Abstract
Objective
To assess the impact of the rate of spontaneous resolution on the relative cost-effectiveness of deferred nasolacrimal duct probing in a surgical facility compared with immediate office-based probing for congenital nasolacrimal duct obstruction (NLDO)
Methods
Data from the literature, Medicare 2009 fee schedule, and consensus assumptions were combined to populate a model of outcomes of two treatment strategies: immediate office-based probing surgery (IOPS) and facility-based probing surgery deferred for 6 months (DFPS). Sensitivity analyses were conducted varying the 6-month spontaneous resolution rate from 50% to 90%. Additional analyses varied factors including surgical cost and each procedure’s probability of success. Outcomes are overall cost of treatment, chance of cure, and months of symptoms avoided by 18 months of life.
Results
Under the base-case assuming a 75% spontaneous resolution during 6 months prior to deferred probing, IOPS is more expensive ($771 vs. $641) and slightly less effective (93.0% vs. 97.5%) than DFPS, although IOPS costs only $44 per month of symptoms avoided. At spontaneous resolution rates between 50% and 68%, IOPS costs less than DFPS (from $2 to $342 less), although it also is slightly less effective (from 2.0% to 3.8% less). At a 90% spontaneous resolution rate, IOPS costs $169 per month of symptoms avoided. As the rate of spontaneous resolution falls, the cost per additional success for DFPS increases to $16,709 at a 50% spontaneous resolution rate.
Conclusion
The relative cost-effectiveness of these strategies for NLDO is critically dependent on the spontaneous resolution rate after presentation.
Introduction
Nasolacrimal duct obstruction (NLDO) is a common condition in the first year of life. Many cases will resolve spontaneously or with massage by 12 months of age.1–4 For children less than 6 months of age, non-surgical treatment is usually administered including antibiotic eyedrops and massage of the lacrimal sac. After 6 months of age, some surgeons offer probing of the nasolacrimal duct (NLD) to clear the blockage in the office setting with topical anesthesia. Other surgeons continue medical management for a time followed by probing under general anesthesia in a hospital outpatient department or ambulatory surgery center, if spontaneous resolution has not occurred. The advantages of early office probing are avoidance of general anesthesia, immediate resolution of symptoms, fewer physician visits, fewer antibiotic prescriptions, and less costly procedures. The advantages of deferring the probing include more subject comfort with the procedure, and complete avoidance of a surgical procedure and its health care costs in the infants who spontaneously resolve.
Controversy remains among clinicians as to the preferred clinical approach. Data that assist in the decision-making include the rate of spontaneous resolution after the age at which an office procedure might be done and the success rates for the probing procedures done early in the office and in a surgical facility after a period of observation. A wide range of spontaneous resolution rates have been reported in the literature (32% to 95%),2, 4, 5 although the spontaneous resolution rate of symptoms present at 6 months of age has been addressed in only one report, a retrospective report which found the rate to be 70% by 12 months of age (26 of 37 subjects).6 The probability of success for NLD probing was determined in a prospective observational study to be 78% overall,7 however, there was a reduced probability of success when the procedure was performed in the office (72%) compared with performance in a surgical facility (80%).7
The purpose of this analysis was to assess the impact of the rate of spontaneous resolution on the relative cost-effectiveness of deferred probing in a surgical facility compared with immediate office-based probing for congenital NLDO.
