Abstract
The mechanism of visceral pain is still less understood compared with that of somatic pain. This is primarily due to the diverse nature of visceral pain compounded by multiple factors such as sexual dimorphism, psychological stress, genetic trait, and the nature of predisposed disease. Due to multiple contributing factors there is an enormous challenge to develop animal models that ideally mimic the exact disease condition. In spite of that, it is well recognized that visceral hypersensitivity can occur due to (1) sensitization of primary sensory afferents innervating the viscera, (2) hyperexcitability of spinal ascending neurons (central sensitization) receiving synaptic input from the viscera, and (3) dysregulation of descending pathways that modulate spinal nociceptive transmission. Depending on the type of stimulus condition, different neural pathways are involved in chronic pain. In early-life psychological stress such as maternal separation, chronic pain occurs later in life due to dysregulation of the hypothalamic–pituitary–adrenal axis and significant increase in corticotrophin releasing factor (CRF) secretion. In contrast, in early-life inflammatory conditions such as colitis and cystitis, there is dysregulation of the descending opioidergic system that results excessive pain perception (i.e., visceral hyperalgesia). Functional bowel disorders and chronic pelvic pain represent unexplained pain that is not associated with identifiable organic diseases. Often pain overlaps between two organs and approximately 35% of patients with chronic pelvic pain showed significant improvement when treated for functional bowel disorders. Animal studies have documented that two main components such as (1) dichotomy of primary afferent fibers innervating two pelvic organs and (2) common convergence of two afferent fibers onto a spinal dorsal horn are contributing factors for organ-to-organ pain overlap. With reports emerging about the varieties of peptide molecules involved in the pathological conditions of visceral pain, it is expected that better therapy will be achieved relatively soon to manage chronic visceral pain.
Keywords: Visceral pain, Visceral afferents, Spinal cord, Pelvic nerve, Splanchnic nerve, Colon, Urinary bladder, Gender difference, Sensitization
1 Introduction
Pain is inherent with any life that is linked with consciousness. It is a major concern in all aspects of human life. Although with the advances of medical science, the acute pain associated with infection and disease can be correctly diagnosed and treated, many chronic pain syndromes still remain a challenge for clinicians. Suffering from chronic pain can significantly deteriorate a person’s quality of life and can often lead to disability. Such chronic pain in the viscera is observed in functional bowel disorders (e.g., noncardiac chest pain, chronic idiopathic dyspepsia, functional abdominal pain, irritable bowel syndrome; IBS) and chronic pelvic pain (e.g., chronic interstitial cystitis, painful bladder syndrome) that are multifaceted problems and still poorly understood. Functional bowel disorders and chronic pelvic pain represent unexplained symptoms that have no readily identifiable infectious, anatomical, or metabolic basis. The pain is diffuse and poorly localized and often it is confused as originating from other visceral organs. One example is noncardiac chest pain, where pain is very similar in nature to that of cardiac angina. About 15–30% of angiograms performed in chest pain patients are normal, and coronary artery disease rarely explains the symptoms (Richter et al. 2000). Among all functional bowel disorders, IBS is the most common and prevalent gastrointestinal (GI) disorder (about 18– 20% of the patient population), having symptoms of cramping, abdominal pain, bloating, constipation, and/or diarrhea. Similarly, chronic pelvic pain affects approximately 15% of women. Interstitial cystitis and painful bladder syndrome are the most common. All functional bowel disorder and chronic pelvic pain patients exhibit one common symptom – lower abdominal pain. There are different factors that can cause or modify these disorders, such as persistent mental and social stress, a previous episode of infection or inflammation, genetic background, and early-life adverse events (e.g., abuse, trauma, and painful experience). Presumably in all functional disorders, patients develop excessive pain to painful stimuli (e.g., hyperalgesia) and may also experience pain to a nonpainful stimulus (e.g., allodynia). This was first documented in a very elegant physchophysical study in IBS patients where patients reported pain to nonpainful colonic distension and were compared with healthy subjects (Ritchie 1973). The general notion for visceral hypersensitivity is the presence of sensitization of the neural pathway (includes primary sensory afferents and spinal ascending neurons) involved in the transmission of visceral sensation to the supraspinal level and the dysregulation of descending pathways that modulate spinal signaling (Mayer and Gebhart 1994). Therefore, recent studies in several laboratories have focused on understanding the response characteristics of primary sensory afferents and spinal neurons to visceral stimuli and their sensitization. In addition, several laboratories are evaluating the cortical processing to painful signal by employing positron emission tomography or field magnetic resonance imaging techniques, which is critical in understanding the ultimate processing mechanism in sensing pain.
Considering the vastness of the subject, this chapter will be restricted to our current knowledge regarding different animal models for evaluating visceral pain, the contribution of different types of visceral sensory afferents, sensitization, and cross-sensitization of visceral afferents in pathological conditions and pharmacological modulation of visceral pain. The chapter will focus only on visceral pain originating from the abdominal viscera and spinal processing will only be mentioned in the context of the sensory afferent’s projection and pain modulation.
2 Behavioral Studies for Visceral Pain in Laboratory Animals
Considering the diverse nature of the disease, it is extremely difficult to design a model of visceral pain in laboratory animals that can exactly mimic all the characteristics of functional visceral pain. The majority of animal models of visceral hyperalgesia have been developed in rats and mice. Pain can be assessed by distending the hollow viscera and recording the somatic muscle reflex (visual observation of abdominal contraction or EMG recordings from the abdominal musculature) or autonomic responses (change in systemic blood pressure and heart rate). These methods have been employed to assess visceral pain from the stomach (Ozaki et al. 2002), colon (Ness and Gebhart 1988, 1990), gall bladder (Cervero 1982), ureter (Olivar and Laird 1999; Giamberardino et al. 1995), urinary bladder (Castroman and Ness 2001; Ness et al. 2001; Cruz and Downie 2006; DeBerry et al. 2007; Su et al. 2008), and uterus (Berkley et al. 1995). To produce hyperalgesia, the viscera are either inflamed with chemicals or the animals are subjected to stress in early life.
2.1 Inflammatory Model
The most commonly used inflammatory models are produced by injecting inflammatory agents into the viscera [e.g., 2,4,6-trinitrobenzene sulfonic acid (TNBS) or zymosan induced colitis, acid-infused esophagitis, cyclophosphamide, or zymosan-induced cystitis]. Although in a strict sense, these inflammatory models cannot be considered as true models for nonpathological functional visceral pain, hyperalgesia manifested by the animals may closely resemble the nature of pain observed in patients.
Instillation of haptens such as TNBS, dinitrobenzene sulfonic acid, or dichlorobenzene sulfonic acid into the colon produces ulcerative colitis-like symptoms in rats (Morris et al. 1989; Rachmilewitz et al. 1989; Elson et al. 1995; Wallace et al. 1995). The inflammation peaks in 4–5 days following the injection and persists for about 1 month (Miampamba and Sharkey 1998). Rats exhibit significant hyperalgesia to colon distension after TNBS-induced inflammation (Morteau et al. 1994a; Fargeas et al. 1995; Sengupta et al. 1999; Friedrich and Gebhart 2000, 2003; Diop et al. 2002; Delafoy et al. 2003; Lamb et al. 2006; Miranda et al. 2007). It has recently been shown that about 24% of the rats treated with TNBS exhibit visceral hyperalgesia for up to 16 weeks following complete remission of the inflammation (Zhou et al. 2008). It appears that these rats behave very similarly to the subset of patients having postinfectious IBS, where pain persists despite the resolution of inflammation. Like TNBS, zymosan, an insoluble carbohydrate from the yeast cell wall, has also been used to induce colon inflammation (Coutinho et al. 1996, 2000; Traub et al. 1999; Jones et al. 2007). However, the severity of inflammation is much less compared with that caused by TNBS and generally does not produce mucosal ulceration.
Urinary bladder inflammation is commonly induced by intravesicular injection of 0.2% acetic acid (Cruz and Downie 2006), zymosan (Randich et al. 2006a, b; DeBerry et al. 2007), or acrolein (Bjorling et al. 2007). Earlier studies used intraperitoneal injection of cyclophosphamide, an antitumoral agent, to produce bladder inflammation (Boucher et al. 2000) and visceral pain. Although the drug produces selective cystitis via its metabolite, acrolein, in the bladder, it has a severe toxic effect in other organs that can complicate the evaluation of bladder pain.
2.2 Neonatal Maternal-Separation-Induced Stress Model
The early neonatal period is a critical time for the development of the nociceptive neural pathways, which require use-dependent activity for normal development. However, abnormal stimuli such as stress, sustained pain, or inflammation in the neonatal period may adversely affect the development and subsequently lead to lower thresholds for pain in later life (Anand 1998; Anand et al. 1999; Lidow et al. 2001; Pattinson and Fitzerald 2004; Fitzerald 2005). The objective of developing the neonatal maternal-separation (MS)-induced stress model was to evaluate the effects of stress early in life on visceral sensitivity in the absence of visceral inflammation. The model possibly mimics the subset of IBS patient having a history of traumatic experiences, including physical, sexual, and emotional abuse and a life-threatening situation in early life (Chitkara et al. 2008). In rats, the neonatal period from postnatal day 2 (P2) to postnatal day 14 (P14) is extremely critical for neurological development. There is a large body of literature suggesting that neonatal MS leads to dysregulation of the hypothalamic–pituitary–adrenal axis resulting in long-term changes in neuroendocrine and neuropeptide secretion (Liu et al. 1997; Caldji et al. 1998; Plotsky et al. 2005; Barreau et al. 2004a, b, 2007; Chung et al. 2007a, b; Ren et al. 2007). Plotsky et al. (2005) have demonstrated that brief (15 min) or prolong (180 min) MS of rat pups at P2 to P14 results in significant elevation of corticotrophin-releasing factor (CRF) messenger RNA (mRNA) and the CRF1 receptor immunoreactivity in the paraventricular nucleus, amygdala, and locus ceruleus. Subsequent studies have shown that MS for 180 min (MS180) results in visceral hyperalgesia to colon distension (Coutinho et al. 2002). The hyperalgesia in MS180 rats is exacerbated following 1 h of water-avoidance stress, and this can be prevented by preemptive administration of a selective CRF1 antagonist, suggesting that CRF indeed may play a critical role in the development of visceral hyperalgesia (Schewtz et al. 2005). The manifestation of visceral hyperalgesia in MS rats is possibly a global pain hyperresponsiveness and may not be somatotopically restricted. In a recent study, it was shown that orogastric suction for 30–45 s in Long-Evans rats during P2 to P14 results in visceral hyperalgesia to colon distension when tested at their adult age (postnatal day 60) (Smith et al. 2007). This result is similar to that reported by a retrospective clinical study that frequent gastric suction in newly born babies has a potential risk for functional disorders in later life (Anand et al. 2004). An animal study has also shown that pretreatment with CRF1 receptor antagonist 10 min prior to orogastric suction averted the visceral hyperalgesia to colon distension (Smith et al. 2007). It is very likely that the stress in early life induces functional changes in the central neurons affecting the descending modulatory system that may contribute to hyperalgesia. For example, in neonatal MS rats there is significantly higher c-fos expression in the cingulate cortex and superficial (I and II) and deeper (V–VI) laminae of the spinal cord to colon distension compared with c-fos expression in naïve non-handled rats (Chung et al. 2007a; Ren et al. 2007). The notion that early-life stress or a noxious stimulus affects the descending modulatory system is also evident in recent behavioral studies where naloxone, a nonselective opioid antagonist, failed to facilitate the visceromotor response (VMR) to colonic distension in MS compared with naïve non-MS rats (Coutinho et al. 2002; DeBerry et al. 2007).
Although the initial concept was that MS-induced stress triggers central sensitization leading to visceral hyperalgesia, later studies showed that stress also affects the immune system of the viscera and subsequent visceral inflammation. It is now well established that mast cells play a critical role in stressed-induced gut inflammation (Barreau et al. 2007). In rats, MS enhances mast cell density and degranulation in the GI tract, resulting in disruption of the mucosal barrier and luminal bacterial translocation into the tissue (Barreau et al. 2004a, 2007; Gareau et al. 2006). Recent studies indicate that peripheral CRF triggers mast cell degranulation via the activation of CRF1 receptors of the cell, leading to the release of serotonin (5-HT), nerve growth factor (NGF), proteases, and proinflammatory cytokines in the gut to trigger the inflammation. NGF released from mast cells possibly enhances tissue permeability and excitation of sensory neurons to produce visceral hypersensitivity, since anti-NGF antibody treatment abolishes these effects (Barreau et al. 2004a, 2007).
2.3 Neonatal Noxious-Stimulus-Induced Visceral Hyperalgesia Model
Population-based studies have demonstrated that approximately 8% of children experience functional recurrent abdominal pain and about 18–61% of these children continue to report abdominal pain or IBS-like symptoms in their adulthood (Chitkara et al. 2005). Similar to MS-induced stress, a neonatal noxious stimulus can lead to visceral hyperalgesia later in life. Recent studies have shown that visceral or somatic noxious stimulation in the early stage of life (P2–P28) can result in chronic hyperalgesia in rats. In all of these studies, the hyperalgesia was present in the absence of persistent inflammation (Al-Chaer et al. 2000; Miranda et al. 2006; Randich et al. 2006a, b; DeBerry et al. 2007). Repetitive noxious colon distension (60 mmHg, two distensions 30 min apart) during postnatal day 8–21 or repetitive instillation of mustard oil (0.2 ml of a 5% solution) results in visceral allodynia in adulthood. In addition, the lumbosacral spinal neurons and colonic afferents in adult rats were shown to be highly sensitized to colon distension and somatic stimuli compared with those of saline-treated pups (Al-Chaer et al. 2000; Lin and Al-Chaer 2003). It is possible that the spinal neuron sensitization observed in Al-Chaer’s study is contributed by sensitization of primary sensory neurons. Interestingly, if the same stimuli are given at a later stage in life (postnatal day 21 or 45), animals do not develop visceral hypersensitivity (Al-Chaer et al. 2000).
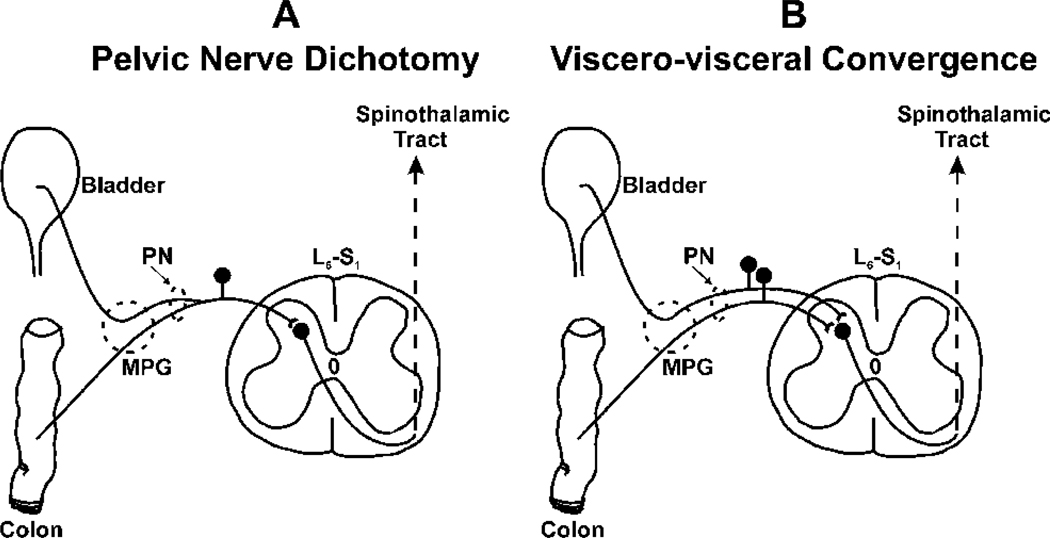
Randich et al. (2006a, b) have shown that cystitis induced in early life by intravesicle injection of zymosan (1%) during postnatal days 14–16 in female rats results in visceral hyperalgesia to bladder distension. These rats exhibited higher micturition frequency (i.e., bladder hyperreflexia) as adults and when these rats were rechallenged with zymosan they exhibited significantly higher response to bladder distension and augmented inflammatory response compared with neonatally saline-injected rats. Following a similar experimental protocol, we have observed that neonatally zymosan-treated rats also exhibit visceral hyperalgesia to colon distension in later life (A. Miranda and J.N. Sengupta, unpublished observation). Although a similar finding has been reported in adult rats where cyclophosphamide-induced cystitis exhibits hyperalgesia to colon distension, it is not known whether the effect is long term (Bielefeldt et al. 2006). Such overlap of sensitization may suggest two possible mechanisms: (1) sensitization of dichotomized (i.e., peripheral axon collaterals) primary sensory neurons innervating two visceral organs (see Fig. 1a; Malykhina et al. 2006; Christianson et al. 2007) and/or (2) sensitization of spinal horn neurons having synaptic input from two organs (i.e., viscerovisceral convergence, see Fig. 1b; McMahon and Morrison 1982; Qin et al. 2004). This is discussed in more detail in Sect. 3.3.
Fig. 1.
