.McClanahan TR, Cinner JE, Maina J, Graham NAJ, Daw T, et al. Conservation action in a changing climate. Conservation Letters. 2008;1:53–59. [Google Scholar]
- 18.Baskett ML, Nisbet RM, Kappel CV, Mumby PJ, Gaine SD. Conservation management approaches to protecting the capacity for corals to respond to climate change: a theoretical comparison. Global Change Biology. 2010;16:1229–1246. [Google Scholar]
- 19.Baker AC, Glynn PW, Riegl B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine, Coastal and Shelf Science. 2008;80:435–471. [Google Scholar]
- 20.Bellwood DR, Fulton CJ. Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea-levels and climate change? Limnology and Oceanography. 2008;53:95–2701. [Google Scholar]
- 21.West JM, Salm RV. Resistance and Resilience to Coral Bleaching: Implications for Coral Reef Conservation and Management. Conservation Biology. 2003;17:956–967. [Google Scholar]
- 22.Crabbe MJC. Climate change, global warming and coral reefs: Modelling the effects of temperature. Computational Biology and Chemistry. 2008;32:311–314. doi: 10.1016/j.compbiolchem.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Selig ER, Casey KS, Bruno JF. New insights into global patterns of ocean temperature anomalies: implications for coral reef health and management. Global Ecology and Biogeography. 2010;19:397–411. [Google Scholar]
- 24.Houk P, Musburger C, Wiles P. Water Quality and Herbivory Interactively Drive Coral- Reef Recovery Patterns in American Samoa. PLoS ONE. 2010;5:e13913. doi: 10.1371/journal.pone.0013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes TP, Graham NJ, Jackson JC, Mumby PJ, Steneck P. Rising to the challenge of sustaining coral reefresilience. Trends in Ecology and Evolution. 2010;11:633–642. doi: 10.1016/j.tree.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Adger NA. Vulnerability. Global Environmental Change. 2006;16:268–281. [Google Scholar]
- 27.Smit B, Wandel J. Adaptation, adaptive capacity and vulnerability. Global Environmental Change. 2006;16:282–292. [Google Scholar]
- 28.Meesters EH, Bak RPM, Westmacott S, Ridgley M, Dollar S. A Fuzzy Model to Predict Coral Reef Development under Nutrient and Sediment Stress. Conservation Biology. 1998;12:957–965. [Google Scholar]
- 29.Silvert W. Ecological impact classification with fuzzy sets. Ecological Modelling. 1997;96:1–10. [Google Scholar]
- 30.Baskett ML, Gaines SD, Nisbet ARM. Symbiont diversity may help coral reefs survive moderate climate change. Ecological Applications. 2009;19:3–17. doi: 10.1890/08-0139.1. [DOI] [PubMed] [Google Scholar]
- 31.Zadeh LA. The concept of a linguistic variable and its application to approximate reasoning. Information Science. 1965;8:199–249. [Google Scholar]
- 32.Eastman JR. IDRISI Kilimanjaro, Guide to GIS and Image Processing. User's guide (ver. 14), Worcester, MA: Clark University Press; 2003. [Google Scholar]
- 33.Mumby PJ, Chisholm JRM, Edwards AJ, Andrefouet S, Jaubert J. Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Marine Ecology progress Series. 2001;222:209–216. [Google Scholar]
- 34.Anthony KRN, Ridd PV, Orpin AR, Larcombe P, Lough J. Temporal variation of light availability in coastal benthic habitats: effects of clouds, turbidity and tides. Limnology and Oceanography. 2004;49:2201–2211. [Google Scholar]
- 35.Anthony KRN, Kerswell AP. Coral mortality following extreme low tides and high solar radiation. Marine Biology. 2007;151:1623–1631. [Google Scholar]
- 36.Dunne RP, Brown BE. Penetration of solar UVB radiation in shallow tropical waters and its potential biological effects on coral reefs; results from the central Indian Ocean and Andaman Sea. Marine Ecology progress Series. 1996;144:109–118. [Google Scholar]
- 37.Loya Y. Effects of water turbidity and sedimentation on community structure Puerto Rican corals. Bulletin of Marine Science. 1976;26:450–466. [Google Scholar]
- 38.Baird AH, Marshall PA. Mortality, growth and reproduction in scleractnian corals following bleaching on the Great Barrier Reef. Marine Ecology progress Series. 2002;237:133–141. [Google Scholar]
- 39.Brown BE, Dunne RP, Goodson MS, Douglas AE. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs. 2002;21:119–126. [Google Scholar]
- 40.Dunne RP, Brown BE. The influence of solar radiation on bleaching of shallow water reef corals in the Andaman Sea 1993–1998. Coral Reefs. 2001;20:201–210. [Google Scholar]