Methods
The Model
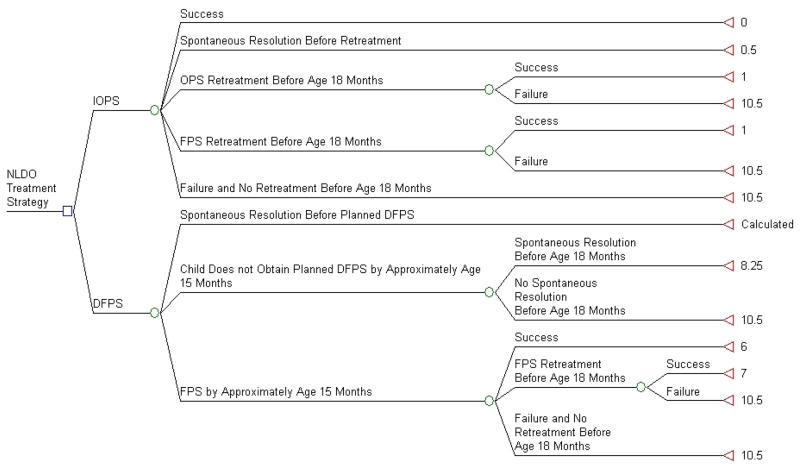
A decision tree was used to compare the costs and outcomes for two common options for treating NLDO in children presenting with symptoms between 6 and 9 months of age: immediate office probing surgery (IOPS) at presentation and deferred facility probing surgery (DFPS) if the condition had not spontaneously resolved within 6 months of presentation. These two options are shown as choices at the initial node in the decision tree (Figure 1). Subsequent to the choice of the initial approach, we determined potential next steps in the management of NLDO after the initial surgery. For IOPS, the initial options are success or failure of the office probing. For initial treatment failures, the options include spontaneous resolution occurring prior to another surgery, a repeat surgery in the office, a repeat surgery in a facility (probing, balloon catheter dilation, or nasolacrimal intubation), and no retreatment occurring before the child is 18 months old. The secondary surgery may also succeed or fail. In our model there is no provision for a third surgical procedure, therefore failure of the second procedure is assumed to be a failure of the entire treatment regimen.
Figure 1. Decision Tree Used in Cost-Effectiveness Analysis.
NLDO: Nasolacrimal duct obstruction
FPS: Facility Probing Surgery
OPS: Office Probing Surgery
DFPS: Deferred Facility Probing Surgery
IOPS: Immediate Office Probing Surgery
Success: Resolution of nasolacrimal duct obstruction symptoms (i.e. absence of epiphora, increased tear lake and mucous discharge) by age 18 months
Failure: presence of at least one symptom of NLDO (epiphora, increased tear lake and mucous discharge)
Outcomes shown at terminal nodes are average months experiencing NLDO after presentation between 6 to 9 months of age.
The DFPS option is more complex. The primary motivation for considering DFPS is the chance for spontaneous resolution and thus avoiding the need for surgery and its associated health care costs. With higher 6-month rates of spontaneous resolution, a smaller number of children will need to have surgery. Among those who do not resolve within a 6-month period, some parents may decide not to obtain surgery for their child. A subset of those cases will resolve with additional observation, while others will be considered treatment failures. Among those children who undergo deferred probing in a facility, the procedure will either succeed or fail, with some of the failed cases undergoing a second surgery. The second surgery (repeat probing in a facility, balloon catheter dilation, or nasolacrimal intubation) also can result in either success or failure. There is no provision for a third surgical procedure, therefore failure at the second procedure is assumed to be a failure of the entire treatment regimen.
Parameters: Costs and Probabilities
The point estimates for costs and probabilities are shown in Table 1.
Table 1.
Cost and Probability Parameters to Populate the Decision Tree
| Parameter Type | Parameter (applicable CPT code) | Sourcea | Point Estimateb | Lower Bound | Upper Bound | Distribution Type |
|---|---|---|---|---|---|---|
| Cost | Initial Physician’s Office Consultation (92004) | 8 | $131 | ---- | ---- | ---- |
| Antibiotic for Children Receiving Deferred Surgery (tobramycin sulfate 0.3%) | c | $16 | ---- | ---- | ---- | |
| Office Procedure: Professional Fee & Procedure Reimbursement (68810) | 8 | $198 | ---- | ---- | ---- | |
| Post Procedure Steroidal Antibiotic (tobramycin - dexamethasone 0.1%) | c | $74 | ---- | ---- | ---- | |
| Established Patient Office Visit (99213) | 8 | $63 | ---- | ---- | ---- | |
| Anesthesia for Facility Surgery | 8 | $250 | ---- | ---- | ---- | |
| Facility Surgery Professional Fee (68811) (initial surgery) | 9 | $172 | ---- | ---- | ---- | |
| Facility Surgery Professional Fee (68811,68815,68816) (secondary surgery) | 9 | $208 | ---- | ---- | ---- | |