Illustrates the pelvic nerve dichotomy (A) and viscero-visceral convergence of colon and bladder afferent fibers on to spinal dorsal horn neuron (B). A: Although the primary sensory neurons are pseudounipolar cells, retrograde labeling of bladder and colon shows colabeling of dorsal root ganglion (DRG) soma in the lumbosacral spinal cord, suggesting that there are two axon collaterals innervating two pelvic structures. Therefore, sensitization of a spinal neuron by inflammation of one organ may influence the sensitivity of the noninflamed organ innervated by the axon collateral. B: Common convergence of two primary sensory afferents from two organs a spinal dorsal neuron is another neural pathway which can exhibit cross-organ sensitization. It has been documented that a small proportion of colorectal distension-sensitive neurons are also sensitive to urinary bladder distension and most of these neurons are in the deeper laminae of the spinal cord.
Manifestation of hyperalgesia to colon distension is not only observed after noxious stimulation of the bladder, but also occurs following a noxious stimulus to the somatic referral site in neonatal rats (Miranda et al. 2006). This phenomenon is due to somatovisceral convergence in the spinal cord and the sensitization occurs at the spinal level, since an ipsilateral noxious stimulus to the hind limb results in contralateral mechanical hyperalgesia. However, there is a distinct difference between rats that received a noxious somatic stimulus in adulthood and rats that received a similar stimulus in their early postnatal (P4–14) life. In adult rats, repetitive low-pH saline injection in the gastrocnemius muscle significantly augments the VMR to colon distension that persists about 2 weeks (Miranda et al. 2004, 2006). In contrast, the adult rats that received low-pH saline injection during postnatal days 8–12 exhibited visceral hyperalgesia lasting up to 5 weeks (Miranda et al. 2006). Although the resultant effect is the same for neonatal stress and a neonatal noxious stimulus, it may or may not share the same neural pathways, neurotransmitters, and neuroendocrine systems. In addition, there could be differential cognitive processing in the brain for different experiences, which is still poorly understood.
3 Contribution of Sensory Afferents in Visceral Pain
3.1 Anatomical Distribution of Visceral Afferents
We have come a long way in our understanding of the existence and contribution of visceral sensory afferents in regards to visceral sensation. Historically, there was a misconception that the visceral organs did not receive any sensory innervation and therefore could not perceive any sensation when the viscera were cut, pinched, or pricked (Von Haller 1755; Lennander 1901). Later, the sensory innervation was recognized, but a notion was formed that visceral dysfunction could only lead to somatic pain since, clinically, muscle tenderness and pain was evident during visceral dysfunction (Mackenzie 1893). It took many years to understand that true visceral pain did exist and that somatic tenderness observed during visceral dysfunction was due to convergence of visceral and somatic sensory afferents in the spinal cord, a phenomenon commonly known as “referred pain” (Bloomfield and Polland 1931; Lewis and Kellgren 1939; Ruch 1946, 1961; Doran 1967).
The majority of thoracic and abdominal visceral organs, except the pancreas, are dually innervated by parasympathetic (craniosacral) and sympathetic (thoracolumbar) outflows. Thoracic viscera and upper abdominal viscera are primarily innervated by the vagus (cranial nerve X) and spinal thoracolumbar outflows. The lower abdominal viscera, including the small and the large intestine and the urogenital organs, are innervated by thoracolumbar (i.e., lumbar splanchnic nerve, LSN, and hypogastric nerve, HGN) and sacral (i.e., pelvic nerve, PN) outflows. For a detailed description of the anatomical organization of visceral afferents, see Jänig and McLachlan (1987) and Sengupta and Gebhart (1994c,1998). The primary sensory afferents are pseudounipolar cells having central and peripheral axonal processes. The peripheral process innervating the visceral organ may have specialized end-organ-like pacinian corpuscles or intraganglionic laminar endings or free nerve endings with multiple parallel branches known as “intramuscular array fiber” (Berthoud et al. 1997; Fox et al. 2000; Phillips and Powley 2000; Zagorodnyuk et al. 2000, 2001, 2003, 2005; Lynn et al. 2003, 2005). The central processes enter the spinal cord via the dorsal root and project primarily to superficial lamina I and deeper laminae V–VII (Cervero 1983; Cervero and Tattersall 1986).
3.2 Response Characteristics of Visceral Afferents
Sensory afferents innervating the visceral organs are not just a homogeneous group of afferents signaling visceral pain to the CNS. It is now a general notion that pain is primarily signaled by spinal afferents, while vagal afferents signal nonpainful sensations such as hunger, satiety, fullness, and nausea. Studies in the esophagus have shown that the response characteristics of vagal afferent fibers to mechanical and chemical stimuli are distinctly different from those of the spinal afferents (for a detailed review, see Sengupta 2006). The vagal afferents may not signal pain, but several human and animal studies have documented that vagal nerve stimulation attenuates somatic and visceral pain. The analgesic effect by vagal activation could be related to its descending inhibitory influence on responses of spinal dorsal horn neurons or via the release of catecholamines from the adrenal medullae (Multon and Schoenen 2005; Sedan et al. 2005; Kirchner et al. 2000; Ness et al. 2000; Jänig et al. 2000; Thurston and Randich 1992; Ren et al. 1991, 1993). Recent studies have also revealed that even the spinal afferents running via the thoracolumbar (LSN) and sacral (PN) pathways are not functionally a homogeneous group of afferents (Brierley et al. 2004, 2005a) and transmit different qualities of signals to the spinal cord (Traub et al. 1992; Wang et al. 2005). In addition, it has been shown that the PN afferents counterbalance the activities of thoracolumbar spinal neurons via the supraspinal descending loop (Wang et al. 2007). Since visceral pain is primarily transmitted via spinal afferents, the following subsections will mainly focus on the functional characteristics of spinal afferents resulting from various types of stimulus.
3.2.1 Visceral Afferents in the Gastrointestinal Tract
In the GI tract, according to the location of the receptive endings in the gut, spinal afferents are classified as mucosal, muscle or tension-sensitive, muscle/mucosal (also known as “tension/mucosal”), serosal, and mesenteric afferents. There is a differential distribution of these afferents in the LSN and PN of mice (Brierley et al. 2004). For example, mesenteric afferents are only identified in the LSN, but not in the PN. Conversely, muscle/mucosal afferents are only found in the PN, but not in the LSN. Although the serosal, muscle, and mucosal afferents are commonly found in both nerves, the population of mucosal (4%) and muscle (10%) afferents in the LSN is much less than the population of those afferents in the PN (23 and 21%, respectively). It is not known whether this differential anatomical distribution of the LSN and the PN holds true in other species.
The majority of mucosal, muscle, muscle/mucosal, serosal, and mesenteric afferents are mechanosensitive, having typical characteristics of response to a mechanical stimulus. Apart from these mechanosensitive afferents, a small group of afferents have been found in the colon mucosa that responded exclusively to chemicals (Lynn et al. 1999). The mucosal mechanosensitive afferents are mostly located in or below the mucosal epithelium. These afferents were previously studied in the vagus nerve, with a major focus on its ability to sense pH and nutrients in the lumen (for review see Mei 1985; Grundy and Scratchard 1989; Sengupta and Gebhart 1994c). Recently, mucosal afferents have been identified and characterized in the LSN and PN afferents (Lynn and Blackshaw 1999; Berthoud et al. 2001; Brierley et al. 2004, 2005a). Under normal conditions, these afferents are either silent or have very low spontaneous firing (< 1 impulse s−1). They exhibit extremely dynamic firing to discrete probing of the mucosal receptive spot, but rarely respond to luminal distension or stretch. It is thought that the mucosal afferents monitor the dynamic events of mechanical positioning of the gut and passage of a bolus through the lumen. The majority of these mucosal mechanosensitive afferents are also chemosensitive and respond to a hyperosmolar solution, acid, bile, 5-HT, ATP, and capsaicin (Lynn and Blackshaw 1999; Berthoud et al. 2001; Brierley et al. 2004, 2005a ; Hicks et al. 2002). Although there is no direct experimental evidence on how spinal mucosal afferent are involved in visceral pain, considering their multimodal character, it can be speculated that these afferents are possibly the first line of the neural pathway that signal pain and discomfort due to a change in the luminal chemical environment, mucosal damage, or inflammation.
The mechanosensitive muscle afferents in the LSN and the PN have been studied extensively in different laboratory animals (Bahns et al. 1987; Blumberg et al. 1983; Clerc and Mei 1983; Haupt et al. 1983; Janig and Koltzenburg 1990, 1991; Sengupta et al. 1990, 1992, 2002; Sengupta and Gebhart 1994a, b; Lynn and Blackshaw 1999; Ozaki and Gebhart 2001; Lynn et al. 2003; Zagorodnyuk et al. 2005). These afferents respond to distension and stretch of the hollow viscera; therefore, they are also known as “distension-sensitive receptors” or “tension receptors.” Typically, muscle afferents demonstrate slow adaptation to tonic distension and the frequency of firing is directly related to tension developed in the smooth muscle. The majority (70–85%) of muscle mechanosensitive afferents have a low threshold for response to luminal distension. However, a small population (15–20%) of afferents exhibit a high threshold (more than 30 mmHg) for response, which could be evidence for their role in visceral nociception (Jänig and Koltzenburg 1990, 1991; Cervero and Jänig 1992; Sengupta et al. 1990, 1992; Sengupta and Gebhart 1994a; Ozaki and Gebhart 2001). Like mucosal afferents, muscle mechanosensitive afferents are also multimodal in character. The effect of the algogenic peptide bradykinin (BK) has been tested in several studies (Haupt et al. 1983; Cervero and Sharkey 1988; Sengupta et al. 1992; Sengupta and Gebhart 1994a; Brierley et al. 2005b). BK excites muscle afferents via the activation of B2 receptors, since the effect is blocked by the selective B2 receptor antagonist, HOE140 (Sengupta et al. 1992; Sengupta and Gebhart 1994a; Brierley et al. 2005b). Interestingly, in the opossum esophagus, muscle afferents in the vagus and splanchnic nerves responded differentially to BK (Sengupta et al. 1992). The splanchnic afferents exhibited greater sensitivity to BK compared with those from the vagus nerve. Activation of vagal afferent fibers by BK was primarily associated with strong smooth muscle contraction, which was attenuated after muscle paralysis. In contrast, the effect of BK on splanchnic afferent fibers was found to be a result of direct action on the nerve endings, since the excitation resulting from exposure to BK was unaffected after paralysis of the smooth muscle (Sengupta et al. 1992). Similar findings were also reported in other areas of the GI tract (Haupt et al. 1983; Cervero and Sharkey 1988; Sengupta and Gebhart 1994a). Studies have also shown that spinal afferents are also sensitive to hot and cold temperatures (Zamiyatina 1954; Su and Gebhart 1998; Ozaki and Gebhart 2001); however, the exact mechanism of the thermosensitivity of these afferents is still not known. It is very likely that the hot and cold sensations are mediated via the activation of TRPV1-2 and TRPM8 channels, respectively. The existence of muscle/mucosal (tension/mucosal) afferents is a relatively new finding in in vitro studies (Page and Blackshaw 1998; Lynn et al. 1999; Brierley et al. 2004, 2005a). The morphological structure of this small subset of afferents is not known. However, from the typical response to muscle tension and mucosal probing, it can be speculated that these afferents have axonal collaterals innervating the muscle layers and mucosa. Alternatively, these afferents can terminate in the submucosal layer and respond to distension and mucosal probing (Paintal 1954). The exact functional role of these afferents has not been suggested, but it is possible that sensitization of the mucosal endings by luminal toxin or chemicals may cross-sensitize the muscular endings, resulting in sensitization of response to distension.
The serosal layer of the GI tract and its adjacent mesentery are innervated by C- and Aδ-afferents. These afferents are commonly found in both the LSN and the PN. One mechanosensitive serosal afferent often has multiple receptive points along the mesenteric blood vessels and at the branching points of the capillary blood vessels supplying the serosal surface (Bessou and Perl 1966; Morrison 1973; Ranieri et al. 1973; Crousillat and Ranieri 1980; Blumberg et al. 1983; Floyd and Morrison 1974; Floyd et al. 1976; Lew and Longhurst 1986; Longhurst and Dittman 1987; Longhurst et al. 1984; Berthoud et al. 2001; Brierley et al. 2004, 2005a, b; Haupt et al. 1983). The majority of serosal afferents have spontaneous firing that does not correlate with movement of the bowel or with changes in intraluminal pressure. Since their receptive fields are close to blood vessels, it can be speculated that serosal afferents possibly sense the serosal blood flow by mechanical sensing of the capillary diameter (Gammon and Bronk 1935). These afferents may also signal an ischemic state of the gut, since occlusion of the descending aorta or mesenteric artery excites these afferents (Sheehan 1932; Haupt et al. 1983; Longhurst and Dittman 1987). Excessive distension due to obstruction may cause blanching of the viscera and excitation of the serosal afferents to signal pain. Serosal mechanosensitive afferents are highly sensitive to several endogenously released substances, such BK, substance P (SP), and 5-HT (Cervero and Sharkey 1988; Haupt et al. 1983; Lew and Longhurst 1986; Longhurst and Dittman 1987; Longhurst et al. 1984). Approximately 65% of serosal mechanosensitive afferents that are sensitive to ischemia are also simulated by BK and prostanoids. It is very likely that the excitation of the serosal afferents during ischemia is due to the release of prostanoids, since treatment with the cyclooxygenase inhibitors aspirin and indomethacin prior to ischemia attenuates excitation (Longhurst et al. 1991).
3.2.2 Visceral Afferents in the Urinary Tract
Under the normal physiological condition, the main function of the urinary bladder is to store and periodically empty (i.e., micturition) urine. This is a reflex process coordinated by three sets of nerves that innervate the lower urinary tract; the PN, the HGN branch of the LSN, and the pudendal nerve (sacral somatic nerves mostly control the external urethral sphincter). There are a large numbers of sensations associated with the process of storage and micturition that range from fullness to urgency, pubic discomfort, and pain. These sensations are transmitted to the CNS via LSN and PN afferents. In the rat, retrograde tracer transport studies have revealed that the LSN bladder afferents enter the spinal cord via L1 and L2 dorsal roots, while PN afferents enter via the L6 and S1 roots (Applebaum et al. 1980; Vera and Nadelhaft 1990, 1992; Nadelhaft and Vera 1991; Pascual et al. 1989, 1993). However, in the cat, LSN afferents run via L2 to L5 dorsal roots, while PN afferents run via S1 to S4 dorsal roots (Applebaum et al. 1980). The variation of segmental distribution has also been observed between females and males and between strains of rats. For example, in female Sprague-Dawley rats, 84% of PN afferents enter the spinal cord via the L6 dorsal root (Vera and Nadelhaft 1990), whereas in male Wistar rats bladder afferents run via the S1 dorsal root (Pascual et al. 1989, 1993). The PN appears to be the major pathway for sensation from the lower urinary tract, because morphological studies in different species of animals clearly indicate that the PN carries a much greater number of afferents than the LSN and selective sacral (S3) nerve denervation in human alleviates bladder pain and urgency (Baron et al. 1985a, b; Hulseboch and Coggeshall 1982; Nadelhaft and Booth 1984; Nadelhaft et al. 1983; Jänig and McLachlan 1987; Schnitzlein et al. 1960; Torrens and Hald 1979). The patterns of innervation of PN and LSN afferents have been studied in the cat bladder by using a selective ganglionectomy induced nerve degenerative technique (Uemura et al. 1973, 1974, 1975). According to these studies, the PN afferents are more abundant in the muscle than in the suburothelium and are widely distributed throughout the bladder, including the dome, body, and trigone area of the bladder. In contrast, LSN afferent innervation is more restricted in the dorsal trigone and neck regions and the nerve terminals are predominantly found in the suburothelium. This strategic innervation by the LSN and the PN may discriminate the mode of sensations arising from the bladder. For example, distension of the bladder can induce sensations in the suprapubic and perineal area, including the perineum and penis. The midline suprapubic sensation associated with bladder overdistension is very likely mediated via LSN afferents entering the thoracolumbar spinal cord, because these segments of the spinal cord innervate the suprapubic dermatomes and myotomes (Morrison 1987). Perineal sensations are thought to be mediated via the PN afferents which enter the sacral spinal segments having a dermatomal distribution in the perineal area.