- 41.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Marine Pollution Bulletin. 2005;50:125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 42.Darling ES, Cote IM. Quantifying the evidence for ecological synergies. Ecology Letters. 2008;11:1278–1286. doi: 10.1111/j.1461-0248.2008.01243.x. [DOI] [PubMed] [Google Scholar]
- 43.Dunne RP. Synergy or antagonism - interactions between stressors on coral reefs. Coral Reefs. 2010;29:145–152. [Google Scholar]
- 44.McClanahan TR, Ateweberhan M, Sebastian CR, Graham NAJ, Wilson SK, et al. Predictability of coral bleaching from synoptic satellite and in situ temperature observations. Coral Reefs. 2007;26:695–701. [Google Scholar]
- 45.Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biology. 2007;5:e124. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams GJ, Aeby GS, Cowie ROM, Davy SK. Predictive Modeling of Coral Disease Distribution within a Reef System. PLoS ONE. 2010;5:e9264. doi: 10.1371/journal.pone.0009264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian SS, King RS, Richardson CJ. Two statistical methods for the detection of environmental thresholds. Ecological Modelling. 2003;166:87–97. [Google Scholar]
- 48.Burrough P, McDonnell R. Principles of Geographical Information Systems. New York: Oxford University Press; 2005. [Google Scholar]
- 49.Kilpatrick KA, Podesta GP, Evans R. Overview of the NOAA/NASA Advanced Very High Resolution Radiometer Pathfinder algorithm for sea surface temperature and associated matchup database. Journal of Geophysical Research-Oceans. 2001;106:9179–9197. [Google Scholar]
- 50.Wang H, Hladik CM, Huang W, Milla K, Edmiston L, et al. Detecting the spatial and temporal variability of chlorophyll-a concentration and total suspended solids in Apalachicola Bay, Florida using MODIS imagery. International Journal of Remote Sensing. 2010;3:439–453. [Google Scholar]
- 51.Zhao D, Xing X, Liu Y, Yang J, Wang L. The relation of chlorophyll-a concentration with the reflectance peak near 700 nm in algae-dominated waters and sensitivity of fluorescence algorithms for detecting algal bloom. International Journal of Remote Sensing. 2010;31:39–48. [Google Scholar]
- 52.Morel A, Prieur L. Analysis of Variations in Ocean Color. Limnology and Oceanography. 1977;22:709–722. [Google Scholar]
- 53.Morel A, Bélanger S. Improved detection of turbid waters from ocean colour sensors information. Remote Sensing of Environment. 2006;102:237–249. [Google Scholar]
- 54.Boss E, Zaneveld JRV. The Effect of Bottom Substrate on Inherent Optical Properties: Evidence of Biogeochemical Processes. Limnology and Oceanography. 2003;48:346–354. [Google Scholar]
- 55.Mumby PJ, Skirving W, Strong AE, Hardy JT, LeDrew EF, et al. Remote sensing of coral reefs and their physical environment. Marine Pollution Bulletin. 2004;48:219. doi: 10.1016/j.marpolbul.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder T, Behnert I, Schaale M, Fischer J, Doerffer R. Atmospheric correction algorithm for MERIS above case-2 waters. International Journal of Remote Sensing. 2007;28:1469–1486. [Google Scholar]
- 57.Zhang HM, Bates JJ, Reynolds RW. Assessment of composite global sampling: Sea surface wind speed. Geophysical Research Letters. 2006;33:L17714. [Google Scholar]
- 58.Zhang HM, Reynolds RW, Lumpkin R, Molinari R, Arzayus K, et al. An integrated global observing system for sea surface temperature using satellites and in situ data: Research to operations. Bullettin of American Meteorological Society. 2009;90:31–38. [Google Scholar]
- 59.Le Provost C, Lyard F, Molines JM, Genco ML, Rabilloud F. A hydrodynamic ocean tide model improved by assimilating a satellite altimeter-derived data set. Geophysical Research Letters. 1998;103:5513–5529. [Google Scholar]
- 60.Lyard F, Lefevre F, Letellier T, Francis O. Modelling the global ocean tides: modern insights from FES2004. Ocean Dynamics. 2006;56:394–415. [Google Scholar]
- 61.Herman JR, Krotkov NA, Celarier E, Larko D, Labow G. Distribution of UV radiation at the Earth's surface from TOMS-measured UV-backscattered radiances. Geophysical Research Letters. 1999;104:12,059–12,076. [Google Scholar]
- 62.Vasilkov A, Krotkov N, Herman J, McClain CR, Arrigo K, et al. Global mapping of underwater UV irradiances and DNA-weighted exposures using Total Ozone Mapping Spectrometer and Sea-viewing Wide Field-of-view Sensor data products. Geophysical Research Letters. 2001;106:27,205–27,219. [Google Scholar]
- 63.McKinlay AF, Diffey BL. Passchier WR, Bosnjakovic BFM, editors. A reference action spectrum for ultraviolet induced erythema in human skin, Human Exposure to Ultraviolet Radiation:Risks and Regulations, Elsevier, Amsterdam. 1987.