| Ambulatory Surgery Center Facility Fee (initial surgery) | 8 | $607 | ---- | ---- | ---- | |
| Ambulatory Surgery Center Facility Fee (secondary surgery) | d | $681 | ---- | ---- | ---- | |
| Hospital Outpatient Department Facility Fee (initial surgery) | 8 | $1276 | ---- | ---- | ---- | |
| Hospital Outpatient Department Facility Fee (secondary surgery) | e | $1408 | ---- | ---- | ---- | |
| Probability | Success of Immediate Office Surgery | f | 0.75 | 0.66 | 0.82 | PERT |
| Spontaneous resolution after Immediate Office Surgery and Prior to Retreatment | Consensus | 0.03 | 0.02 | 0.04 | PERT | |
| Failure to Present for Repeat Surgery After Initial Office Failure | Consensus | 0.05 | 0.03 | 0.07 | PERT | |
| Success of Office Surgery After Initial Office Failure | 11 | 0.56 | 0.33 | 0.76 | PERT | |
| Repeat Office Surgery After Initial Office Failure | Consensus | 0.10 | 0.05 | 0.15 | PERT | |
| Success of Repeat Surgery After Initial Surgery Failure | g | 0.78 | 0.71 | 0.83 | PERT | |
| Spontaneous Resolution Prior to Deferred Facility Surgery | Consensus | 0.75 | 0.5 | 0.9 | Calculated | |
| Children Resolving who Resolve Before Antibiotic Prescription Renewal | Consensus | 0.5 | 0.4 | 0.6 | PERT | |
| Children Who Reach Date of Scheduled Surgery with a Return Clinic Visit | Consensus | 0.5 | ---- | ---- | ---- | |
| Children Scheduled for Deferred Surgery Not Presenting | Consensus | 0.05 | 0.03 | 0.07 | PERT | |
| Spontaneous Resolution Between 15 and 18 Months | Consensus | 0.02 | 0.01 | 0.03 | PERT | |
| Facility Surgeries at Ambulatory Surgery Center | 7 | 0.32 | 0.3 | 1 | PERT | |
| Facility Surgeries at Hospital | 7 | 0.68 | 0.0 | 1 | PERT | |
| Success of Initial Facility Surgery | 7 | 0.80 | 0.74 | 0.85 | PERT | |
| Failure to Present for Repeat Facility Surgery After Facility Failure | Consensus | 0.05 | 0.03 | 0.07 | PERT |
CPT = Current Procedural Terminology (copyright American Medical Association, 2009)
PERT = Program Evaluation and Review Technique
Numbers represent reference numbers.
Point estimates for costs are given in US dollars.
Cost of drug at www.drugstore.com accessed August 8, 2009
Based on Medicare Physician Fee Schedule 20099 ambulatory surgery centers costs estimating that the distribution of type of surgery would approximate that found in a prospective observational study of secondary procedures: balloon catheter dilation ($780) in 43% of cases, nasolacrimal intubation ($607) in 45%, and repeat probing ($607) in 12%.10, 11
Based on Medicare Physician Fee Schedule 20099 hospital outpatient department costs estimating that the distribution of type of surgery would approximate that found in a prospective observational study of secondary procedures: balloon catheter dilation ($1582 including $306 cost of balloon catheter) in 43% of cases, nasolacrimal intubation ($1276) in 45%, and repeat probing ($1276) in 12%10, 11
Data from unpublished subset of data published by PEDIG in 20087
Based on the success rates reported in an prospective observational study of secondary procedures -- balloon catheter dilation (77%), nasolacrimal intubation (84%) and repeat probing (56%) -- weighted according to the distribution of procedures in the study (43% of patients received balloon catheter dilation, 45% received nasolacrimal intubation, and 12% received repeat probing). The confidence interval is also based on the weighted data.10, 11
Data on costs were obtained primarily from the 2009 Medicare Fee Schedule.8, 9 We assumed full phase-in of ambulatory surgery rates at 59% of the hospital outpatient surgery schedule. The cost of a facility surgery as a follow up to a failed surgery (either an office probing or a facility probing) is based on the Medicare Fee Schedule 2009 costs8, 9 assuming that the distribution of type of secondary surgery would approximate that found in a prospective observational study:10, 11 balloon catheter dilation in 43% of cases, nasolacrimal intubation in 45%, and repeat probing in 12%.