Until recently, most electrophysiology studies characterized the response of bladder afferents in intact animals by employing intravesicle fluid distension or by punctate probing of the serosal surface, which demonstrated the presence of distension and/or stretch-sensitive afferents (Evans 1936; Talaat 1937; Iggo 1955; Floyd et al. 1976, 1977; Winter 1971; Clifton et al. 1976; Bahns et al. 1986; Häbler et al. 1988a, 1990; Jänig and Koltzenburg 1990; Sengupta and Gebhart 1994b; Su et al. 1997a, b; Dmitrieva and McMahon 1996; Shea et al. 2000; Roppolo et al. 2005). Recently, bladder afferents in the LSN and the PN have been characterized by using an in vitro electrophysiology recording technique from isolated bladders of rats, mice, and guinea pigs (Namasivayam et al. 1999; Rong et al. 2002; Zagorodnyuk et al. 2007; Daly et al. 2007; Xu and Gebhart 2008). These studies revealed different classes of afferents, which are quite similar to the types found in the GI tract. Xu and Gebhart (2008) demonstrated four classes of afferents from the LSN and the PN: serosal, muscle, muscle/urothelial, and urothelial. Interestingly, in the LSN, serosal afferents are predominant (67%) and devoid of urothelial afferents, whereas in the PN, muscle afferents are predominant (63%) compared with serosal afferents (14%). The distribution of receptive fields of LSN and PN afferents in mice is very similar to that reported in cats, where LSN receptive fields, irrespective of class of afferents, are mostly located at the base of the bladder and PN afferents are widely distributed throughout the whole organ (Xu and Gebhart 2008). The only difference between cats and mice is that in cats the LSN afferents innervate urothelium, whereas such innervation is absent in mice (Uemura et al. 1973, 1974, 1975; Xu and Gebhart 2008). Species difference is also evident in the guinea pig, where serosal afferents are nonexistent (Zagorodnyuk et al. 2007). The recordings in this study were done from the nerve trunks in close proximity to the bladder and therefore the pathway of the recorded afferents was not known. Unlike colon mechanosensitive afferents in the LSN and the PN of mice that exhibit differential mechanosensitivity (see Sect. 3.2.1; Brierley et al. 2004, 2005a), bladder mechanosensitive afferents in the LSN and the PN of mice do not exhibit any difference in mechanosensitivity (Xu and Gebhart 2008). However, multiunit recordings from HGN and PN afferents have demonstrated that HGN afferents are sensitive to intravesicle KCl, while PN afferents rarely (1/15 units) responded (Moss et al. 1997).
In addition to the four classes of mechanosensitive afferents, there is a subset of afferents that are unresponsive to a noxious mechanical stimulus (Häbler et al. 1990). These afferents, commonly known as “silent nociceptors,” have been identified by electrical stimulation of the PN trunk (Häbler et al. 1990) or by chemical stimulation with α,β-methylene ATP in the isolated bladder (Rong et al. 2002). Interestingly, instillation of irritant substances such as mustard oil or turpentine oil into the bladder sensitizes these afferents to become mechanosensitive. Due to technical limitation and lack of knowledge of adequate natural stimuli, these afferents have not been studied systematically. It is still not known whether these afferents are exclusively chemospecific and how they become mechanosensitive following chemical sensitization. It is generally thought that these afferents participate in pain signaling following tissue inflammation.
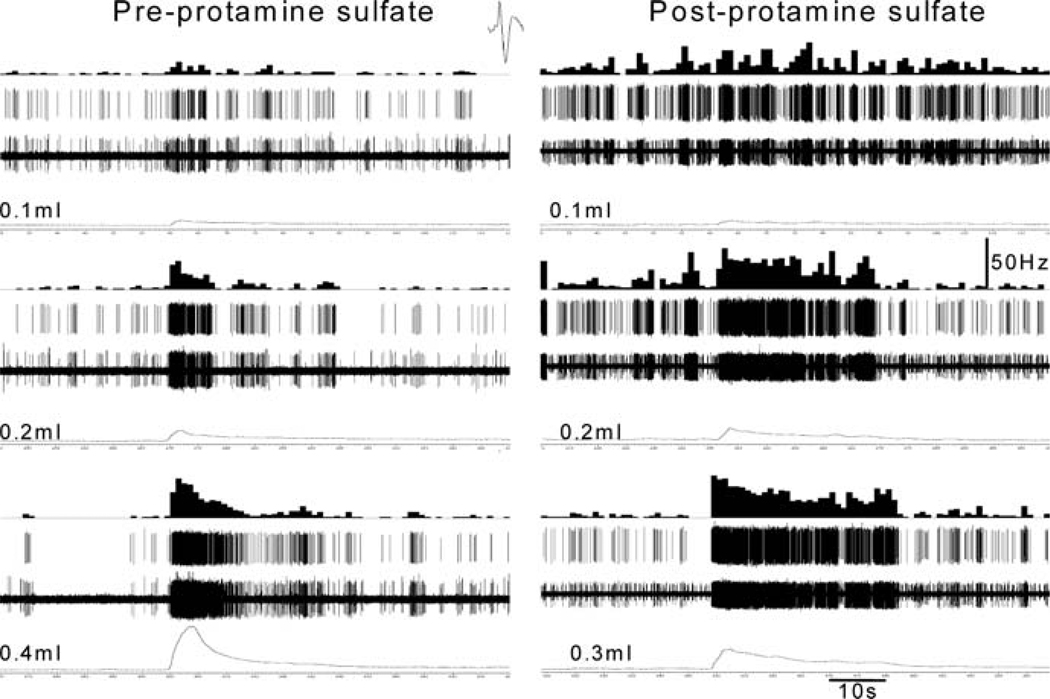
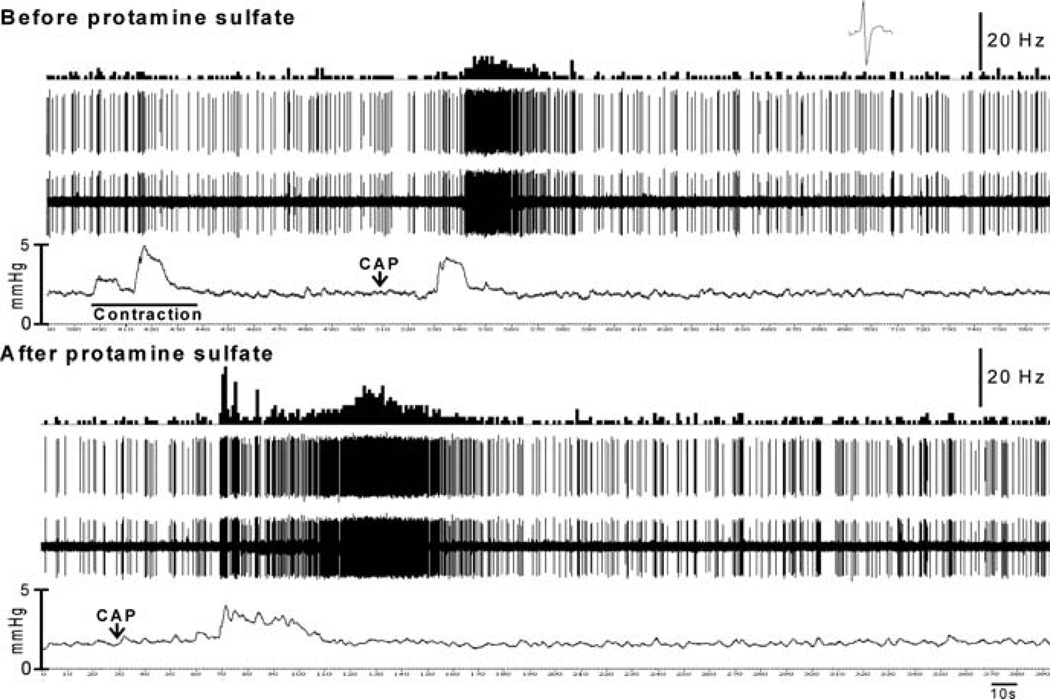
In the PN, muscle afferents exhibit ongoing resting firing when the bladder is empty (Evans 1936; Talaat 1937; Sengupta and Gebhart 1994b; Su et al. 1997a, b; Shea et al. 2000). The spontaneous firing of the muscle afferents has also been reported in in vitro experiments from isolated bladder of mice (Rong et al. 2002; Daly et al. 2007). This is in contrast to some studies in the cat where PN afferent fibers did not exhibit spontaneous firing (Bahns et al. 1987; Häbler et al. 1990, 1993; Iggo 1955). The muscle afferents respond to passive distension of the bladder and the majority of them exhibit a linear increasing response to a graded increase in intravesicle pressure (see the example in Fig. 2) (Sengupta and Gebhart 1994b; Shea et al. 2000; Roppolo et al. 2005) or muscular stretch (Zagorodnyuk et al. 2007; Xu and Gebhart 2008). However, some afferent fibers, mostly those innervating the body of the bladder, do not exhibit a linear relationship with increasing intravesicle pressure (Shea et al. 2000; Daly et al. 2007). These fibers exhibit peak firing at lower pressure and reach a plateau or a decline in firing when the intravesicle pressure reaches the maximum. Although it has been reported that bladder afferents in the cat HGN have a high threshold for response to distension (Evans 1936), their existence in the urinary bladder and GI tract was questioned by investigators (Jänig and Morrison 1986; Morrison 1987). Often it was thought that afferents exhibiting a high threshold for response were not adequately stimulated, as their receptive fields were away from the site of the stimulus. It has now been well documented in several studies, including in vitro studies where the stimulus can be applied to the organ more precisely, that there is a large proportion (approximately 75%) of afferents having a low threshold for response and a small proportion (approximately 25%) of fibers having a high threshold for response (Häbler et al. 1990, 1991, 1993; Sengupta and Gebhart 1994b; Shea et al. 2000; Rong et al. 2002; Daly et al. 2007; Xu and Gebhart 2008). In humans, a sensation of bladder fullness generally occurs at an intravesicle pressure of 5–15 mmHg. A sense of urgency to void occurs when the intravesicle pressure reaches 20–25 mmHg and a pressure exceeding 30 mmHg gives rise to a sense of discomfort and suprapubic pain (Morrison 1987). In rats, the majority of low-threshold afferents respond to an intravesicle pressure below 15 mmHg, while high-threshold afferents start to respond at a pressure above 25 mmHg (Fig. 3). Since low-threshold afferents respond to small increments of intraluminal pressure, they exhibit a change in firing rate during the spontaneous contraction of the detrusor muscle, suggesting that they constantly monitor bladder filling to signal the urgency of micturition. On the other hand, high-threshold afferents do not respond until the intravesicle pressure reaches a certain degree, which signals the sense of discomfort and pain (Fig. 4). A recent report indicates that the excitability of low-threshold, but not high-threshold, afferents is associated with transient receptor potential vanilloid receptor 1 (TRPV1) (Daly et al. 2007). The mechanotransduction of low-threshold afferents can be attenuated by the TRPV1 antagonist capsazepine in wild-type mice. Similarly, muscle afferents from TRPV−/− mice exhibit significantly less response to distension compared with wild-type littermates. Therefore, it is concluded that TRPV1 channels may play an important role in normal bladder function. The mechanosensitive muscle afferents are also sensitive to several chemical stimuli, including hypertonic NaCl, KCl, 50 mM HCl, capsaicin, BK, 5-HT, and histamine. In addition, endogenous substances such as ATP, NGF, and prostaglandins are released by the bladder afferents, the urothelium, and by inflammatory cells, which leads to excitation and sensitization of response bladder afferents to a mechanical stimulus (Chuang et al. 2001; Rong et al. 2002; Yu and de Groat 2008). It appears that the urothelial layer play a critical role in the bladder sensory mechanism and disruption of the urothelial barrier by protamine sulfate may sensitize responses of mechanosensitive bladder afferents to a mechanical (Fig. 5) and a chemical (Fig. 6) stimulus. The effect of ATP and the P2X3 agonist α,β-methylene ATP has been widely tested on bladder afferents and they are known to excite the bladder afferents and sensitize their response to distension (Namasivayam et al. 1999; Rong et al. 2002; Zagorodnyuk et al. 2007; Yu and de Groat 2008). The sensitization by ATP and α,β-methylene ATP is most likely via the activation of P2X3 receptors, since the selective P2X antagonist trinitrophenyl ATP blocks the effect (Rong et al. 2002).
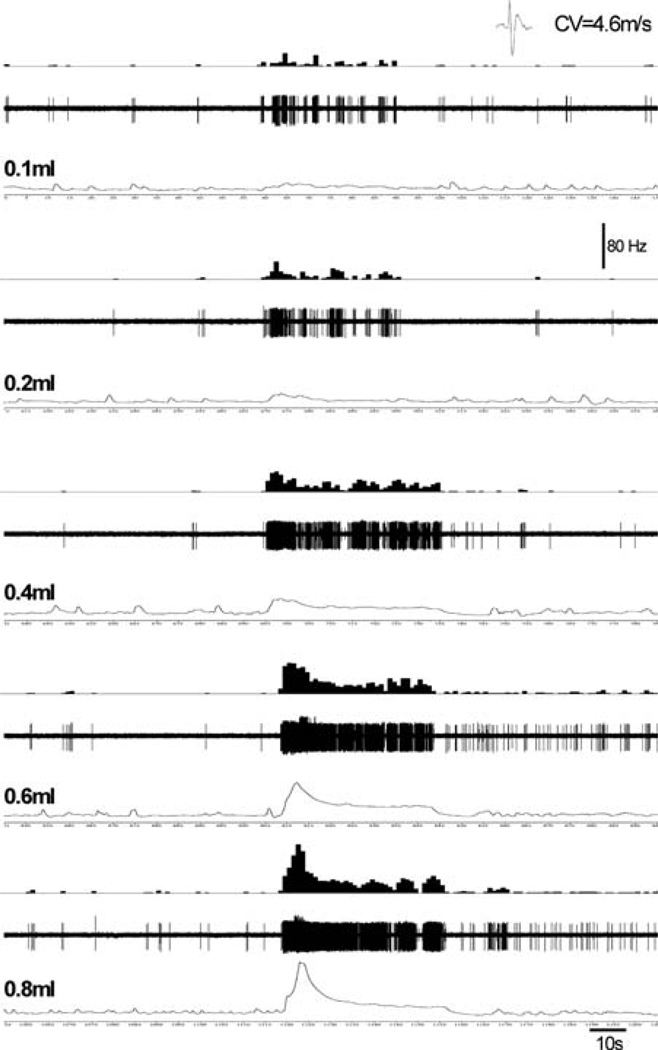
Fig. 2.
Illustrates response characteristics of a thinly myelinated (conduction velocity 4.6 ms−1) pelvic nerve afferent fiber to isovolumic distension. The fiber exhibited low ongoing spontaneous firing when the bladder was empty. With increasing volume of distension (0.1–0.8 ml), the firing frequency of the fibers progressively increased and with higher volume (0.6 and 0.8 ml) it developed a post-stimulus increase in spontaneous firing even when the bladder was empty. In each panel, the top trace represents the frequency histogram (1s binwidth), the middle trace shows nerve action potentials, and the bottom trace shows the isovolumic distension of the bladder. (unpublished data of Sengupta JN)
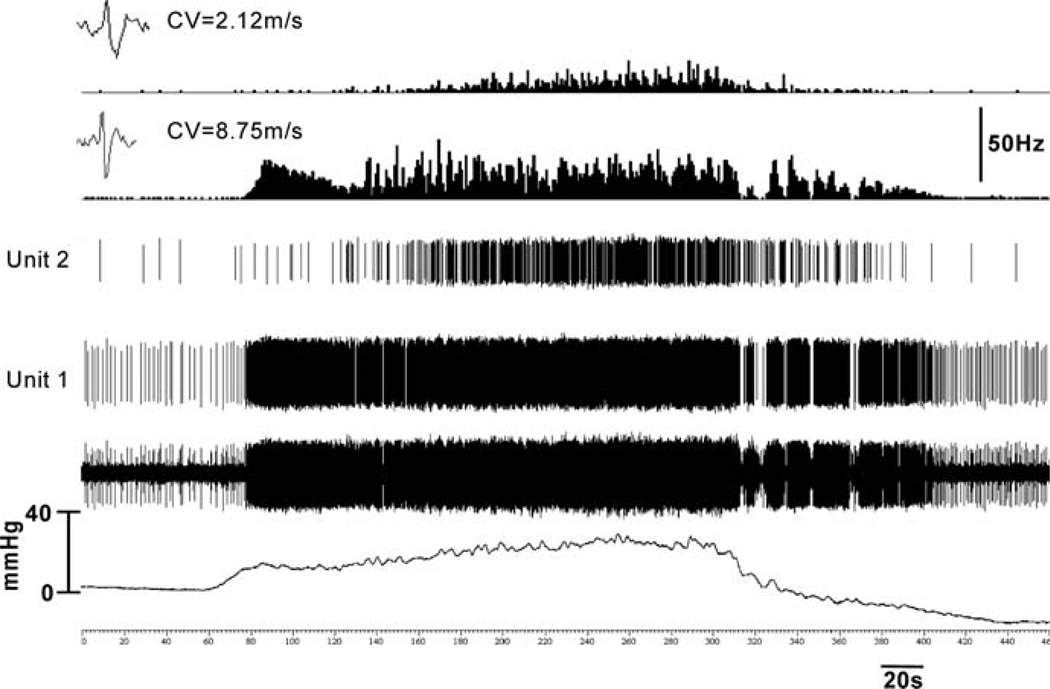
Fig. 3.