- 64.Dunne RP. The use of remotely sensed solar radiation data in modeling suscepti bility of coral reefs to environmental stress: Comment on Maina et al. [Ecol. Model. 212 (2008) 280-199]. Ecological modeling. 2008;218:188–191. [Google Scholar]
- 65.Vasilkov AP, Herman JR, Ahmad Z, Kahru M, Mitchell BG. Assessment of the ultraviolet radiation field in ocean waters from space-based measurements and full radiative-transfer calculations. Applied Optics. 2005;44:2863–2869. doi: 10.1364/ao.44.002863. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Newchurch MJ, Kim JH. Occurrence of ozone anomalies over cloudy areas in TOMS version-7 level-2 data. Atmospheric Chemistry and Physics. 2003;3:187–223. [Google Scholar]
- 67.Parinet B, Lhote A, Legube B. Principal component analysis: an appropriate tool for water quality evaluation and management-application to a tropical lake system. Ecological Modelling. 2004;178:295–311. [Google Scholar]
- 68.Li A, Wang A, Liang S, Zhoua W. Eco-environmental vulnerability evaluation in mountainous region using remote sensing and GIS-A case study in the upper reaches of Minjiang River, China. Ecological Modelling. 2006;192:175–187. [Google Scholar]
- 69.An P, Moon WM, Rencz A. Application of fuzzy set theory to integrated mineral exploration. Canadian Journal of Exploration Geophysics. 1991;27:1–11. [Google Scholar]
- 70.Ateweberhan M, McClanahan TR. Relationship between historical sea-surface temperature variability and climate change-induced coral mortality in the western Indian Ocean. Marine Pollution Bulletin. 2010;60:964–970. doi: 10.1016/j.marpolbul.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 71.Legendre P, Legendre L. Numerical ecology, second English ed. Elsevier Science BV, Amsterdam; 1998. [Google Scholar]
- 72.Coles SL, Brown BE. Coral bleaching - Capacity for acclimatization and adaptation. Advances in Marine Biology. 2003;46:183–223. doi: 10.1016/s0065-2881(03)46004-5. [DOI] [PubMed] [Google Scholar]
- 73.McClanahan TR, Ateweberhan M, Omukoto J, Pearson L. Recent seawater temperature histories, status, and predictions for Madagascar's coral reefs. Marine Ecology Progress Series. 2009;380:117–128. [Google Scholar]
- 74.Mora M. A clear human footprint in the coral reefs of the Caribbean. Proceedings of Royal Society B. 2008;275:767–773. doi: 10.1098/rspb.2007.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palumbi SR. Germ theory for ailing corals. Nature. 2005;434:713–714. doi: 10.1038/434713a. [DOI] [PubMed] [Google Scholar]
- 76.Carreiro-Silva M, McClanahan TR, Kiene WE. Effects of inorganic nutrients and organic matter on microbial euendolithic community composition and microbioerosion rates. Marine Ecology Progress Series. 2009;392:1–15. [Google Scholar]
- 77.Yentsch CS, Yentsch CM, Cullen JJ, Lapointe B, Phinney DA, et al. Sunlight and water transparency: cornerstones in coral research. Journal of Experimental Marine Biology and Ecology. 2002;268:171–183. [Google Scholar]
- 78.Wooldridge SA, Done TJ. Improved water quality can ameliorate effects of climate change on corals. Ecological Applications. 2009;19:1492–1499. doi: 10.1890/08-0963.1. [DOI] [PubMed] [Google Scholar]
- 79.McClanahan TR, Obura DO. Sedimentation effects on shallow coral communities in Kenya. Journal of Experimental Marine Biology and Ecology. 1997;209:103–122. [Google Scholar]
- 80.Fleitmann D, Dunbar RB, McCulloch M, et al. East Africa soil erosion recorded in a 300 year old coral colony from Kenya. Geophysical Research Letters. 2007;34:L04401. doi: 04410.01029/02006GLO028525. [Google Scholar]
- 81.Rakotoarison HF. Evaluation Economique des Bénéfices Hydrologiques du Programme Environment III a Madagascar. 2003. Mémoire de fin d'Etudes. Université d'Antananarivo, Madagascar.