A previous study of NLDO treatment by the PEDIG group was the main source of probability data on clinical outcomes.7 The probability of success for an office-based probing was 75% based on unpublished data on patients reported in a PEDIG study in 20087 but limited to a subset of 98 patients who received office probing between 6 to <10 months of age. The probability of success for a facility-based probing was 80% based on unpublished data on patients reported in PEDIG, 20087 but limited to a subset of 171 patients who received facility probing between 12 to <18 months of age. The probability of success for a facility surgery that was a follow-up to failed surgery was 78% based on the success rates reported from a prospective observational study of secondary procedures10, 11--balloon catheter dilation (77%), nasolacrimal intubation (84%), and repeat probing (56%)--weighted according to the distribution of procedures in the study (balloon catheter dilation – 43%, nasolacrimal intubation – 45%, and repeat probing – 12%). The probability of success for an office surgery that was a follow-up to failed surgery was assumed to be 56%, based on data from a prospective study of 20 subjects.11 Other estimates included in Table 1 were made by expert consensus.
Outcomes
The outcomes in the model include an indicator of clinical success and the number of months of NLDO symptoms. Success was defined as the absence of epiphora, increased tear lake and mucous discharge. The number of symptomatic months experienced when reaching each terminal node was calculated through 18 months of age. Months are counted from the time of the initial office consultation at 6 to 9 months of age (average 7.5 months) because the focus is on the number of months of symptoms experienced subsequent to the choice of treatment option. Combining the outcome at each end node with the probability of reaching each end node allows us to describe the expected success rate and calculate the expected number of months with the condition.
For the IOPS option, the number of months with symptoms depends on the outcome of the immediate office probing. Initial success is consistent with no months of symptoms after diagnosis. When retreatment is needed, it is assumed to occur after one additional month of symptoms. Success ends symptoms, while those for whom surgery fails a second time are assumed not to resolve and to experience an average of 10.5 months of symptoms through 18 months of age. This number of months is based on the assumed uniform distribution of presentation between 6 and 9 months. For children who fail the first treatment, but whose symptoms resolve spontaneously prior to retreatment, the resolution is assumed to be distributed uniformly; thus, the average time to resolution is 0.5 months from the initial surgery.
For the DFPS option, the cases that spontaneously resolve before the surgery scheduled after six months of observation are assumed to resolve with a uniform distribution with an average of three months of symptoms. Children successfully treated at the first surgery will experience 6 months of symptoms. For children who do not resolve in the first 6 months and who do not undergo surgery, their condition may still resolve. This average duration of symptoms is derived from first presentation for treatment between 6 and 9 months, 6 months of symptoms prior to the possible deferred surgery at 12 to 15 months, and a uniform distribution of resolution between 12 to 15 months through 18 months of age. A child initially presenting at 6 months would have an expected duration of symptoms of 9 months. A child initially presenting at 9 months would have an expected duration of 7.5 months. The midpoint between these two is 8.25 months. Those children who do not resolve between 15 and 18 months will experience an average of 10.5 months of symptoms. Those children who require retreatment and are successful on that second treatment will experience 7 months of symptoms. Those who do not have a successful second treatment will experience an average of 10.5 months of symptoms through 18-months of age.
Cost-Effectiveness Analysis
All costs are reported in 2009 U.S. dollars. The costs used for the analysis are only the direct medical care costs and do not include costs of missed wages or travel. Thus, the perspective of this analysis is that of the health care insurer. The base-case incremental cost-effectiveness analysis assumed a spontaneous resolution rate of 75%. The base-case results are reported both in terms of overall success at 18 months of age and by months of symptoms avoided at 18 months of age. Microsoft Excel was used for the primary and sensitivity analyses.
Sensitivity Analysis
Three sensitivity analyses (i.e. analyses changing assumptions to determine how critical those assumptions are to determining the result) were conducted. First, we systematically varied the rate of spontaneous resolution from 50% to 90%, while using all other parameters from the base-case. For each spontaneous resolution proportion, the results will indicate which treatment alternative costs less, which has the higher probability of success, and the difference in cost to achieve each additional success.
Second, in addition to varying the spontaneous resolution rate, we varied the relative cost of the immediate office surgery and deferred facility surgery. Although changing the costs of both surgical procedures at once would change the cost per additional success, it would not change the spontaneous resolution rate at which one treatment approach dominates (i.e. is both less expensive and more effective) because the costs in the current health care system move in the same direction. Thus a change in cost would simply expand or contract the incremental cost ratio by the percentage by which the costs are changed. Instead, because the facility fee for performing the surgery in a hospital outpatient department (HOPD) is more than double the facility fee for performing the surgery in an ambulatory surgery center (ASC), we varied the relative cost by varying the probability of a deferred facility surgery case being performed at an ASC from 0% to 100%. Examining the impact of site of service for deferred probing results in wide variation in the relative cost of the immediate and deferred probing options and allows us to determine how the results might change in markets with different availability of free-standing ambulatory surgery centers.