In rats, majority of low-threshold afferent fibers respond to intravesicle pressure < 15 mmHg, while a small proportion of high-threshold afferent fibers respond at a pressure > 25 mmHg. This figure illustrates examples of two pelvic nerve afferent fibers in a multiunit recording. Unit 1 having conduction velocity 8.75 ms−1 exhibited a low threshold for response (approximately 17 mmHg), whereas unit 2 (conduction velocity 2.12 ms−1) began to respond at about 32 mmHg. The low-threshold afferent (unit 1) had relatively higher spontaneous firing compared with the high-threshold afferent (unit 2). (unpublished data of Sengupta JN)
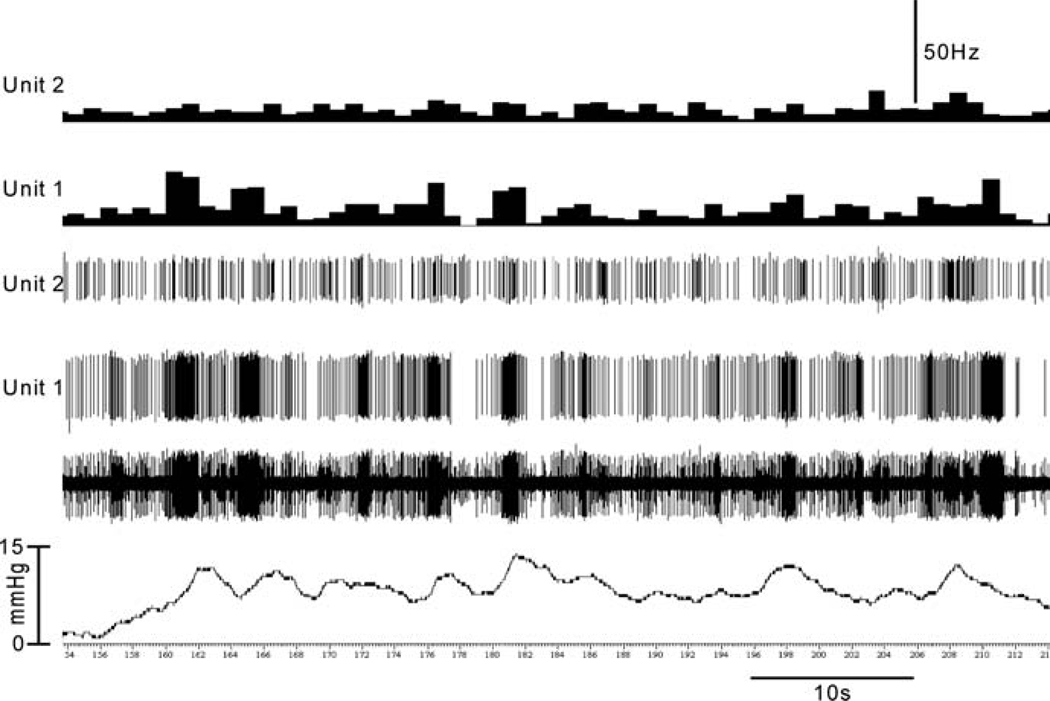
Fig. 4.
Illustrates response characteristics of the same two units shown in figure 3 during intravesicle pressure change when the bladder is empty. Low-threshold afferent fiber (unit 1) responds to small increments of intravesicle pressure and exhibits a change in firing rate during the spontaneous contraction of the detrusor muscle. Therefore, these afferents can constantly monitor bladder filling to signal the urgency of micturition. On the other hand, high-threshold afferent fiber (unit 2) does not respond until the intravesicle pressure reaches a certain degree to signal the sense of discomfort and pain. (unpublished data of Sengupta JN)
Fig. 5.
Instillation of protamine sulfate in to the bladder can sensitize mechanosensitive afferent fiber to urinary bladder distension (UBD). The left column illustrates responses of a pelvic nerve afferent fiber to 0.1, 0.2, and 0.4 ml of distension, respectively. Thirty minutes following the instillation of protamine sulfate (1 mgml−1) the fiber exhibited greater response (right column) to UBD. Note that the fibers exhibited markedly higher response at lower volume (0.3 ml) of distension
Fig. 6.
Sensitization of a bladder afferent fiber to chemical stimulus following protamine sulfate treatment. Before protamine sulfate application, intravesicular injection of capsaicin (10 µl of 1 mgml−1) produced detrusor muscle contraction and an increase in firing of the fiber. However, the firing was not associated with the detrusor muscle contraction, suggesting that capsaicin (CAP) directly stimulated the fiber. Following protamine sulfate treatment, the fiber exhibited markedly greater response to the same dose of CAP. The initial dynamic increase in firing was possibly associated with muscle contraction. However, a prolonged increase in firing was maintained after the intravesicle pressure returned to the baseline. (unpublished data of Sengupta JN)
Clinical studies have shown that rapid filling of the bladder with ice-cold water causes immediate detrusor muscle contraction in paraplegic patients. This is known as the “bladder cooling reflex” (Bors and Blinn 1957). During ice-cold-water infusion, patients report a cold sensation in the urethra or in the suprapubic regions. Patients with painful bladder syndrome report significantly higher pain in the suprapubic area following ice-water instillation compared with the same volume (100 ml) of distension with saline at room temperature, suggesting that cold temperature elicits a painful signal (Mukerji et al. 2006b). Experimental studies in the cat and humans have indicated that cold-saline-induced bladder cooling reflex is not initiated by activation of bladder-distension-sensitive afferents, since the reflex occurs at a volume and pressure that is much less than the threshold intensity to activate bladder afferents. This observation suggests that the cold temperature possibly excites thermospecific afferents (Fall et al. 1990; Lindström and Mazieres 1991; Giersson et al. 1993, 1999). Thermospecific afferents could be located in the urothelium, since immunoreactivity of cold- and menthol-sensitive TRPM8 channels has been detected in nerve fibers scattered in the suburothelium of human bladder tissue (Mukerji et al. 2006a). Alternatively, it is possible that urothelial mechanosensitive afferents are multimodal in character and sensitive to temperature, but this is yet to be documented. A recent study has shown that saline (38 °C) containing menthol (0.6 mM) decreases the voiding threshold in guinea pigs and menthol pretreatment significantly enhances cold-saline-induced bladder cooling reflex (Tsukimi et al. 2005). The existence of cold- and menthol-sensitive bladder afferents in the PN of the cat has been previously documented (Jiang et al. 2002). All the afferents responding to cold temperature are unmyelinated C-fibers unresponsive to bladder distension and their response characteristics resemble those of cutaneous cold receptors. However, the exact location of these afferents in the bladder is not known. The notion that cold-sensitive afferents are unmyelinated C-fibers has been supported by an immunohistochemistry study showing that TRPM8 expression is predominantly in small-diameter neurons in the S1 sacral dorsal root ganglion (DRG) of the guinea pig (Tsukimi et al. 2005). The exact functional role of cold-sensitive afferents in the bladder is not known. However, there are indications that these afferents may participate in nociception. For example, the density of TRPM8 immunoreactivity in suburothelium nerve fibers is significantly higher in patients with painful bladder syndrome and idiopathic detrusor overactivity (Mukerji et al. 2006a). The functional role of cold-sensitive afferents under pathological conditions is difficult to understand because it is not known what the endogenous ligand is for the TRPM8 channel.
Pain is the only conscious sensation arising from the ureter. Afferents from the ureter are mostly found in the L2–L3 and S1–S2 DRGs in the guinea pig (Semenenko and Cervero 1992). Interestingly, retrograde injection of a dye in one ureter revealed a large number of labeled cells in the DRGs of the contralateral side, suggesting that pain originating from one ureter can spread bilaterally to a wide referral site. The majority of labeled neurons were small-diameter neurons containing SP or calcitonin gene-related peptide (CGRP) and about 65% of them exhibited colocalization of SP and CGRP. This result is in agreement with a subsequent study in chickens showing that the ureter is primarily innervated by afferents rich in SP and CGRP (Sann et al. 1997). Electrophysiology recordings from the ureter nerve of chickens and guinea pigs have revealed a large proportion (64–90%) of afferents having a high threshold (range 25–40 mmHg) for response to intraluminal distension, with about 10–30% of afferents having a low threshold (range 7–10 mmHg) for response (Hammer et al. 1993; Sann et al. 1997; Sann 1998). Both low- and high-threshold ureter afferents in the guinea pig were sensitive to algogenic substances such as ATP, α,β-methylene ATP, BK, capsaicin, and KCl, with the exception for SP, that selectively excited high-threshold afferents (Rong et al. 2004; Sann 1998; San et al. 1997). Similar to bladder afferents, both high- and low-threshold ureteric afferents exhibit sensitization of response to distension following intraluminal infusion of ATP or α,β-methylene ATP (Rong et al. 2004). Recordings from the thoracolumbar (T12-L1) spinal dorsal horn neurons have documented that all excitatory neurons respond to distending pressures > than 20 mmHg (Laird et al. 1996), thus suggesting that ureter afferents are largely involved in signaling a painful stimulus.
3.2.3 Visceral Afferents in the Female Reproductive Organs
Like the colon, bladder, and ureter, the female internal reproductive organs, including the uterus, cervix, and vaginal canal, are dually innervated by the HGN branch of the LSN and the PN. Retrograde labeling has revealed a topographical organization of innervation of the vagina, cervix, and uterine horns (Berkley et al. 1993). In rats, afferents from the vaginal canal predominantly enter the spinal cord via the lumbosacral (L6–S1) dorsal roots, while the projections from the mid portion of the uterine cervix are equally distributed in lumbosacral (L6–S1) and the first two lumbar (L1–L2) roots. Projections from the uterine horn run through the lumbar (L1–L2) roots. The majority of afferents innervating the reproductive organs are multimodal in character and respond to both mechanical (uterine distension, punctuate probing, stretching) and chemical (BK, KCl, 5-HT, NaCN, and capsaicin) stimuli (Berkley et al. 1988, 1990, 1993; Hong et al. 1993). Similar to colonic afferents of the HGN and PN afferents in mice, the uterine afferents in these two pathways differ in their sensitivity to a mechanical stimulus. PN afferents are more sensitive to mechanical stimulation compared with HGN afferents (Berkley et al. 1993). HGN afferents mostly respond to discrete probing of the surface of the uterine horn and respond only to a high intensity of uterine distension. This response characteristic is very similar to that of LSN serosal afferents in the colon. In contrast, PN afferents respond best to vaginal and cervical distension and often to probing the internal surface of the cervix (Berkley et al. 1990). Unlike colonic afferents in mice, where PN afferents are less sensitive to BK, uterine PN afferents in rats are more sensitive to BK and other chemicals (5-HT and NaCN) than their HGN counterparts. This difference is not thought to be influenced by the estrous cycle (Berkley et al. 1990). Considering the pattern of innervation and response to mechanical and chemical stimuli, it appears that PN and HGN afferents signal different types of sensations from the reproductive organs. The intensity of pain sensation is variable at different stages of the estrous cycle and is primarily influenced by estrogen (Berkley et al. 1995). It has been shown that both the PN and HGN afferents in rats are more sensitive, including an expanded receptive field, in the proestrous stage compared with diestrous/metestrous stages (Berkley et al. 1988, 1990; Robbins et al. 1990, 1992).
3.3 Sensitization and Cross-Sensitization of Visceral Afferents
Behavioral studies (see Sect. 2) in laboratory animals indicate that inflammation-induced hyperalgesia requires hyperexcitability of afferents to initiate the central (spinal and supraspinal) sensitization. In several studies, acute sensitization of spinal visceral afferents was achieved following application of irritants (mustard oil, turpentine), algogenic substances (BK, capsaicin, ATP, NGF), an inflammatory cocktail (5-HT, histamine, BK, prostaglandin), and inflammogens (zymosan) (Häbler et al. 1993; Dmitrieva and McMahon 1996; Coutinho et al. 2000; Ozaki and Gebhart 2001; Rong and Burnstock 2004; Rong et al. 2002; Xu and Gebhart 2008; Mitsui et al. 2001; Wynn et al. 2004). It is not known how long the afferent sensitization lasts following chemical application and whether the sensitization is always associated with the tissue inflammation. Another important question is whether a long-term ongoing sensitization of afferents is required to maintain the visceral hyperalgesia. There is a dearth of systematic studies that have addressed these questions and the available results are conflicting and incomplete. Sensitization of afferents and its duration could be variable depending on several factors, including the time required for a particular chemical to produce inflammation, the number of applications of a certain chemical, the duration of inflammation, and the stages of life (neonate or adult) during which the stimulus is applied. For example, single intracolonic application of zymosan in adult Sprague-Dawley rats maximally sensitized low- and high-threshold and mechanically unresponsive afferents to colon distension 30 min after injection, but normal sensitization was achieved within 1 h. However, when the VMR response to colon distension is tested at different time periods (1, 2, 3, 4, 5, 6, and 24 h), rats exhibit progressive hyperalgesia (Coutinho et al. 2000). This result suggests that the hyperalgesia outlasts the sensitization of PN afferents and sustained sensitization of afferents is not required to maintain the hyperalgesia. This should be regarded as acute hyperalgesia, since zymosan-induced visceral hyperalgesia generally normalizes by 48 h (Randich et al. 2006a, b). In dextran sulfate sodium induced small intestinal inflammation, mesenteric afferents exhibit significantly higher responses to 5-HT and capsaicin during acute inflammation and after recovery from the inflammation (21 days), but not to mechanical stimulation (Coldwell et al. 2007). Thus, from these two studies, it appears that depending on the type of chemical and the duration of inflammation, visceral afferents exhibit a differential sensitization to mechanical and chemical stimuli in adult rats. In contrast to these reports in adult rats, afferents in neonatally challenged rats behave completely differently to mechanical stimulation. In neonatal rats (postnatal days 8–12) that received repetitive (three times) colonic irritation with mustard oil or noxious distension, PN afferents exhibited a long-term sensitization of response to colon distension when the rats were tested during adulthood when there is complete absence of inflammation (Lin and Al-Chaer 2003). Therefore, it appears that noxious insult during the neonatal period resulted in a long-term phenotypic change of the colonic afferents. Such long-term sensitization of afferents in neonatally challenged rats could be due to sustained upregulation of several receptor molecules, including TRPV1 and P2X purine receptors in the DRGs (Winston et al. 2007; Xu et al. 2008). This is different from adult rats, where a high expression of TRPV1 and SP in DRGs was associated with tissue inflammation (Miranda et al. 2007; Banerjee et al. 2007). In adult rats, premptive treatment with a selective TRPV1 antagonist prevented the development of colonic inflammation and normalized TRPV1 expression in DRGs and hypersensitivity to colon distension, whereas in nontreated rats hypersensitivity was still observed even though TRPV1 expression following inflammation was no different from that of noninflamed naïve rats (Miranda et al. 2007). This difference in duration of overexpression of TRPV1 in the DRGs between neonatally inflamed and adult-inflamed rats may explain the short- and long-term sensitization of afferents observed in electrophysiology studies (Coutinho et al. 2000; Lin and Al-Chaer 2003; Coldwell et al. 2007). However, more systematic study is needed to establish the fact that neonatal noxious insult produces chronic peripheral sensitization.
There are several clinical reports documenting an overlap between IBS and chronic pelvic pain (Longstreth 1994; Longstreth and Drossman 2002; Talley et al. 2003). Approximately 35% of patients with chronic pelvic pain showed significant improvement when treated for IBS (Williams et al. 2004, 2005). In recent years, it has been documented in rats that there is an organ-to-organ cross-sensitization of pelvic viscera, including colon, bladder, and female reproductive organs, which may contribute to the overlap of lower abdominal pain (Pezzone et al. 2005; Malykhina 2007; Ustinova et al. 2006; Qin et al. 2004, 2005; Winnard et al. 2006). At the peripheral and spinal level, there are two potential pathways involved in such cross-sensitization: (1) axonal dichotomy of visceral sensory afferents (Fig. 1A) and (2) convergence of two visceral afferents from two organs on spinal neurons (Fig. 1B). Injections of two different retrograde tracers in the bladder and colon, respectively, have revealed approximately 7–14% colabeled soma in the lumbosacral and thoracolumbar DRGs, suggesting an axonal dichotomy in the pelvic viscera (Malykhina et al. 2006; Christianson et al. 2007). VMR recordings in anesthetized rats demonstrated that bladder irritation with protamine sulfate and KCl significantly increased the VMR to colon distension. In a similar fashion, TNBS-induced colon inflammation induces bladder hyperreflexia (Pezzone et al. 2005) and sensitized responses of bladder afferents to bladder distension (Ustinova et al. 2006, 2007). It has been suggested that such cross-organ sensitization is due to initiation of axon reflex of dichotomized afferents innervating the urinary bladder to produce neurogenic inflammation and an increase in mast cell density in the bladder (Ustinova et al. 2007; Liang et al. 2007). Another recent study has shown that inflammation of the colon or uterus can produce significant inflammation of the bladder. Interestingly, there is no such cross-organ inflammation between the colon and the uterus, suggesting that the bladder is more vulnerable to cross-organ inflammation (Winnard et al. 2006). This study also documented that following HGN sectioning (with intact PN) there is a significant reduction of bladder inflammation, indicating that the HGN plays a major role in cross-organ sensitization in the pelvic viscera. However, this result does not fit well with retrograde tracing data, which have shown a greater number of colabeled cells in the sacral S1 DRG (20%) compared with lumbar L1 DRG (7%) (Malykhina et al. 2006). The cross-sensitization of response to bladder distension has been observed in lumbosacral spinal neurons following inflammation of the colon (Qin et al. 2005). The sensitization of spinal neurons observed in this study could involve viscerovisceral convergence of afferents from two sensitized organs or synaptic input from dichotomized sensory afferents. In addition to the roles of dichotomized afferents and viscerovisceral convergence in the spinal cord, there could be another potential mechanism that can explain organ-to-organ cross-sensitization. This involves axon collaterals of visceral afferents synapsing on secreto/motor (S/M) neurons at the prevertebral ganglia (Aldskogius et al. 1986; Matthews et al. 1987). If these S/M neurons from the prevertebral ganglia (e.g., major pelvic ganglion) innervate different pelvic viscera, then it will be logical to think that the sensitization of afferents can excite the S/M resulting in altered function of the innervated viscera.