- 82.Albietz JM. Watershed protection for ecosystem services in the Makira Forest Area, Madagascar: a preliminary biophysical assessment. 2006. Unpublished paper prepared for Wildlife Conservation Society, Antananarivo, Madagascar.
- 83.Furnas M. Catchments and corals: Terrestrial runoff to the Great Barrier Reef. 2003. Australian Institute of Marine Science, Queensland.
- 84.McKergow LA, Prosser PI, Hughes AO, Brodie J. Sources of sediment to the Great Barrier Reef World Heritage Area. Marine Pollution Bulletin. 2005;51:200–211. doi: 10.1016/j.marpolbul.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 85.Cooper TF, Uthicke S, Humphrey C, Fabricius KE. Gradients in water column nutrients, sediment parameters, irradiance and coral reef development in the Whitsunday Region, central Great Barrier Reef Estuarine. Coastal and Shelf Science. 2007;74:458–470. [Google Scholar]
- 86.Devlin MJ, Brodie J. Terrestrial discharge into the Great Barrier Reef Lagoon: nutrient behaviour in coastal waters. Marine Pollution Bulletin. 2005;5:9–22. doi: 10.1016/j.marpolbul.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 87.Sheppard CRC. Coral decline and weather patterns over 20 years in the Chagos Archipelago, central Indian Ocean. Ambio. 1999;28:472–478. [Google Scholar]
- 88.Sheppard CRC, Harris A, Sheppard ALS. Archipelago-wide coral recovery patterns since 1998 in the Chagos Archipelago, central Indian Ocean. Marine Ecology Progress Series. 2008;362:109–117. [Google Scholar]
- 89.Schutte VGW, Selig ER, Bruno JF. Regional spatio-temporal trends in Caribbean coral reef benthic communities. Marine Ecology Progress Series. 2010;402:115–1222. [Google Scholar]
- 90.Aronson RB, Macintyre IG, Precht WF, Murdoch TJT, Wapnick CM. The expanding scale of species turnover events on coral reefs in Belize. Ecological Monographs. 2002;72:233–249. [Google Scholar]
- 91.Gibson J, Mcfield M, Wells S. Coral reef management in Belize: an approach through Integrated Coastal Zone Management. Ocean & Coastal Management. 1998;39:229–244. [Google Scholar]
- 92.McClanahan TR, Muthiga NA. An ecological shift in a remote coral atoll of Belize over 25 years. Environmental Conservation. 1998;25:122–130. [Google Scholar]
- 93.McClanahan TR, Sala E, Stickels PA, Cokos BA, Baker AC, et al. Interaction between nutrients and herbivory in controlling algal communities and coral condition on Glover's Reef, Belize. Marine Ecology Progress Series. 2003;261:135–147. [Google Scholar]
- 94.Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R. Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Functional Ecology. 2009;23:539–550. [Google Scholar]
- 95.Guidetti P, Sala E. Community-wide effects of marine reserves in the Mediterranean Sea. Marine Ecology Progress Series. 2007;335:43–56. [Google Scholar]
- 96.Isukapalli SS, Georgopoulos PG. Computational Methods for Sensitivity and Uncertainty Analysis for Environmental and Biological Models. New Jersey: Environmental and Occupational Health Sciences Institute; 2001. [Google Scholar]
- 97.Maynard JA, Marshall PA, Johnson JE, Harman S. Building resilience into practical conservation: identifying local management responses to global climate change in the southern Great Barrier Reef. Coral Reefs. 2010;29:381–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A summary of conceptual deductions of reef coral responses to environmental variables (adopted from [16] ).
(DOC)
A conceptual framework adopted for the analysis of ocean color data.
(TIF)
Normal cumulative density functions fitted on respective environmental parameters (log transformed except for SST and UV).
(TIF)
A table of coral exposure indices i.e. radiation, reducing, reinforcing, each set of coordinates represents coral reef location within respective ocean provinces.
(DOC)