Third, we performed a probabilistic sensitivity analysis varying all assumed probabilities (see the ranges defined in Table 1) to explore the impact on the qualitative conclusions. Direct medical costs were held constant in the probabilistic sensitivity analysis. For all probabilities except the spontaneous resolution rate, the PERT (Program Evaluation and Review Technique, named because of the focus on expert opinion) distribution was used to allow for a minimum (lower bound), a most likely (point estimate), and a maximum (upper bound) while having a distribution shape that is not triangular. The distributions of the probabilities were based on confidence intervals in cases in which data was available, otherwise on expert consensus. When data for estimates came from multiple sources we used a weighted average and a confidence interval based on similar weights. A probabilistic sensitivity analysis was performed at the base spontaneous resolution rate (75%) to explore the impact of changes in these variables. The analysis used 1000 repeated simulations to examine the effect of possible random variation in the probabilities of success and appropriate follow-up.
Results
The results of the base-case analysis using a spontaneous rate of resolution of 75% are shown in Table 2. The average cost for the DFPS option is $641, while the average cost of IOPS is $771. Using resolution of NLDO symptoms at 18 months as the outcome, DFPS would have a probability of success of 97.5%, compared with 93.0% for IOPS. The average number of months to symptom resolution with IOPS is 0.9, while the average number of months of symptoms with DFPS is 3.9 using a spontaneous rate of resolution of 75%. Thus, the choice of IOPS avoids an average 3.0 months of symptoms. The additional health care expenditures for IOPS per month of symptoms avoided is $44.
Table 2.
Base-case Analysis (75% Spontaneous Resolution Rate from Enrollment at 6 to 9 Months of Age Prior to Deferred Probing after 6 Months of Observation)
| Initial Option | Cost* | Incremental Cost | Probability of Success | ICER ($/Success) | Months of Symptoms | Months of Symptoms Averted | ICER ($/Month of Symptoms Averted) |
|---|---|---|---|---|---|---|---|
| Deferred Facility Probing Surgery | $641 | ---- | 97.5% | ---- | 3.9 | ---- | ---- |
| Immediate Office Probing Surgery | $771 | $130 | 93.0% | Dominated** | 0.9 | 3.0 | $44 |
Values are rounded to the nearest US dollar.
Dominated implies that the option is more expensive and less effective. Options like this receive no further economic consideration.
ICER = Incremental Cost-Effectiveness Ratio (additional dollars spent per additional unit of outcome)
Suspecting that the spontaneous resolution rate would be of paramount importance in this analysis, we performed sensitivity analyses in which the spontaneous resolution rate was systematically varied from 50% to 90% (eTable 1 – online only). The incremental expense with DFPS ranges from a cost of $342 per case at 50% spontaneous resolution to savings of $413 per case at 90% spontaneous resolution.
Using the model, we evaluated the impact of spontaneous resolution rate on overall proportions of success at 18 months of age (eTable 1 – online only). The probability of success of DFPS increases from 95% to 99% as the spontaneous resolution rate increases from 50 to 90%. Compared with IOPS, the probability of success of DFPS is 2% higher when the spontaneous resolution rate is 50% and 6% higher when the spontaneous resolution rate is 90%. Thus, DFPS always has a higher probability of eventually alleviating the symptoms before the child is 18 months of age.