It is well recognized that visceral pain can influence the sensitivity of somatic structures, including the skin and muscle (i.e., viscerosomatic hypersensitivity). For example, IBS patients often experience somatic and cutaneous hyperalgesia, which is thought to be the result of central sensitization (Verne et al. 2001, 2003; Verne and Price 2002; Price et al. 2006). These clinical observations have been confirmed in a recent study of experimental cystitis, where mice exhibited cutaneous thermal hyperalgesia (Bielefeldt et al. 2006). Similar to viscerovisceral hypersensitivity, chronic somatic pain can also influence the sensitivity of the visceral organs. Approximately 64% of patients with somatic pain syndromes such as fibromyalgia have frequent abdominal pain and a large proportion of these patients (30–70%) have IBS-like symptoms (Veale et al. 1991; Sperber et al. 1999; Triadafilopolous et al. 1991; Caldarella et al. 2006). Recent studies have documented that a chronic painful stimulus in the somatic referral site alters visceral sensitivity in rats (Miranda et al. 2004; Bielefeldt et al. 2006; Cameron et al. 2007). Miranda et al. (2004) first documented that somatic pain, in the form of low-pH (4.0) saline injections in the gastrocnemius muscle of rat, results in somatic and colonic hypersensitivity. The somatic hyperalgesia in these rats was also observed on the contralateral hind limb, suggesting that this was a result of central sensitization. In a subsequent electrophysiology study, it was documented that spinal neurons responsive to colon distension exhibit sensitization of response to distension following acute low pH saline injection in the gastrocnemius muscle. The sensitized response of these neurons was unaffected by cervical spinal transection, confirming that the sensitization occurs at the spinal level. Since it was an acute experiment, it could be also secondary to hyperexcited primary sensory afferent input. The study documented that the sensitization was primarily driven by glutamates as N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) antagonists significantly attenuated the sensitization (Peles et al. 2004). These behavioral and electrophysiology studies suggest that colonic hypersensitivity following noxious somatic stimulation is due to somatovisceral convergence in the spinal cord and is unlikely due to axonal dichotomy, since the existence of somatovisceral dichotomized afferents is very rare (Häbler et al. 1988b).
3.4 Pharmacological Modulation of Visceral Afferents and Visceral Pain
Considering the fact that afferent nerve sensitization initiates visceral hypersensitivity, attempts have been made to pharmacologically modulate the excitability of the afferents to alleviate visceral sensitivity. The advantage of targeting visceral afferents with a peripherally restricted drug is to avoid unnecessary CNS complications. Among many target receptors, κ-opioid receptors (KOR), P2X purine receptors, 5-HT3 and 5-HT4 seretonin receptors, NMDA receptors (NMDAr), tachykinin (NK1, NK2, and NK3) receptors, TRPV1, and GABAB receptors have been documented to have modulating effects on responses of sensory afferents and spinal processing of pain.
3.4.1 κ-Opioid Receptor
Behavioral studies in rats have shown that unlike μ- and δ-opioid receptor agonists, KOR agonists have no effect when injected spinally, but exhibit significant antinociceptive effects to noxious colon distension when injected systemically (Danzebrink and Gebhart 1995; Sengupta et al. 1999). Thus, this suggests that the effect of KOR agonists is either peripherally or supraspinally mediated. In subsequent studies, it was shown that the majority of arylacetamide KOR agonists such as U50,488, U69,488, EMD 61,753, and IC204,488 dose-dependently attenuate the responses of colonic and bladder mechanosensitive PN afferents to noxious distension (Sengupta et al. 1996, 1999; Su et al. 1997a, b). Interestingly, the inhibitory effects of these KOR agonists were not blocked by the selective KOR antagonist nor-binaltorphimine (nor-BNI) or the nonselective antagonist naloxone. Further evaluation revealed that similar to the local anesthetic arylacetamide, KOR agonists attenuate the mechanotransduction of visceral afferents by blocking the tetrodotoxin-sensitive and tetrodotoxin-resistant Na+ channels of sensory afferents (Joshi et al. 2000, 2003; Su et al. 2002).
3.4.2 P2X Purine Receptors
Among different subtypes of P2X receptors, P2X2, and P2X3 receptors are thought to be involved in mechano- and chemosensory transduction and these two receptors are primarily expressed in small-diameter sensory neurons in DRGs. It is well recognized that ATP and P2X receptors play important roles in bladder pain associated with the inflammation (Burnstock 2002, 2006). Studies in rodents and humans have documented that both P2X2 and P2X3 receptor expression is upregulated in cystitis and possibly contribute to bladder hyperreflexia and pain (Tempest et al. 2004; Nazif et al. 2007; Dang et al. 2008). Exogenous application of ATP and the P2X-selective agonist α,β-methylene ATP sensitizes the responses of afferents to distension. These responses can be attenuated by the nonselective P2X receptor blockers trinitrophenyl ATP and pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (Rong and Burnstock 2004; Rong et al. 2002; Wynn et al. 2003). Therefore, selective antagonists for P2X2 and P2X3 receptors could be useful in the modulation of bladder pain.
3.4.3 5-HT3 and 5-HT4 Seretonin Receptors
The GI tract is the largest source of seretonin (5-HT), located primarily in the enterochromaffin and mast cells. The presence of toxins in the gut triggers the release of 5-HT from these cells, resulting in altered gut motility, nausea, vomiting, and abdominal pain. It is now well recognized from human and animal studies that 5-HT3 and 5-HT4 receptors play critical roles in visceral hypersensitivity in IBS (Gershon and Liu 2007; Spiller 2007; Greenwood-van Meerveld 2007). Behavioral studies in rats have documented that both 5-HT3 antagonists and 5-HT4 agonists have antinociceptive effects resulting from noxious colon distension and this effect is thought to be peripherally mediated (Morteau et al. 1994b; Kozlowski et al. 2000; Greenwood-van Meerveld et al. 2006). Immunohistochemical studies have revealed the existence of 5-HT3 receptors in LSN and PN afferents, indicating that these receptors are involved in peripheral 5-HT-mediated signaling to the brain. This was confirmed in electrophysiology recordings from the LSN where serosal, muscle, and mucosal afferents responded to 5-HT and a selective 5-HT3 agonist. The effects of 5-HT and 5-HT3 agonist were blocked by alosetron, suggesting that the 5-HT effect in GI sensory afferents is mediated via 5-HT3 receptors (Hicks et al. 2002; Coldwell et al. 2007). These studies showed that 5-HT3 receptors are not involved in mechanotransduction of LSN afferents, since alosetron did not affect the responses to mechanical stimulation. Therefore, the question arises how 5-HT3 antagonists produced antinociceptive effects in behavioral studies where mechanical distension was employed to stimulate the colonic mechanosensitive afferents (Morteau et al. 1994b; Kozlowski et al. 2000). It has recently been documented that alosetron significantly attenuates intracolonic glycerol-induced visceral hyperalgesia, but not to colonic distension (Mori et al. 2004). It is possible that intraluminal injection of glycerol produces visceral pain by releasing 5-HT from the enterochromaffin cells to stimulate the sensory afferents and this activation of afferents by 5-HT can be blocked by alosetron. In addition to its peripheral effect to modulate the chemically induced hyperalgesia, it may also be possible that the antinociceptive effect of 5-HT3 antagonist is partly a central effect. Recent studies have shown that intrathecal injection of alosetron into the lumbosacral spinal cord attenuates VMR to colon distension in sensitized rats (Miranda et al. 2006; Bradesi et al. 2007). The 5-HT4 agonists are more known for their prokinetic effect in the GI tract by enhancing motility and water and electrolyte secretion. However, in recent years, it has been documented that the 5-HT4 agonist tegaserod has antinociceptive effects in the viscera (Greenwood-van Meerveld et al. 2006). The effects of 5-HT4 agonists is possibly peripherally mediated, since the antinociceptive effects were observed only when the agonists were injected systemically (intraperitoneal), but not when they were injected centrally into the ventricular space (Greenwood-van Meerveld et al. 2006). However, recent study in our laboratory indicates that antinociceptive effect of tegaserod is via the activation supraspinal 5-HT4 receptors linked with opioidergic descending inhibitory pathway. Intracerebroventricular (icv) injection of tegaserod produces visceral analgesia, which can be blocked by selective 5-HT4 receptor antagonist GR113808 and non-selective opioid receptor antagonist naloxone. The drug fails to attenuate the mechanotransduction of colonic mechanosensitive afferents. In addition, our immunohistochemistry study indicates that in LSN (T13 and L1 DRGs) and PN (L6 and S1 DRGs) nerves mostly large and medium diameter cells are 5-HT4 positive. None of the isolectin B4 (IB4) positive small diameter cells and very few SP-containing DRG cells are 5-HT4 positive. Therefore, it is very unlikely that 5-HT4 has any role in peripheral nociceptive transmission. On the other hand, we have found that endorphin or enkephalin containing neurons in the rostroventral medulla (RVM) are 5-HT4 positive. We believe that 5-HT4 agonist tegaserod activates opiodergic neurons in the RVM to release endogenous opioids to produce descending inhibition of spinal neurons and that results in visceral analgesia.
3.4.4 N-Methyl-d-aspartate Receptor (NMDAr)
Glutamate is the major excitatory neurotransmitter that plays a critical role in the development of visceral hyperalgesia. Animal and human studies have documented that ionotropic glutamate NMDAr antagonists can modulate visceral pain (Olivar and Laird 1999; Zhai and Traub 1999; McRoberts et al. 2001; Castroman and Ness 2002; Gaudreau and Plourde 2004; Ji and Traub 2001; Strigo et al. 2005; Willert et al. 2004, 2007). In the human esophagus, acid-induced secondary hyperalgesia is significantly attenuated by the NMDA channel blocker ketamine (Willert 2004). This drug has been found to be more effective in attenuating visceral pain than somatic pain (Willert 2004, 2007; Strigo et al. 2005). Animal studies have provided substantial evidence that NMDA antagonists modulate visceral pain by attenuating responses of spinal neurons to noxious colon distension (Kohlekar and Gebhart 1994, 1996; Zhai and Traub 1999; Ji and Traub 2001; Traub et al. 2002; Peles et al. 2004). The excitation of central terminals of sensory afferents leads to the release of glutamate to activate the postsynaptic NMDAr and induce spinal neuron sensitization. In addition, glutamate released at the synaptic junction also activates presynaptic NMDAr to regulate the release of SP from the afferent terminals (Marvizon et al. 1997). Although NMDAr are ubiquitously present in the CNS, their presence in spinal visceral afferents has been documented in rats. It has also been shown that colonic inflammation upregulates and phosphorylates the NR1 subunit of the receptor, resulting a functional change (Marvizon et al. 2002; Li et al. 2006). Therefore, other than its effect in the spinal cord, NMDAr antagonist may also attenuate responses of spinal afferents. Olivar and Laird (1999) reported that vasopressor response induced by ureter distension in rats was significantly inhibited by the NMDA channel blockers memantine and ketamine as well as by the NR1 glycine-B site modulator MRZ 2/576. Similarly, memantine attenuated the visceral sensitivity to colon distension when it was injected intravenously, but not intrathecally. Since memantine dose-dependently attenuated the responses of PN afferents to colon distension, the analgesic effect of the drug was thought to be via peripheral NMDAr (McRoberts et al. 2001). Recent studies have documented that the female gonadal hormone estrogen influences the function of NMDAr, which is possibly one of the reasons for the fluctuation of pain sensitivity observed at different phases of the menstrual cycle (Tang et al. 2008; McRoberts et al. 2007). A behavioral study showed that intrathecal injection of an NMDAr antagonist attenuates the VMR response more effectively in overectomized rats compared with overectomized rats supplemented with estradiol, indicating that estradiol modulates the function of the NMDAr channel. In addition, estrogen receptors α and NMDArs coexist in spinal neurons and activation of estrogen receptors α enhances pain signaling by increasing the NR1 subunit expression and phosphorylation of the subunit (Tang et al. 2008). Similarly, in dissociated DRG cells, the average current density of NMDAr was 2.8-fold greater in cells from female rats than in those from male rats. Further, exogenous application of estradiol enhances the current significantly more in female DRGs (55%) than that in male DRGs (19%) (McRoberts et al. 2007). Thus, this suggests that female sex hormones influence the intensity of pain by modulating the function of the receptor molecules, which could be one of the factors accounting for sexual dimorphism in pain sensation.
3.4.5 Tachykinin Receptors: NK1, NK2, and NK3
Three tachykinins SP such as, neurokinin A, and neurokinin B are constitutively present in small-diameter sensory neurons. Although SP is the preferred ligand for NK1, neurokinin A for NK2, and neurokinin B for NK3, all three ligands bind to all three receptors with different affinities. It is well recognized that SP is one of the major neurotransmitters involved in visceral pain and deletion of the NK1 gene in mice significantly affects visceral pain (Laird et al. 2000, 2001a, b). However, regarding the effects of neurokinin receptor antagonists in alleviating visceral pain, reports are conflicting. This is largely due to several factors, including the selection of animal models, lack of selectivity and affinity of the antagonists, bioavailability, and the lack of receptor homology among the species. For the same reasons, many compounds designed for human neurokinin receptors failed to exhibit any analgesic effect in animal studies. Regardless, several animal studies have documented that selective antagonists for all three receptors (NK1, NK2, and NK3) attenuate visceral pain in several animal species, including mice, rats, rabbits, guinea pigs and gerbils (Gardeau and Plourde 2003; Julia et al. 1994, 1999; Laird et al. 2001a, b; Kamp et al. 2001; Fioramonti et al. 2003; Birder et al. 2003; Greenwood-van Meerveld et al. 2003; Bradesi et al. 2003; Okano et al. 2002; Kakol-Palm et al. 2008). Although in many of these studies, the sites of action of the antagonists were not well characterized, presumably most of the antagonists modulate spinal processing by blocking presynaptic and/or postsynaptic neurokinin receptors in the spinal cord. Similarly, there are a few reports of the evaluation of the peripheral effects of these drugs. Two reports have documented the inhibitory effects of NK2 and NK3 receptor antagonists on colonic PN afferents (Julia et al. 1999; Birder et al. 2003).
3.4.6 Transient Receptor Potential Vanilloid 1
The TRPV1 receptor is a ligand-gated, nonselective cation channel sensitive to many natural stimuli, including noxious heat (42–53 °C), acidic pH (5.0–6.0), lipid derivatives, anandamide, and H2S (Caterina et al. 1999; Caterina and Julius 2001; Olah et al. 2001; Trevisani et al. 2005). Traditionally, it was thought that TRPV1 was associated with somatic thermal hyperalgesia in tissue inflammation (Caterina and Julius 2001); however, recent studies suggest that the TRPV1 channel plays a significant role in visceral hyperalgesia associated with tissue inflammation (Apostolidis et al. 2005; Dinis et al. 2004; Fujino et al. 2004, 2006; Matthews et al. 2004; Schicho et al. 2004; Yiangou et al. 2001). In inflammatory bowel disease and esophagitis, the expression of TRPV1 receptors in the lamina propria markedly increases, suggesting that the channel is closely associated with inflammatory process of the GI tract (Matthews et al. 2004; Yiangou et al. 2001). In immunohistochemical examination of tissues from rectosigmoid biopsies, it has been reported that the TRPV1 immunoreactivity was significantly higher (3.5-fold) in the nerve fibers from IBS patients compared with controls. The high TRPV1 immunoreactivity was associated with significantly high SP immunoreactivity in the nerve fibers, mast cells, and lymphocytes in the tissues in the IBS group. This study also reported that high TRPV1 immunoreactive fibers and tissue mast cells closely correlated with the abdominal pain score in patients. Increased TRPV1 immunoreactive nerve fibers were observed in IBS together with a low-grade inflammatory response (Akbar et al. 2008). Similarly, the expression of TRPV1 significantly increases in the DRGs of rats following exposure of the esophagus and stomach to acid (Schicho et al. 2004; Banerjee et al. 2007). In a rat model of cystitis, TRPV1 plays an important role in bladder hyperreflexia, which can be significantly attenuated by blocking the TRPV1 channels (Dinis et al. 2004). It has been shown that hyperreflexia in cystitis is associated with activation of the TRPV1 channel through inflammation-induced release of anandamide, an endogenous ligand for TRPV1 channels (Dinis et al. 2004). It appears that the TRPV1 channel functions via positive feedback during the inflammatory process and visceral sensitivity. For example, the activation of the channel in sensory neurons leads to neurogenic inflammation by release of SP and CGRP. Once the inflammation of the tissue has set in, inflammatory products, including cycloxygenase derivatives of arachidonic acid and other inflammatory cytokines, activate the channel to further enhance the inflammatory process. As indicated in Sect. 3.2.2, TRPV1 plays an important role in mechanotransduction properties of muscle afferents and for that reason visceral sensitivity to mechanical distension of the colon was found to be significantly reduced in TRPV1 knockout mice (Daly et al. 2007; Jones et al. 2005). Therefore, it is very likely that blocking the TRPV1 channel may reduce visceral pain. This has been confirmed in recent studies documenting that pretreatment or posttreatment of a selective TRPV1 antagonist significantly improves colonic inflammation and attenuates the visceral hypersensitivity (Fujino et al. 2004; Miranda et al. 2007; Winston et al. 2007).