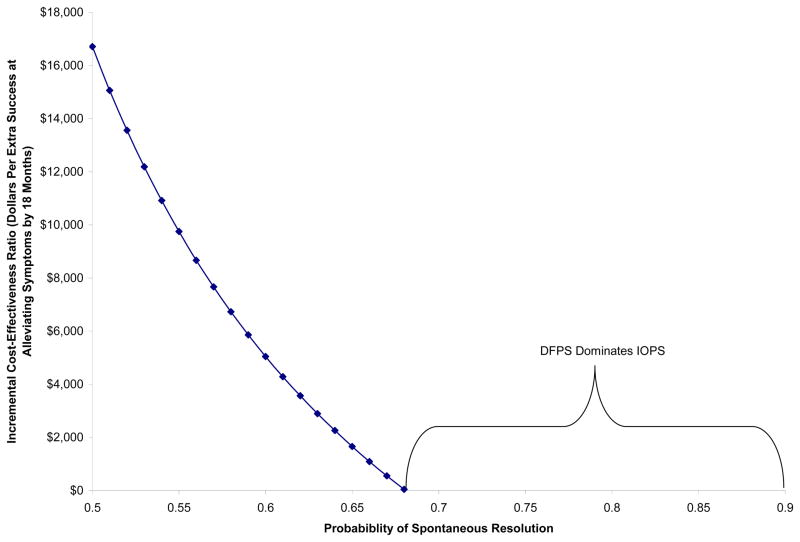
The incremental cost-effectiveness ratio of extra dollars spent on DFPS for each additional successfully-resolved case is sensitive to the spontaneous resolution rate. Figure 2 shows that at spontaneous resolution rates of 68% or more, DFPS is both less expensive and more effective (i.e. DFPS dominates IOPS). For spontaneous resolution rates of less than 68%, DFPS is more expensive although slightly more effective. The incremental cost-effectiveness ratio increases to $16,709 dollars per additional DFPS success when the spontaneous resolution rate is only 50%.
Figure 2.
Dollars Per Additional Success Using Deferred Facility Probing Surgery (DFPS) at Probabilities of Spontaneous Resolution in the 50–90% Range
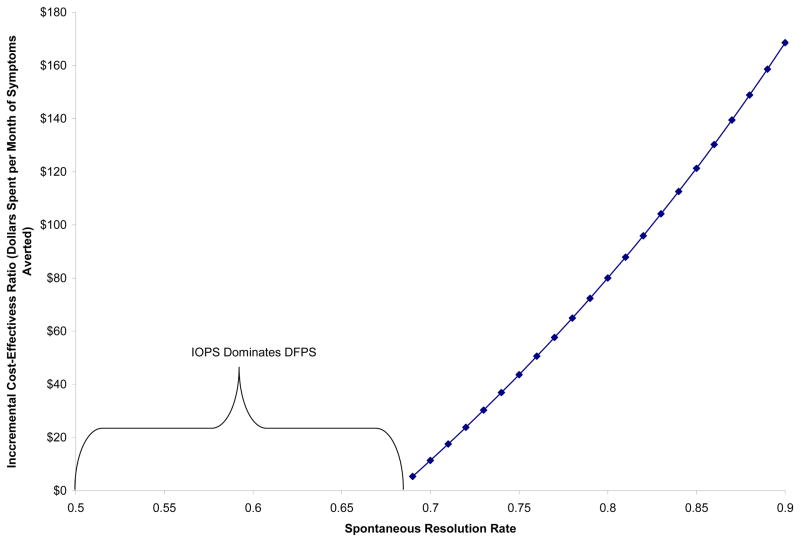
When focusing on months of symptoms averted by 18 months of age, IOPS always results in fewer months of symptoms at spontaneous resolution rates between 50% and 90%. At a spontaneous resolution rate of 50%, IOPS is associated with 3.9 months of symptoms averted by 18 months of life, while at 90% spontaneous resolution, IOPS is associated with 2.4 months of symptoms averted. Figure 3 shows the cost per additional month of symptoms averted by using IOPS rather than DFPS. At the highest spontaneous resolution rate we evaluated (90% - the least favorable for IOPS), the extra cost per month of symptoms averted by IOPS is $169. At spontaneous resolution rates between 69% and 90%, IOPS is more expensive but results in fewer months of symptoms. At rates of spontaneous resolution less than 69%, IOPS both costs less and has fewer months of symptoms, thus dominating DFPS.
Figure 3.
Dollars Per Month of Symptoms Averted Using Immediate Office-Based Probing Surgery (IOPS) at Probabilities of Spontaneous Resolution in the 50–90% Range
In the second sensitivity analysis, changing the relative cost of the two surgeries did not result in a substantial change in the rate of spontaneous resolution at which DFPS becomes less expensive. In the model with all base values, the threshold is 68%. If all deferred facility probings are assumed to be performed in an ASC, the threshold is 64% whereas if all deferred facility probings are assumed to be performed in a HOPD, the threshold is 70%. Thus, changes in the relative cost lead to changes in the threshold that span at most a 6 percentage point range. When all deferred surgeries are performed at ASCs, and the spontaneous resolution rate is only 50%, an extra $9,145 is spent per extra DFPS success; in contrast when all deferred surgeries are performed in a HOPD, the incremental cost-effectiveness ratio is $20,269 per extra DFPS success. In contrast, when the spontaneous resolution rate is 90% and all deferred surgeries are performed in ASCs, the incremental cost-effectiveness ratio is $147 per month of symptoms averted by IOPS; when all deferred surgeries are performed in HOPDs the incremental cost-effectiveness ratio is $179 per month of symptoms averted by IOPS.
In the probabilistic sensitivity analysis using repeated sampling from the random distributions of the probabilities defined by range, type, and data source in Table 1, holding costs constant, assuming a 75% spontaneous resolution rate, and using success as the outcome of interest, the DFPS is always less expensive and more likely to succeed. When focusing on months of symptoms as the outcome, IOPS is always more expensive but results in fewer months of symptoms. The range of extra health care spending on IOPS at a 75% spontaneous resolution rate varies from less than $10 to $102 per month of symptoms averted. The values for the 2.5%ile and 97.5%ile are $19 and $81, respectively.
Discussion
There is uncertainty about whether to treat infantile NLDO immediately in the office or wait for a period of months for spontaneous resolution and operate if necessary in a surgical facility. In this report we have modeled the costs and probabilities of success for these two treatment regimens. The rate of spontaneous resolution is a key factor in predicting the relative cost-effectiveness and clinical outcomes to be expected from these approaches. Most estimates of the rate of spontaneous resolution include children less than 6 months of age who are expected to have a high rate of spontaneous resolution and in whom no surgery would be planned immediately.2,4,5 There are few data which address the clinically pertinent question of what to do for the symptomatic child at 6 to 9 months of age for whom a surgeon might do an office probing immediately or decide to wait for up to 6 months to do a facility probing. Paul found that among 37 eyes with NLDO symptoms at 6 months of age, 26 (70%) cleared without surgical intervention by 12 months of age and that among 23 eyes with NLDO symptoms at 9 months, 12 (52%) cleared by 12 months.6
In this cost-effectiveness model we used a 6-month spontaneous resolution rate of 75% for children between 6 and 9 months of age with symptoms of NLDO who either underwent IOPS or waited 6 months for DFPS. Children whose procedures failed could be retreated once and the outcomes assessed at 18 months of age. The IOPS and DFPS approaches have nearly the same cost and probability of success. DFPS is $130 less expensive and slightly (4.5%) more successful. However, the DFPS approach is associated with an average of 3.0 extra months of symptoms. The average extra cost per month of symptoms avoided when using IOPS rather than DFPS is $44.
Our sensitivity analyses varied the rate of spontaneous resolution and showed how critical spontaneous resolution is in our model. A difference of as little as 8% in the spontaneous resolution rate (68% vs. 60%) means the difference between the DFPS approach being more effective and less costly and DFPS costing over $5000 per expected extra successful treatment. Clinically relevant changes in relative costs change the threshold rate of spontaneous resolution above which DFPS becomes less costly and more effective, although all thresholds are within 6 percentage points and are below the base case spontaneous resolution rate of 75%. More certainty about the costs (as related to the local market structure in which clinical decisions are being made) would only become important if future definitive studies reveal a spontaneous resolution rate lower than 75%. The sensitivity analyses on costs also reveal how important it is for stakeholders to understand the impact of care at ambulatory surgery centers, which substantially changes the cost profile for DFPS by more than doubling the cost of the initial surgery when switching from all deferred surgeries being done in the ASC as compared with the HOPD. The changes in relative cost had a much larger effect on the incremental cost-effectiveness ratio for DFPS with respect to extra successes when it did not dominate than it had for IOPS with respect to months of symptoms avoided when it did not dominate.
If these results are to guide policy development, it is useful to consider how the decision to choose the IOPS or DFPS option causes stress for parents. For instance, how much additional out of pocket cost would parents be willing to pay to avoid additional months of symptoms? How much of a cost savings would cause them to select the less expensive option, despite the longer duration of symptoms? While we had to make many assumptions for this model, repeated simulations indicated a middle 95% range from $19 to $81 per month of symptoms avoided under the IOPS option. It seems that this additional cost, even if not covered by insurers, might be small enough that parents would consider paying the uncovered difference to alleviate the symptoms sooner.