3.4.7 GABAB Receptor
γ-Aminobutyric acid (GABA), a major inhibitory neurotransmitter, plays an important role in antinociception. The effect is largely mediated by GABAB receptors, which are ubiquitously present in the brain and spinal cord. Baclofen, a GABAB receptor agonist, produces antinociception in the rat model of visceral pain (Abelli et al. 1989; Hara et al. 1999). It has been shown that in conscious rats subcutaneous injection of baclofen prevents the behavioral responses of pain produced by instilling xylene into the urinary bladder (Abelli et al. 1989). Similarly, intrathecal injection of baclofen increases the threshold for VMR to colon distension (Hara et al. 1999). The c-fos expression in the lumbosacral spinal cord from intracolonic mustard oil induced inflammation was markedly reduced by intraperitoneal injection of baclofen (Lu and Westlund 2001). The effect was primarily via the activation of presynaptic GABAB receptors that regulate the release of SP from the afferent terminals in the spinal cord (Barber et al. 1978; Malcangio and Bowery 1996; Marvizon et al. 1999; Riley et al. 2001). Electrophysiology studies have documented that GABAB receptor agonists modulate responses of vagal mucosal and muscle afferents innervating the esophagus and proximal stomach, suggesting the presence of functional GABAB receptors at the receptive terminals of the vagal afferent fibers (Page and Blackshaw 1999; Partosoedarso et al. 2001; Smid et al. 2001). The existence of GABAB receptor and receptor mRNA has been documented in DRGs (Towers et al. 2000). GABAB receptor mRNA is highly expressed in both small- and large-diameter DRG cells. A recent electrophysiology study showed that baclofen dose-dependently attenuates responses of mechanosensitive PN afferents to noxious colon distension, providing direct evidence that GABAB receptor agonist can modulate visceral pain by acting at the peripheral site (Sengupta et al. 2002). Since GABAB receptor agonist other than its antinociceptive effect has multiple undesirable CNS effects, including sedation, tolerance, respiratory depression, and motor deficiency, its therapeutic use for visceral pain could be seriously limited. However, GABAB is a unique G protein coupled receptor, which requires dimerization of two subunits (GABAB1 and GABAB2) to form a functional receptor. A number of splice variants of the GABAB1 subunit (GABA1a, GABA1b, GABA1c, and GABA1d) have been identified in the rat and human, which are differentially expressed in different tissues (Isomoto et al. 1998). For example, the GABAB1 subunit is ubiquitously present in the CNS and nonneuronal tissues, whereas the GABAB2 subunit is present only in the neurons of the brain and spinal cord (Bolser et al. 1994). Therefore, a peripherally restricted agonist targeting for the GABAB2 splice variant may be a useful to modulate the visceral pain.
4 Conclusion
Despite the large number of reports emerging, the mechanism of visceral pain is still less understood than that of somatic pain. This is primarily due to the diverse nature of visceral pain compounded by multiple factors such as sexual dimorphism, psychological stress, genetic trait, and the nature of predisposed disease. Sensitization of primary sensory afferents is an important underlying mechanism for visceral hypersensitivity and hyperalgesia and the duration of sensitization is possibly the determinant of the chronic nature of the pain. In short-term sensitization, excitation of afferent fibers lasting 1–2 h may lead to a widespread increase in cell responsiveness in the CNS owing to enhanced synaptic strength, whereas in long-term sensitization there could be morphological changes of neurons (e.g., sprouting), central synaptic connectivity, expression of receptor molecules, an altered descending modulatory system, and cortical processing. Findings in animal studies have indicated that the mechanism of neonatal stress-induced visceral hyperalgesia could be different from that in adults. Stress and a noxious stimulus early in life permanently alter the hypothalamic–pituitary–adrenal axis, the descending pain modulatory system, and the expression profile of receptor molecules, and these animals exhibit chronic hyperalgesia later in their life. Such a pain mechanism is possibly different from that observed in postinfectious, diarrhea- or constipation-predominant IBS or in painful bladder syndrome. Similarly, sexual dimorphism plays a critical role in differential pain sensation. Considering this diverse mechanism of visceral pain, the treatment strategy and therapeutic intervention will depend on the disease symptoms.
Acknowledgements
The author acknowledges the support of NIH (RO1 DK062312-A2) to obtain unpublished data reported in this chapter. The author also acknowledges Adrian Miranda and Bidyut K. Medda for their comments and suggestions.
References
- Abelli L, Conte B, Somma V, Maggi CA, Giulaini S, Meli A. A method of studying pain arising from the urinary bladder in conscious, freely-moving rats. J Urol. 1989;141:148–151. doi: 10.1016/s0022-5347(17)40629-x. [DOI] [PubMed] [Google Scholar]
- Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1 expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Aldskogius H, Elfvin LG, Forsman CA. Primary sensory afferents in the inferior mesenteric ganglion and related nerves of the guinea pig. An experimental study with anterogradely transported wheat germ agglutinin-horseradish peroxidase conjugate. J Auton Nerv Syst. 1986;15:179–190. doi: 10.1016/0165-1838(86)90013-5. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thirvikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pedatr. 2004;144:449–454. doi: 10.1016/j.jpeds.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65:400–405. doi: 10.1016/j.urology.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Applebaum AE, Vance WH, Coggeshall RE. Segmental localization of sensory cell that innervate the bladder. J Comp Neurol. 1980;192:203–209. doi: 10.1002/cne.901920202. [DOI] [PubMed] [Google Scholar]
- Bahns E, Ernsberger U, Jänig W, Nelke A. Functional characteristics of lumbar visceral afferent from the urinary bladder and urethra in the cat. Pflügers Arch. 1986;407:510–518. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- Bahns E, Halsband U, Jänig W. Responses of visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch. 1987;410:296–303. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- Banerjee B, Medda BK, Lazarova Z, Bansal N, Shaker R, Sengupta JN. Effect of reflux-induced inflammation on transient receptor potential vanilloid one (TRPV1) expression in primary sensory neurons innervating the oesophagus of rats. Neurogastroenterol Motil. 2007;19:681–691. doi: 10.1111/j.1365-2982.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- Barber RP, Vaughn JE, Saito K, McLaughlin BJ, Roberts E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978;141:35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- Baron R, Jänig W, McLachlan EM. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. I. The hypogastric nerve. J Comp Neurol. 1985a;238:135–146. doi: 10.1002/cne.902380202. [DOI] [PubMed] [Google Scholar]
- Baron R, Jänig W, McLachlan EM. The afferent and sympathetic components of the lumbar spinal outflow to the colon and pelvic organs in the cat. II. The lumbar splanchnic nerves. J Comp Neurol. 1985b;238:147–157. doi: 10.1002/cne.902380203. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004a;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004b;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. New insight in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr Res. 2007;62:240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Afferent fibers supplying the uterus in the rat. J Neurophysiol. 1988;59:142–163. doi: 10.1152/jn.1988.59.1.142. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hotta H, Robbins A, Sato Y. Functional properties of afferent fibers supplying reproductive and other pelvic organs in pelvic nerve of female rat. J Neurophysiol. 1990;63(2):256–272. doi: 10.1152/jn.1990.63.2.256. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J Neurophysiol. 1993;69:533–544. doi: 10.1152/jn.1993.69.2.533. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. Pain. 1995;61:121–131. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol. 1997;195:183–1891. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol. 2001;280:R1371–R1381. doi: 10.1152/ajpregu.2001.280.5.R1371. [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. A movement receptor of the small intestine. J Physiol Lond. 1966;182:404–426. doi: 10.1113/jphysiol.1966.sp007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G658–G665. doi: 10.1152/ajpgi.00585.2005. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kiss S, de Groat WC, Lecci A, Maggi CA. Effect of nepadutant, a neurokinin 2 tachykinin receptor antagonist, on immediate-early gene expression after trinitrobenzene sulfonic acid-induced colitis in the rat. J Pharmacol Exp Ther. 2003;304:272–276. doi: 10.1124/jpet.102.042077. [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Elkahwaji JE, Bushman W, Janda LM, Boldon K, Hopkins WJ, Wang ZY. Acute acrolein-induced cystitis in mice. BJU Int. 2007;99:1523–1529. doi: 10.1111/j.1464-410X.2007.06773.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield AL, Polland WS. Experimental referred pain from the gastrointestinal tract. Part II. Stomach, duodenum and colon. J Clin Invest. 1931;10:453–473. doi: 10.1172/JCI100364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H, Haupt P, Jänig W, Kohler W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibers from the colon. Pflugers Arch. 1983;398:33–40. doi: 10.1007/BF00584710. [DOI] [PubMed] [Google Scholar]
- Bolser DC, DeGennaro FC, O’Reilly S, Chapman RW, Kreutner W, Egan RW, Hey JA. Peripheral and central site of action of GABA-B agonists to inhibit the cough reflex in the cat and guineapig. Br J Pharmacol. 1994;113:1344–1348. doi: 10.1111/j.1476-5381.1994.tb17145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bors EH, Blinn KA. Spinal reflex activity from the vesical mucosa in paraplegic patients. AMA Arch Neurol Psychiatry. 1957;78:339–354. doi: 10.1001/archneurpsyc.1957.02330400013002. [DOI] [PubMed] [Google Scholar]
- Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203–208. [PubMed] [Google Scholar]
- Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain. 2003;102:227–234. doi: 10.1016/S0304-3959(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Bradesi S, Lao L, McLean PG, Winchester WJ, Lee K, Hicks GA, Mayer EA. Dual role of 5-HT3 receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain. 2007;130:56–65. doi: 10.1016/j.pain.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RCW, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005a;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Xu L, Gebhart GF, Blackshaw LA. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol Motil. 2005b;17:854–862. doi: 10.1111/j.1365-2982.2005.00710.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Caldarella MP, Giamberardino MA, Sacco F, Affaitati G, Milano A, Lerza R, Balatsinou C, Laterza F, Pierdomenico SD, Cuccurullo F, Neri M. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782–2789. doi: 10.1111/j.1572-0241.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain. 2007;9:246–253. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Lett. 2001;306:97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- Castroman PJ, Ness TJ. Ketamine, an N-methyl-d-aspartate receptor antagonist, inhibits the spinal neuronal responses to distension of the rat urinary bladder. Anesthesiology. 2002;96:1410–1419. doi: 10.1097/00000542-200206000-00021. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Cervero F. Afferent activity evoked by natural stimulation of the biliary system in the ferret. Pain. 1982;13:137–151. doi: 10.1016/0304-3959(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Cervero F. Somatic and visceral inputs to the thoracic spinal of the cat. J Physiol. 1983;337:51–67. doi: 10.1113/jphysiol.1983.sp014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Jänig W. Visceral nociceptor: a new world order? Trends Neurosci. 1992;15:374–378. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- Cervero F, Sharkey KA. An electrophysiological and anatomical study of intestinal afferent fibers in the rat. J Physiol Lond. 1988;401:381–397. doi: 10.1113/jphysiol.1988.sp017168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Tattersall JEH. Somatic and visceral sensory integration in the thoracic spinal cord. Prog Brain Res. 1986;67:189–205. doi: 10.1016/s0079-6123(08)62763-6. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastrol. 2005;100:1868–1875. doi: 10.1111/j.1572-0241.2005.41893.x. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastrol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol. 2001;165:975–979. [PubMed] [Google Scholar]
- Chung EKY, Zhang X, Li Z, Zhang H, Xu HX, Bian ZX. Neonatal maternal separation enhances central sensitivity to noxious colorectal distension in rat. Brain Res. 2007a;1153:68–77. doi: 10.1016/j.brainres.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Chung EKY, Zhang XJ, Li Z, Xu HX, Sung JJY, Bian ZX. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neural plasticity in rat spinal cord. Neuroscience. 2007b;149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Clerc N, Mei N. Thoracic esophageal mechanoreceptor connected with fibers following sympathetic pathways. Brain Res Bull. 1983;10:1–7. doi: 10.1016/0361-9230(83)90065-5. [DOI] [PubMed] [Google Scholar]
- Clifton GL, Coggeshall RE, Vance WH, Willis WD. Receptive fields of unmyelinated ventral root afferent fibers. J Physiol Lond. 1976;256:573–600. doi: 10.1113/jphysiol.1976.sp011340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell JR, Phillis BD, Sutherland K, Howarth GS, Blackshaw LA. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol. 2007;579:203–213. doi: 10.1113/jphysiol.2006.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Su X, Sengupta JN, Gebhart GF. Role of sensitized pelvic nerve afferents from the inflamed rat colon in the maintenance of visceral hyperalgesia. Prog Brain Res. 2000;129:375–387. doi: 10.1016/S0079-6123(00)29029-8. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Crousillat J, Ranieri F. Mecanorecepteurs splanchniques de la voie biliaire et dedon peritoine. Exp Brain Res. 1980;40:146–153. doi: 10.1007/BF00237532. [DOI] [PubMed] [Google Scholar]
- Cruz Y, Downie JW. Abdominal muscle activity during voiding in female rats with normal or irritated bladder. Am J Physiol. 2006;290:R1436–R1445. doi: 10.1152/ajpregu.00556.2005. [DOI] [PubMed] [Google Scholar]
- Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2007;583:663–674. doi: 10.1113/jphysiol.2007.139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol. 2008;99:49–59. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzebrink RM, Green SA, Gebhart GF. Spinal mu and delta, but not kappa, opioid-receptor agonists attenuate responses to noxious colorectal distension in the rat. Pain. 1995;63:39–47. doi: 10.1016/0304-3959(94)00275-J. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Ness TJ, Robbins MT, Alan R. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distension: modulation of endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105:489–497. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F. Aandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci. 2004;24:11253–11263. doi: 10.1523/JNEUROSCI.2657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop L, Raymond F, Fargeau H, Petoux F, Chovet M, Doherty AM. Pregabalin (CI-1008) inhibits the trinitrobenzene sulfonic acid-induced chronic colonic allodynia in the rat. J Pharmacol Exp Ther. 2002;302:1013–1022. doi: 10.1124/jpet.302.3.1013. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- Doran FSA. The site to which pain is referred from the common bile duct in man and implication for the theory of referred pain. Br J Surg. 1967;54:599–606. doi: 10.1002/bjs.1800540708. [DOI] [PubMed] [Google Scholar]
- Elson CO, Sartor BR, Tennyson GS, Riddel RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Evans JP. Observations on the nerves of supply to the bladder and urethra of the cat, with a study of their action potentials. J Physiol. 1936;86:396–414. doi: 10.1113/jphysiol.1936.sp003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall M, Lindström S, Maziéres L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargeas MJ, Theodorou V, More J, Wal JM, Fioramonti J, Bueno L. Boosted systemic immune and local responsiveness after intestinal inflammation in orally sensitized guinea pigs. Gastroenterology. 1995;109:53–62. doi: 10.1016/0016-5085(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Fioramonti J, Gaultier E, Toulouse M, Sanger GJ, Bueno L. Intestinal anti-nociceptive behaviour of NK3 receptor antagonism in conscious rats: evidence to support a peripheral mechanism of action. Neurogastroenterol Motil. 2003;15:363–369. doi: 10.1046/j.1365-2982.2003.00420.x. [DOI] [PubMed] [Google Scholar]
- Fitzerald M. The development of nociceptive circuits. Nat Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Floyd K, Morrison JFB. Splanchnic mechanoreceptor in the dog. Q J Exp Physiol Cogn Med Sci. 1974;59:361–366. doi: 10.1113/expphysiol.1974.sp002279. [DOI] [PubMed] [Google Scholar]
- Floyd K, Hick EV, Morrison JFB. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol Lond. 1976;259:457–471. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Hick EV, Koley J, Morrison JFB. The effect of bradykinin on afferent units in intra-abdominal sympathetic nerve trunks. Q J Exp Physiol Cogn Med Sci. 1977;62:19–25. doi: 10.1113/expphysiol.1977.sp002373. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Effects of spinal cholecystokinin receptor antagonists on morphine antinociception in a model of visceral pain in the rat. J Pharmacol Exp Ther. 2000;292:538–544. [PubMed] [Google Scholar]
- Friedrich AE, Gebhart GF. Modulation of visceral hyperalgesia by morphine and cholecystokinin from the rat rostroventral medial medulla. Pain. 2003;104:93–101. doi: 10.1016/s0304-3959(02)00469-4. [DOI] [PubMed] [Google Scholar]