Regardless of how the results are used, a more precise estimate of the spontaneous resolution rate is critical for establishing which approach is the clinically or economically obvious choice or whether tradeoffs between costs and outcomes need to be considered carefully. The PEDIG network has launched such a prospective randomized clinical trial with the primary objective to more precisely estimate the rate of spontaneous resolution. An additional primary objective is to assess cost-effectiveness for IOPS and DFPS for children 6 to 9 months of age with symptoms from congenital NLDO.
The present study is not without limitations. These include, as in many cost-effectiveness analyses, estimates of costs and clinical outcomes drawn from different sources and a model constructed to simulate what is likely to happen. Some of the studies from which data were gathered are not definitive, particularly the true rate of spontaneous resolution. The cost data are not actual costs, but are Medicare-allowed reimbursements for participating providers. The Medicare fee schedule likely represents a reasonable national weighting of fees for physician and facility, though actual values vary under an assortment of private and government programs. The proportion of facility cases performed in the hospital outpatient department compared with an ambulatory surgical center was drawn from a multi-center observational study7 for our base case and may not represent current clinical practice, although we assessed the impact of site of service from 0% to 100% ASC utilization. In addition, a number of estimates needed to be developed by consensus. Also, the value of parental time obtaining medical care for children is not included. If parental time were included, the cost per month of symptoms averted by IOPS would be lower. The type of choices we made in our model are standard in economic evaluation, but primary data collected during an effectiveness-oriented randomized trial would be superior.
In conclusion, for a 6 to 9 month old infant with NLDO, under the assumption that the spontaneous resolution rate is 75%, the clinician’s choice of immediate probing in the office or delaying for 6 months and then probing in a surgical facility has little impact in terms of overall success rate or cost for a population. Because cost-effectiveness is critically dependent on the spontaneous resolution rate, prospective data are needed to refine this point estimate.
Supplementary Material
Acknowledgments
Supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health(Bethesda, Maryland), Department of Health and Human Services (EY011751).
References
- 1.Price HW. Dacryostenosis. J Pediatr. 1947;30:300–5. doi: 10.1016/s0022-3476(47)80165-9. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RA, Robb RM. The natural course of congenital obstruction of the nasolacrimal duct. J Pediatr Ophthalmol Strabismus. 1978;15(4):246–50. doi: 10.3928/0191-3913-19780701-14. [DOI] [PubMed] [Google Scholar]
- 3.Paul TO, Shepherd R. Congenital nasolacrimal duct obstruction: natural history and the timing of optimal intervention. J Pediatr Ophthalmol Strabismus. 1994;31(6):362–7. doi: 10.3928/0191-3913-19941101-04. [DOI] [PubMed] [Google Scholar]
- 4.Nelson LB, Calhoun JH, Menduke H. Medical management of congenital nasolacrimal duct obstruction. Ophthalmology. 1985;92(9):1187–90. doi: 10.1016/s0161-6420(85)33878-2. [DOI] [PubMed] [Google Scholar]
- 5.Ghuman T, Gonzales C, Mazow MM. Treatment of congenital nasolacrimal duct obstruction. Am Orthoptic J. 1999;49:163–8. [Google Scholar]
- 6.Paul TO. Medical management of congenital nasolacrimal duct obstruction. J Pediatr Ophthalmol Strabismus. 1985;22(2):68–70. doi: 10.3928/0191-3913-19850301-09. [DOI] [PubMed] [Google Scholar]
- 7.Pediatric Eye Disease Investigator Group. Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology. 2008;115:577–84. doi: 10.1016/j.ophtha.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Federal Register. Dept. of Health and Human Services. Centers for Medicare & Medicaid Services; Nov 18, 2008. Medicare Program: Changes to the Hospital Outpatient Prospective Payment System and CY 2009 Payment Rates; p. 68930.p. 9082. [Google Scholar]
- 9.Federal Register. Dept. of Health and Human Services. Centers for Medicare & Medicaid Services; Nov 19, 2008. Medicare Program: Payment Policies Under the Physician Fee; p. 70061. [Google Scholar]
- 10.Pediatric Eye Disease Investigator Group. Balloon catheter dilation and nasolacrimal intubation for treatment of nasolacrimal duct obstruction following a failed probing. Arch Ophthalmol. 2009;127(5):633–9. doi: 10.1001/archophthalmol.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric Eye Disease Investigator Group. Repeat probing for treatment of persistent nasolacrimal duct obstruction. J AAPOS. 2009;13(3):306–7. doi: 10.1016/j.jaapos.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.