- Fujino K, Takami Y, Sebastian G, de la Fuente, Ludwig KA, Christopher R, Mantyh MD. Inhibition of the vanilloid receptor subtype-1 attenuates TNBS-colitis. J Gastrointest Surg. 2004;8:842–847. doi: 10.1016/j.gassur.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Fujino K, de la Fuente SG, Takami Y, Takahashi T, Mantyh CR. Attenuation of acid-induced oesophagitis in VR-1 deficient mice. Gut. 2006;55:34–40. doi: 10.1136/gut.2005.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon GD, Bronk DW. The discharges of impulses from pacinian corpuscles in the mesentery and its relation to vascular changes. Am J Physiol. 1935;114:77–84. [Google Scholar]
- Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–88. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- Gaudreau GA, Plourde V. Role of tachykinin NK1, NK2 and NK3 receptors in the modulation of visceral hypersensitivity in the rat. Neurosci Lett. 2003;351:59–62. doi: 10.1016/s0304-3940(03)00414-2. [DOI] [PubMed] [Google Scholar]
- Gaudreau GA, Plourde V. Involvement of N-methyl-d-aspartate (NMDA) receptors in a rat model of visceral hypersensitivity. Behav Brain Res. 2004;150:185–189. doi: 10.1016/j.bbr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Lindström S, Fall M. The bladder cooling reflex in man – characteristics and sensitivity to temperature. Br J Urol. 1993;71:675–680. doi: 10.1111/j.1464-410x.1993.tb16064.x. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Lindström S, Fall M. The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. J Urol. 1999;162:1890–1896. doi: 10.1016/S0022-5347(05)68062-7. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil. 2007;19 Suppl 2:19–24. doi: 10.1111/j.1365-2982.2007.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino MA, Valente R, de Bigontina P, Vecchiet L. Artificial ureteral calculosis in rats: behavioural characterization of visceral pain episodes and their relationship with referred lumbar muscle hyperalgesia. Pain. 1995;61:459–469. doi: 10.1016/0304-3959(94)00208-V. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B. Importance of 5-hydroxytryptamine receptors on intestinal afferents in the regulation of visceral sensitivity. Neurogastroenterol Motil. 2007;19 Suppl 2:13–18. doi: 10.1111/j.1365-2982.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gibson MS, Johnson AC, Venkova K, Sutkowski-Markmann D. NK1 receptor-mediated mechanisms regulate colonic hypersensitivity in the guinea pig. Pharmacol Biochem Behav. 2003;74:1005–1013. doi: 10.1016/s0091-3057(03)00032-7. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Venkova K, Hicks G, Dennis E, Crowell MD. Activation of peripheral 5-HT receptors attenuates colonic sensitivity to intraluminal distension. Neurogastroenterol Motil. 2006;18(1):76–86. doi: 10.1111/j.1365-2982.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- Grundy D, Scratcherd T. Handbook of physiology; gastrointestinal system. vol 1. Bethesda: American Physiological Society; 1989. Sensory afferents from the gastrointestinal tract; pp. 593–620. [Google Scholar]
- Häbler J, Jänig W, Koltzenburg M. A novel type of unmyelinated chemosensitive nociceptor in the acutely inflamed urinary bladder. Agent Act. 1988a;25:219–212. doi: 10.1007/BF01965016. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Dichotomizing unmyelinated afferents supplying pelvic viscera and perineum are rare in the sacral segments of the cat. Neurosci Lett. 1988b;94:119–124. doi: 10.1016/0304-3940(88)90281-9. [DOI] [PubMed] [Google Scholar]
- Häbler J, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibers by mechanical stimuli and inflammation of the urinary bladder of the cat. J Physiol Lond. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häbler J, Jänig W, Koltzenburg M. Spinal cord integration of colon function: Afferent and efferent pathways. In: Tache Y, Wingate D, editors. Brain–gut interactions. Boca Raton: CRC; 1991. pp. 147–160. [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol. 1993;463:449–460. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer K, Sann H, Pierau FK. Functional properties of mechanosensitive units from the chicken ureter in vitro. Pflugers Arch. 1993;425:353–361. doi: 10.1007/BF00374186. [DOI] [PubMed] [Google Scholar]
- Hara K, Saito Y, Kirihara Y, Yamada Y, Sakura S, Kosaka Y. The interaction of anti-nociceptive effects of morphine and GABA receptor agonists within the rat spinal cord. Anesth Analg. 1999;89:422–427. doi: 10.1097/00000539-199908000-00032. [DOI] [PubMed] [Google Scholar]
- Haupt P, Jänig W, Kohler W. Response patterns of visceral afferent fibers, supplying the colon, upon chemical and mechanical stimuli. Pflugers Arch. 1983;398:41–47. doi: 10.1007/BF00584711. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Coldwell JR, Schindler M, Ward PA, Jenkins D, Lynn PA, Humphrey PP, Blackshaw LA. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002;544:861–869. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SK, Han HC, Yoon YW, Chung JM. Response properties of hypogastric afferent fibers supplying the uterus in the cat. Brain Res. 1993;622(1–2):215–225. doi: 10.1016/0006-8993(93)90822-5. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Coggeshall RE. An analysis of the axon populations in the nerve to the pelvic viscera in the rat. J Comp Neurol. 1982;211:1–10. doi: 10.1002/cne.902110102. [DOI] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and urinary bladder. J Physiol Lond. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomoto S, Kaibara M, Sakurai-Yamashita Y, Nagayama Y, Uenzo Y, Yano K, Taniyama K. Cloning and tissue distribution of novel splice variants of the rat GABAB receptor. Biochem Biophys Res Comm. 1998;253:10–15. doi: 10.1006/bbrc.1998.9706. [DOI] [PubMed] [Google Scholar]
- Jänig W, Koltzenburg M. On the function of spinal primary afferent fibers supplying colon and urinary bladder. J Auton Nerv Syst. 1990;30:S89–S96. doi: 10.1016/0165-1838(90)90108-u. [DOI] [PubMed] [Google Scholar]
- Jänig W, Koltzenburg M. Receptive properties of sacral primary afferent neurons supplying the colon. J Neurophysiol. 1991;65:1067–1077. doi: 10.1152/jn.1991.65.5.1067. [DOI] [PubMed] [Google Scholar]
- Jänig W, McLachlan EM. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987;67:1332–1404. doi: 10.1152/physrev.1987.67.4.1332. [DOI] [PubMed] [Google Scholar]
- Jänig W, Morrison JFB. Visceral sensation. Progress in brain research. vol 67. Amsterdam: Elsevier; 1986. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception; pp. 87–114. [DOI] [PubMed] [Google Scholar]
- Jänig W, Khasar SG, Levine JD, Miao FJ. The role of vagal visceral afferents in the control of nociception. Prog Brain Res. 2000;122:273–287. doi: 10.1016/s0079-6123(08)62145-7. [DOI] [PubMed] [Google Scholar]
- Ji Y, Traub RJ. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. J Neurophysiol. 2001;86:1783–1791. doi: 10.1152/jn.2001.86.4.1783. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Mazieres L, Lindström S. Cold- and menthol-sensitive C afferents of cat urinary bladder. J Physiol. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, III, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Su X, Porreca F, Gebhart GF. Kappa-opioid receptor agonists modulate visceral nociception at a novel, peripheral site of action. J Neurosci. 2000;20:5874–5879. doi: 10.1523/JNEUROSCI.20-15-05874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SK, Lamb K, Bielefeldt K, Gebhart GF. Arylacetamide kappa-opioid receptor agonists produce a tonic- and use-dependent block of tetrodotoxin-sensitive and -resistant sodium currents in colon sensory neurons. J Pharmacol Exp Ther. 2003;307:367–372. doi: 10.1124/jpet.103.052829. [DOI] [PubMed] [Google Scholar]
- Julia V, Morteau O, Buéno L. Involvement of neurokinin 1 and 2 receptors in viscerosensitive response to rectal distension in rats. Gastroenterology. 1994;107:94–102. doi: 10.1016/0016-5085(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Julia V, Su X, Buéno L, Gebhart GF. Role of neurokinin 3 receptors on responses to colorectal distention in the rat: electrophysiological and behavioral studies. Gastroenterology. 1999;116:1124–1131. doi: 10.1016/s0016-5085(99)70015-6. [DOI] [PubMed] [Google Scholar]
- Kakol-Palm D, Brusberg M, Sand E, Larsson H, Martinez V, Johansson A, von Mentzer B, Påhlman I, Lindström E. Role of tachykinin NK(1) and NK(2) receptors in colonic sensitivity and stress-induced defecation in gerbils. Eur J Pharmacol. 2008;582:123–131. doi: 10.1016/j.ejphar.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Kamp EH, Beck DR, Gebhart GF. Combinations of neurokinin receptor antagonists reduce visceral hyperalgesia. J Pharmacol Exp Ther. 2001;299:105–113. [PubMed] [Google Scholar]
- Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55(8):1167–1171. doi: 10.1212/wnl.55.8.1167. [DOI] [PubMed] [Google Scholar]
- Kolhekar R, Gebhart GF. NMDA and quisqualate modulation of visceral nociception in the rat. Brain Res. 1994;651:215–226. doi: 10.1016/0006-8993(94)90700-5. [DOI] [PubMed] [Google Scholar]
- Kolhekar R, Gebhart GF. Modulation of spinal visceral nociceptive transmission by NMDA receptor activation in the rat. J Neurophysiol. 1996;75:2344–2353. doi: 10.1152/jn.1996.75.6.2344. [DOI] [PubMed] [Google Scholar]
- Kozlowski CM, Green A, Grundy D, Boissonade FM, Bountra C. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetized rat. Gut. 2000;46:474–480. doi: 10.1136/gut.46.4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Roza C, Cervero F. Spinal dorsal horn neurons responding to noxious distension of the ureter in anesthetized rats. J Neurophysiol. 1996;5:3239–3248. doi: 10.1152/jn.1996.76.5.3239. [DOI] [PubMed] [Google Scholar]
- Laird JM, Olivar T, Roza C, De Felipe C, Hunt SP, Cervero F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience. 2000;98:345–352. doi: 10.1016/s0306-4522(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Laird JM, Roza C, De Felipe C, Hunt SP, Cervero F. Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain. 2001a;90:97–103. doi: 10.1016/s0304-3959(00)00394-8. [DOI] [PubMed] [Google Scholar]
- Laird JM, Olivar T, Lopez-Garcia JA, Maggi CA, Cervero F. Responses of rat spinal neurons to distension of inflamed colon: role of tachykinin NK2 receptors. Neuropharmacology. 2001b;40:696–701. doi: 10.1016/s0028-3908(00)00205-7. [DOI] [PubMed] [Google Scholar]
- Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol. 2006;290:G451–G457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- Lennander KB. Ueber die sensibilität der bauchhohle und ueber lokale und allgemeine anasthesie bei bruch und bauchoperationen. Zentralbl Chir. 1901;28:200–223. [Google Scholar]
- Lew WYW, Longhurst JC. Substance-P, 5-hydroxytryptamine, and bradykinin stimulate abdominal visceral afferent fiber endings in cats. Am J Physiol. 1986;250:R465–R473. doi: 10.1152/ajpregu.1986.250.3.R465. [DOI] [PubMed] [Google Scholar]
- Lewis T, Kellgren JH. Observation related to referred pain, viscerosomatic reflexes and other associated phenomena. Clin Sci. 1939;4:47–71. [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol. 2006;291:G219–G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Liang R, Ustinova EE, Patnam R, Fraser MO, Gutkin DW, Pezzone MA. Enhanced expression of mast cell growth factor and mast cell activation in the bladder following the resolution of trinitrobenzene sulfonic acid (TNBS) colitis in female rats. Neurourol Urodyn. 2007;226:887–893. doi: 10.1002/nau.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Lindström S, Mazières L. Effect of menthol on the bladder cooling reflex in the cat. Acta Physiol Scand. 1991;141:1–10. doi: 10.1111/j.1748-1716.1991.tb09037.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Dittman LE. Hypoxia, bradykinin and prostaglandins stimulate ischemically sensitive visceral afferents. Am J Physiol. 1987;253:H556–H567. doi: 10.1152/ajpheart.1987.253.3.H556. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Kaufman MP, Ordway GA, Musch TI. Effects of bradykinin and capsaicin on endings of afferent fibers from abdominal visceral organs. Am J Physiol. 1984;247:R552–R559. doi: 10.1152/ajpregu.1984.247.3.R552. [DOI] [PubMed] [Google Scholar]
- Longhurst JC, Rotto DM, Kaufman MP, Stahl GL. Ischemically sensitive abdominal visceral afferents: response to cyclooxygenase blockade. Am J Physiol. 1991;261:H2075–H2081. doi: 10.1152/ajpheart.1991.261.6.H2075. [DOI] [PubMed] [Google Scholar]
- Longstreth GF. Irritable bowel syndrome and chronic pelvic pain. Obstet Gynecol Surv. 1994;49:505–507. doi: 10.1097/00006254-199407000-00027. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Drossman DA. New developments in the diagnosis and treatment of irritable bowel syndrome. Curr Gastroenterol Rep. 2002;4:427–434. doi: 10.1007/s11894-002-0014-2. [DOI] [PubMed] [Google Scholar]
- Lu Y, Westlund KN. Effects of baclofen on colon inflammation-induced Fos, CGRP and SP expression in spinal cord and brainstem. Brain Res. 2001;889:118–130. doi: 10.1016/s0006-8993(00)03124-3. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol. 1999;518:271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786–794. doi: 10.1016/s0016-5085(03)01050-3. [DOI] [PubMed] [Google Scholar]
- Lynn P, Zagorodnyuk V, Hennig G, Costa M, Brookes S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J Physiol. 2005;564:589–601. doi: 10.1113/jphysiol.2004.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J. Some points bearing on the association of sensory disorders and visceral disease. Brain. 1893;16:321–353. [Google Scholar]
- Malcangio M, Bowery NG. GABA and its receptors in the spinal cord. Trends Pharmacol Sci. 1996;17:457–462. doi: 10.1016/s0165-6147(96)01013-9. [DOI] [PubMed] [Google Scholar]
- Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660–672. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI. Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil. 2006;18:936–948. doi: 10.1111/j.1365-2982.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Marvizón JC, Martínez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neuroscience. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABAB receptors. Eur J Neurosci. 1999;11:417–426. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Marvizón JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-d-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Matthews MR, Connaughton M, Cuello AC. Ultrastructure and distribution of substance P-immunoreactive sensory collaterals in the guinea pig prevertebral sympathetic ganglia. J Comp Neurol. 1987;258:28–51. doi: 10.1002/cne.902580103. [DOI] [PubMed] [Google Scholar]
- Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increase Capsaicin Receptor TRPV1 nerve fibers in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF. Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol. 1982;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizón JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-d-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Li J, Ennes HS, Mayer EA. Sex-dependent differences in the activity and modulation of N-methyl-d-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148(4):1015–1020. doi: 10.1016/j.neuroscience.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N. Intestinal chemosensitivity. Physiol Rev. 1985;65:211–237. doi: 10.1152/physrev.1985.65.2.211. [DOI] [PubMed] [Google Scholar]
- Miampamba M, Sharkey K. Distribution of calcitonin gene-related peptide, somatostatin, substance P and vasoactive intestinal polypeptide in experimental colitis in rats. Neurogastroenterol Motil. 1998;10:315–329. doi: 10.1046/j.1365-2982.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Shaker R, Rudolph C, Sengupta JN. Neonatal nociceptive somatic stimulation differentially modifies the activity of spinal neurons in rats and results in altered somatic and visceral sensation. J Physiol. 2006;572:775–785. doi: 10.1113/jphysiol.2006.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Smith C, Sengupta JN. The Role of TRPV1 in Mechanical and Chemical Visceral Hyperalgesia Following Experimental Colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T. Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J Neurophysiol. 2001;86:2276–2284. doi: 10.1152/jn.2001.86.5.2276. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawano K, Shishikura T. 5-HT3-receptor antagonist inhibits visceral pain differently in chemical and mechanical stimuli in rats. J Pharmacol Sci. 2004;94:73–76. doi: 10.1254/jphs.94.73. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Morrison JFB. Splanchnic slowly adapting mechanoreceptor with punctate receptive fields in the mesentery and gastrointestinal tract of the cat. J Physiol Lond. 1973;233:340–361. doi: 10.1113/jphysiol.1973.sp010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JFB. Sensation arising from the lower urinary tract. In: Torrens M, Morrison JFB, editors. Physiology of the lower urinary tract. New York: Springer; 1987. pp. 89–131. [Google Scholar]
- Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994a;39:1239–1248. doi: 10.1007/BF02093789. [DOI] [PubMed] [Google Scholar]
- Morteau O, Julia V, Eeckhout C, Bueno L. Influence of 5-HT3 receptor antagonists in visceromotor and nociceptive responses to rectal distension before and during experimental colitis in rats. Fundam Clin Pharmacol. 1994b;8:553–562. doi: 10.1111/j.1472-8206.1994.tb00837.x. [DOI] [PubMed] [Google Scholar]
- Moss NG, Harrington WW, Tucker MS. Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol. 1997;272:R695–R703. doi: 10.1152/ajpregu.1997.272.2.R695. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006a;6:6–13. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Waters J, Chessell IP, Bountra C, Agarwal SK, Anand P. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006b;6:31–42. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man … and back. Acta Neurol Belg. 2005;105(2):62–67. [PubMed] [Google Scholar]
- Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution the distribution of the visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons labelled after the application of tracer to the distal stump of the transected hypogastric nerve in the rat. J Auton Nerv Syst. 1991;36:87–96. doi: 10.1016/0165-1838(91)90104-b. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Roppolo C, Morgan C, De Groat WC. Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. J Comp Neurol. 1983;216:36–52. doi: 10.1002/cne.902160105. [DOI] [PubMed] [Google Scholar]
- Namasivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. Br J Urol. 1999:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69:24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Metcalf AM, Gebhart GF. A psychophysical study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain. 2000;86:81–85. doi: 10.1016/s0304-3959(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. J Urol. 2001;165:968–975. [PubMed] [Google Scholar]
- Okano S, Ikeura Y, Inatomi N. Effects of tachykinin NK1 receptor antagonists on the viscerosensory response caused by colorectal distention in rabbits. J Pharmacol Exp Ther. 2002;300:925–931. doi: 10.1124/jpet.300.3.925. [DOI] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- Olivar T, Laird JM. Differential effects of N-methyl-d-aspartate receptor blockade on nociceptive somatic and visceral reflexes. Pain. 1999;79:67–73. doi: 10.1016/S0304-3959(98)00152-3. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Gebhart GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol. 2001;281:G1449–G1459. doi: 10.1152/ajpgi.2001.281.6.G1449. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Bielefeldt K, Sengupta JN, Gebhart GF. Models of gastric visceral hyperalgesia. Am J Physiol. 2002;283:G666–G676. doi: 10.1152/ajpgi.00001.2002. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw AL. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Blackshaw AL. GABAB receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS. A method of location of the receptors of visceral afferent fibers. J Physiol Lond. 1954;124:166–172. doi: 10.1113/jphysiol.1954.sp005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partosoedarso ER, Young RL, Blackshaw AL. GABAB receptors on vagal afferent pathways: peripheral and central inhibition. Am J Physiol Gastrointest Liver Physiol. 2001;280:G658–G668. doi: 10.1152/ajpgi.2001.280.4.G658. [DOI] [PubMed] [Google Scholar]
- Pascual JI, Insausti R, Gonzalo LM. The pelvic innervation in the rat: different spinal origin and projections in Sprague-Dawley and Wistar rats. Brain Res. 1989;480:397–402. doi: 10.1016/0006-8993(89)90742-7. [DOI] [PubMed] [Google Scholar]
- Pascual JI, Insausti R, Gonzalo LM. Urinary bladder innervation in male rat: termination of primary afferents in the spinal cord as determined by transganglionic transport of WGA-HRP. J Urol. 1993;150:500–504. doi: 10.1016/s0022-5347(17)35535-0. [DOI] [PubMed] [Google Scholar]
- Pattinson D, Fitzerald M. The neurobiology of infant pain: development of excitatory and inhibitory neurotransmission in the spinal dorsal horn. Reg Anes Pain Med. 2004;29:36–44. doi: 10.1016/j.rapm.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Peles S, Miranda A, Shaker R, Sengupta JN. Acute nociceptive somatic stimulus sensitizes neurons in the spinal cord to colonic distension in the rat. J Physiol. 2004;560:291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Price DD, Zhou Q, Moshiree B, Robinson ME, Nicholas Verne G. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport. 2004;15:467–471. doi: 10.1097/00001756-200403010-00017. [DOI] [PubMed] [Google Scholar]
- Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Simon PL, Schwartz LW, Griswold DE, Fondacaro JD, Wasserman MA. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989;97:326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006a;6:2–8. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Cannon R, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006b;7:468–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Mei N, Crousillat J. Splanchnic afferent arising from gastrointestinal and peritoneal mechanoreceptor. Exp Brain Res. 1973;16:276–290. doi: 10.1007/BF00233331. [DOI] [PubMed] [Google Scholar]
- Ren K, Randich A, Gebhart GF. Effects of electrical stimulation of vagal afferents on spinothalamic tract cells in the rat. Pain. 1991;44:311–319. doi: 10.1016/0304-3959(91)90102-4. [DOI] [PubMed] [Google Scholar]
- Ren K, Zhuo M, Randich A, Gebhart GF. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol. 1993;69(5):1530–1540. doi: 10.1152/jn.1993.69.5.1530. [DOI] [PubMed] [Google Scholar]
- Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol. 2007;292:G849–G856. doi: 10.1152/ajpgi.00400.2006. [DOI] [PubMed] [Google Scholar]
- Richter JE, Heading RC, Janssens J, Wilson J. Functional esophageal disorders. In: Drossman DA, editor. Rome II; the functional gastrointestinal disorders. 2nd edn. chap 5. Degnon, McLean: 2000. [Google Scholar]
- Riley RC, Trafton JA, Chi SI, Basbaum AL. Presynaptic regulation of spinal cord tachykinin signaling via GABAB but not GABAA receptor activation. Neuroscience. 2001;103:725–737. doi: 10.1016/s0306-4522(00)00571-6. [DOI] [PubMed] [Google Scholar]
- Ritchie J. Pain from the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A, Sato Y, Hotta H, Berkley KJ. Responses of hypogastric nerve afferent fibers to uterine distension in estrous or metestrous rats. Neurosci Lett. 1990;110:82–85. doi: 10.1016/0304-3940(90)90791-7. [DOI] [PubMed] [Google Scholar]
- Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- Rong W, Burnstock G. Activation of ureter nociceptor by exogenous and endogenous ATP in guinea pig. Neuropharmacology. 2004;47:1093–1101. doi: 10.1016/j.neuropharm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppolo JR, Tai C, Booth AM, Buffington CA, de Groat WC, Birder LA. Bladder Adelta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol. 2005;173(3):1011–1015. doi: 10.1097/01.ju.0000145591.35569.9e. [DOI] [PubMed] [Google Scholar]
- Ruch TC. Visceral sensation and referred pain. In: Fulton JF, editor. Howell’s textbook of physiology. 15th edn. Saunders, Philadelphia: 1946. pp. 385–401. [Google Scholar]
- Ruch TC. Pathophysiology of pain. In: Ruch TC, Patton JD, Woodbury JW, Towe AL, editors. Medical physiology and biophysics. 9th edn. Saunders, Philadelphia: 1961. pp. 350–368. [Google Scholar]
- Sann H. Chemosensitivity of nociceptive, mechanosensitive afferent nerve fibres in the guinea-pig ureter. Eur J Neurosci. 1998;10:1300–1311. doi: 10.1046/j.1460-9568.1998.00141.x. [DOI] [PubMed] [Google Scholar]
- Sann H, Hammer K, Hildesheim IF, Pierau FK. Neurons in the chicken ureter are innervated by substance P- and calcitonin gene-related peptide-containing nerve fibres: immunohisto-chemical and electrophysiological evidence. J Comp Neurol. 1997;380:105–118. doi: 10.1002/(sici)1096-9861(19970331)380:1<105::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schicho R, Waltraud F, Liebmann I, Holzer P, Lippe IT. Increased expression of TRPV1 receptor in dorsal root ganglia by acid insult of the rat gastric mucosa. Eur J Neurosci. 2004;19:1811–1818. doi: 10.1111/j.1460-9568.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- Schnitzlein HN, Hoffman HH, Tucker CC, Quigley MB. The pelvic splanchnic nerves of the male Rheusus monkey. J Comp Neurol. 1960;114:51–65. doi: 10.1002/cne.901140105. [DOI] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Taché Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Sedan O, Sprecher E, Yarnitsky D. Vagal stomach afferents inhibit somatic pain perception. Pain. 2005;113:354–359. doi: 10.1016/j.pain.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Semenenko FM, Cervero F. Afferent fibres from the guinea-pig ureter: size and peptide content of the dorsal root ganglion cells of origin. Neuroscience. 1992;47:197–201. doi: 10.1016/0306-4522(92)90132-l. [DOI] [PubMed] [Google Scholar]
- Sengupta JN. GI motility online Nature. New York: 2006. Esophageal sensory physiology. [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994a;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1994b;72:2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and visceral sensations. In: Johnson LR, et al., editors. Physiology of the gastrointestinal tract. Raven, New York: 1994c. pp. 483–519. [Google Scholar]
- Sengupta JN, Gebhart GF. The sensory innervation of the colon and its modulation. Curr Opin Gastrol. 1998;14:15–20. [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Stimulus-response function studies of esophageal mechanosensitive nociceptor in sympathetic afferents of opossum. J Neurophysiol. 1990;64:796–812. doi: 10.1152/jn.1990.64.3.796. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Saha JK, Goyal RK. Differential sensitivity of bradykinin to esophageal distension-sensitive mechanoreceptor in vagal and sympathetic afferents of the opossum. J Neurophysiol. 1992;68:1053–1067. doi: 10.1152/jn.1992.68.4.1053. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111:968–980. doi: 10.1016/s0016-5085(96)70064-1. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK, Shaker R. Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol. 2002;283:G1343–G1351. doi: 10.1152/ajpgi.00124.2002. [DOI] [PubMed] [Google Scholar]
- Shea VK, Cai R, Crepps B, Mason JL, Perl ER. Sensory fibers of the pelvic nerve innervating the Rat’s urinary bladder. J Neurophysiol. 2000;84:1924–1933. doi: 10.1152/jn.2000.84.4.1924. [DOI] [PubMed] [Google Scholar]
- Sheehan D. The afferent nerve supply of the mesentery and significance in the causation of abdominal pain. J Anat. 1932;67:233–249. [PMC free article] [PubMed] [Google Scholar]
- Smid SD, Young RL, Cooper NJ, Blackshaw AL. GABABR expressed on vagal afferent neurons inhibit gastric mechanosensitivity in ferret proximal stomach. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1494–G1501. doi: 10.1152/ajpgi.2001.281.6.G1494. [DOI] [PubMed] [Google Scholar]
- Smith C, Nordstrom E, Sengupta JN, Miranda A. Neonatal gastric suctioning results in chronic somatic and visceral hyperalgesia: role of corticotropin releasing factor. Neurogastroenterol Motil. 2007;19:692–699. doi: 10.1111/j.1365-2982.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- Sperber AD, Atzmon Y, Neumann L, Weisberg I, Shalit Y, Abu-Shakrah M, Fich A, Buskila D. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94:3541–3546. doi: 10.1111/j.1572-0241.1999.01643.x. [DOI] [PubMed] [Google Scholar]
- Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19 Suppl 2:25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Bushnell MC, Boivin M, Wainer I, Rodriguez Rosas ME, Persson J. The effects of racemic ketamine on painful stimulation of skin and viscera in human subjects. Pain. 2005;113:255–264. doi: 10.1016/j.pain.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat polymodal in character. J Neurophysiol. 1998;80:2632–2644. doi: 10.1152/jn.1998.80.5.2632. [DOI] [PubMed] [Google Scholar]
- Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997a;77:1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- Su X, Sengupta JN, Gebhart GF. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997b;78:1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- Su X, Joshi SK, Kardos S, Gebhart GF. Sodium channel blocking actions of the kappa-opioid receptor agonist U50,488 contribute to its visceral antinociceptive effects. J Neurophysiol. 2002;87:1271–1279. doi: 10.1152/jn.00624.2001. [DOI] [PubMed] [Google Scholar]
- Su X, Riedel ES, Leon LA, Laping NJ. Pharmacologic evaluation of pressor and visceromotor reflex responses to bladder distension. Neurourol Urodyn. 2008;27:249–253. doi: 10.1002/nau.20469. [DOI] [PubMed] [Google Scholar]
- Talaat M. Afferent impulses in the nerves supplying the urinary bladder. J Physiol Lond. 1937;89:1–13. doi: 10.1113/jphysiol.1937.sp003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW, Crowell MD. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344–1348. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- Thurston CL, Randich A. Electrical stimulation of the subdiaphragmatic vagus in rats: inhibition of heat-evoked responses of spinal dorsal horn neurons and central substrates mediating inhibition of the nociceptive tail flick reflex. Pain. 1992;51:349–365. doi: 10.1016/0304-3959(92)90221-V. [DOI] [PubMed] [Google Scholar]
- Torrens M, Hald T. Bladder denervation procedures. Urol Clin North Am. 1979;6:283–293. [PubMed] [Google Scholar]
- Towers S, Princivalle A, Billinton A, Edmunds M, Bettler B, Urban L, Castro-Lopes J, Bowery NG. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur J Neurosci. 2000;12:3201–3210. doi: 10.1046/j.1460-9568.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Pechman P, Iadarola MJ, Gebhart GF. Fos-like proteins in the lumbosacral spinal cord following noxious and non-noxious colorectal distention in the rat. Pain. 1992;49:393–403. doi: 10.1016/0304-3959(92)90247-9. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptides. 1999;20:267–273. doi: 10.1016/s0196-9781(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Zhai Q, Ji Y, Kovalenko M. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention-induced Fos expression in the spinal cord and the visceromotor reflex. Neuroscience. 2002;113:205–211. doi: 10.1016/s0306-4522(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci. 1991;36:59–64. doi: 10.1007/BF01300088. [DOI] [PubMed] [Google Scholar]
- Tsukimi Y, Mizuyachi K, Yamasaki T, Niki T, Hayashi F. Cold response of the bladder in guinea pig: involvement of transient receptor potential channel, TRPM8. Urology. 2005;65:406–410. doi: 10.1016/j.urology.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Dirks VA, Bradley WE. Distribution of sacral afferent axons in cat urinary bladder. Am J Anat. 1973;136:305–313. doi: 10.1002/aja.1001360305. [DOI] [PubMed] [Google Scholar]
- Uemura E, Fletcher TF, Bradley WE. Distribution of lumbar afferent axons in muscle in muscle coat of cat urinary bladder. Am J Anat. 1974;139:389–398. [Google Scholar]
- Uemura E, Fletcher TF, Bradley WE. Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anat Rec. 1975;183:579–587. doi: 10.1002/ar.1091830408. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Fraser MO, Pezzone MA. Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol. 2006;290:F1478–F1487. doi: 10.1152/ajprenal.00395.2005. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Gutkin DW, Pezzone MA. Sensitization of pelvic nerve afferents and mast cell infiltration in the urinary bladder following chronic colonic irritation is mediated by neuropeptides. Am J Physiol. 2007;292:F123–F130. doi: 10.1152/ajprenal.00162.2006. [DOI] [PubMed] [Google Scholar]
- Veale D, Kavanagh G, Fielding JF, Fitzeral O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenic process. Br J Rheumatol. 1991;30:220–222. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- Vera PL, Nadelhaft I. Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Res. 1990;520:83–89. doi: 10.1016/0006-8993(90)91693-b. [DOI] [PubMed] [Google Scholar]
- Vera PL, Nadelhaft I. Afferent and sympathetic innervation of the dome and the base of the urinary bladder of the female rat. Brain Res. 1992;29:651–658. doi: 10.1016/0361-9230(92)90134-j. [DOI] [PubMed] [Google Scholar]
- Verne GN, Price DD. Irritable bowel syndrome as a common precipitant of central sensitization. Curr Rheumatol Rep. 2002;4:322–328. doi: 10.1007/s11926-002-0041-x. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- Von Haller A. A dissertation of the sensible and irritable parts of animals. London: Nourse; 1755. [Google Scholar]
- Wallace JL, Le T, Carter L, Appleyard CB, Beck P. Hapten-induced colitis in the rat: alternatives to trinitrobenzene sulfonic acid. J Pharmacol Toxicol Methods. 1995;33:237–239. doi: 10.1016/1056-8719(95)00001-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J Neurophysiol. 2005;94:3788–3794. doi: 10.1152/jn.00230.2005. [DOI] [PubMed] [Google Scholar]
- Wang G, Tang B, Traub RJ. Pelvic nerve input mediates descending modulation of homovisceral processing in the thoracolumbar spinal cord of the rat. Gastroenterology. 2007;133:1544–1553. doi: 10.1053/j.gastro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-d-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- Willert RP, Delaney C, Kelly K, Sharma A, Aziz Q, Hobson AR. Exploring the neurophysiological basis of chest wall allodynia induced by experimental oesophageal acidification – evidence of central sensitization. Neurogastroenterol Motil. 2007;19:270–278. doi: 10.1111/j.1365-2982.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- Williams RE, Hartmann KE, Sandler RS, Miller WC, Steege JF. Prevalence and characteristics of irritable bowel syndrome among women with chronic pelvic pain. Obstet Gynecol. 2004;104:452–458. doi: 10.1097/01.AOG.0000135275.63494.3d. [DOI] [PubMed] [Google Scholar]
- Williams RE, Hartmann KE, Sandler RS, Miller WC, Savitz LA, Steege JF. Recognition and treatment of irritable bowel syndrome among women with chronic pelvic pain. Am J Obstet Gynecol. 2005;192:761–767. doi: 10.1016/j.ajog.2004.10.634. [DOI] [PubMed] [Google Scholar]
- Winnard KP, Dmitrieva N, Berkley KJ. Cross-organ interactions between reproductive, gastrointestinal, and urinary tracts: modulation by estrous stage and involvement of the hypogastric nerve. Am J Physiol. 2006;291(6):R1592–R1601. doi: 10.1152/ajpregu.00455.2006. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Winter DL. Receptor characteristics and conduction velocites in bladder afferents. J Psychiatr Res. 1971;8:225–235. doi: 10.1016/0022-3956(71)90021-5. [DOI] [PubMed] [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2008;99:244–253. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57(9):1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NHC, Chan CLH, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Yu Y, de Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJH. Transduction sites of vagal mechanoreceptors in the guinea-pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJH. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol. 2005;289:G397–G406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ, Gregory SJ. Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol. 2007;585:147–163. doi: 10.1113/jphysiol.2007.140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamyatina ON. Electrophysiological characteristics and functional significance of afferent impulses originating in the intestinal wall. Transaction of I.P. Pavlov Institute of Physiology. 1954;3:193–208. [PubMed] [Google Scholar]
- Zhai QZ, Traub RJ. The NMDA receptor antagonist MK-801 attenuates c-Fos expression in the lumbosacral spinal cord following repetitive noxious and non-noxious colorectal distention. Pain. 1999;83(2):321–329. doi: 10.1016/s0304-3959(99)00116-5. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Caudle RM, Verne N. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]