Abstract
To explore the role of IKKβ in gastric carcinogenesis, we established a conditional gastric mucosal epithelium IKKβ knockout mouse (IkkβΔST) that did not differ phenotypically or histologically from control mice (IkkβF/F). Gastric cancer was induced using N-methyl-N-nitrosourea (MNU). After 8 months, the mean number (±SE) of tumors per body was significantly less in the IkkβΔST group (0.80 ± 0.23) than in the IkkβF/F group (2.21 ± 0.48), although the size of tumors did not differ between groups (2.75 ± 0.99 vs. 2.89 ± 1.12 mm, respectively). We investigated the levels of several inflammatory cytokines in the stomach after a single oral dose of MNU and found that IL-1α was significantly up-regulated in control mice compared to IkkβΔST mice, whereas the levels of IL-1β, IL-6, and TNF-α were unchanged. MNU significantly increased apoptotic cell death in control mice compared to IkkβΔST mice, and apoptosis was dependent on decreased IL-1α expression. IL-1α also induced the proliferation of gastric cancer cells. Fewer tumors were observed in IL-1 receptor knockout mice (Il-1r−/−; 1.17 ± 0.44) than in control mice (2.42 ± 0.52). These results suggest that IKKβ regulates gastric carcinogenesis via IL-1α expression, which is associated with anti-apoptotic signaling and cell proliferation.
Introduction
The development of cancer in various organs is often associated with chronic inflammation, suggesting a strong relationship between inflammation and carcinogenesis (1). Abnormal cellular alterations accompanying inflammation, such as oxidative stress, gene mutation, epigenetic changes, and inflammatory cytokine-induced cell proliferation, have been proposed as carcinogenic factors. The signal transduction pathways of carcinogenesis are also under study (2). Understanding the mechanism by which inflammation leads to carcinogenesis may enable the development of drugs targeted at important molecules in this process, potentially providing a powerful tool for preventing cancer development.
The nuclear factor-kappa B (NF-κB) pathway is thought to play a role in the process leading from inflammation to carcinogenesis and thus may be a candidate for targeted intervention (3). Many pro-inflammatory stimuli activate NF-κB, primarily through inhibitor of κB (IκB) kinase (IKK)-dependent phosphorylation and degradation of IκB proteins. Once activated, NF-κB dimers stimulate the transcription of genes encoding cytokines, growth factors, chemokines, and anti-apoptotic factors (4). IKK consists of two catalytic subunits, IKKκ and IKKβ, and a regulatory component, IKKγ/NEMO. IKK and NF-κB activation by most stimuli depend largely upon IKKβ (4), the absence of which increases susceptibility to tumor necrosis factor-α (TNFα)-induced apoptosis and causes the loss of innate immunity (5). NF-αB is instrumental for tumor promotion in a colitis-associated cancer (CAC) model (6) and inflammation-associated liver cancer (7, 8). However, the inhibition of NF-αB in keratinocytes promotes squamous cell carcinoma (9). Such results suggest that the impact of NF-αB on cancer development may be cell type- or model-specific. NF-αB activation is likely most critical in cancers in which inflammation acts as a tumor promoter, but a more general role for NF-αB, and the inflammation driven by it, in cancers that are not associated with chronic inflammation remains to be established.
Gastric cancer is the second leading cause of cancer deaths worldwide, although its incidence has been declining in recent decades. A high-salt diet, smoked foods rich in nitrates, and water contaminated with Helicobacter pylori appear to be major environmental inducers of gastric cancer (10–13). A high-salt diet disrupts the gastric mucosal barrier, and N-nitroso mutagens formed from dietary nitrates in a hypochlorhydric stomach can damage DNA (10). The role of H. pylori in causing mucosal effects has been heavily investigated, because of the possibility that some cancers could be prevented with antibiotic treatment against H. pylori (13). However, the molecular signals that actually initiate the program of irreversible transformation remain unclear.
To explore the role of IKKβ in gastric carcinogenesis, we established a conditional gastric mucosal epithelium IKKβ knockout mouse (IkkβΔST) and analyzed it in an N-methyl-N-nitrosourea (MNU)-mediated gastric cancer model. Mice lacking IKKβ in the gastric epithelium exhibited a marked decrease in MNU-induced gastric carcinogenesis relative to control mice. This inhibition of tumor development was correlated with a decrease in the expression of IL-1α, which is associated with anti-apoptotic effects and cell proliferation. Thus, IL-1α expression regulated via IKKβ signaling may represent a therapeutic target in the prevention and treatment of gastric cancer.
Results
Generation of gastric epithelium IKKβ deficient mice
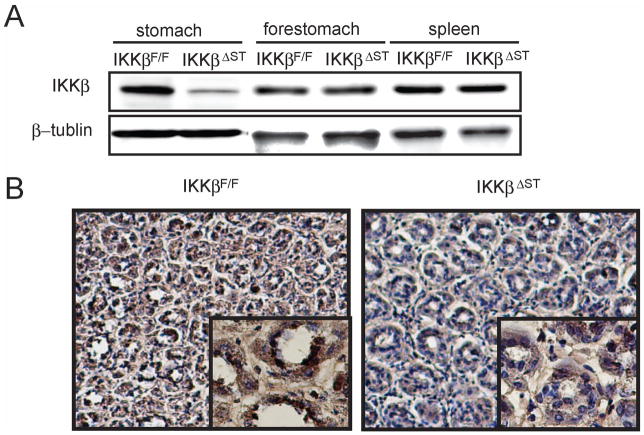
To generate conditional gastric mucosal epithelium IKKβ mutant mice (IkkβΔST), IkkβF/F mice (14) were crossed with Foxa3-Cre transgenic mice expressing Cre recombinase, which is active beginning on embryonic day 8.5 (E8.5) in the ventral foregut endoderm (15). Cre-mediated recombination was assessed using Western blot analysis of whole stomach extract; this analysis confirmed that stomachs from IkkβΔST mice produced less than 20% of the normal level of IKKβ, whereas no change was observed in the forestomach or spleen, in which Cre recombinase is not expressed (Figure 1A). Because the gastric epithelium represents only 70–80% of the total gastric mass, the residual IKKβ expression was most likely due to other cell types in the stomach.
Figure 1.
Generation of IKKβ gastric epithelium knockout mice.
(A) Gastric mucosa, forestomach, and spleen of IkkβF/F and IkkβΔST mice were lysed, gel-separated, and immunoblotted with antibodies against the indicated proteins. (B) IkkβF/F and IkkβΔST mice were administered 10 mg of lipopolysaccharide (LPS); after 1 h, the stomachs were extracted and stained with anti-p65 antibody. Left panel, IkkβF/F (×200, ×800); right panel, IkkβΔST (×200, ×800).
IkkβΔST mice were born at Mendelian frequencies and exhibited no developmental abnormality or morbidity. At 12 weeks of age, the body weights of male IkkβF/F and IkkβΔST mice (26.9 ± 3.3 and 25.1 ± 2.2 g, respectively) were not significantly different. The gross anatomy and histological organization of the IkkβΔST stomach were normal, and no spontaneous gastric inflammation or tumor was observed for at least 1 year after birth (Supplemental Figure 1 and data not shown). Thus, IKKβ is not required for stomach development or long-term survival under normal husbandry.
To determine whether NF-κB activity decreased in response to IKKβ knockout, IkkβF/F and IkkβΔST mice were treated with lipopolysaccaride (LPS), a potent NF-κB activator, and frozen sections were immunohistochemically stained with an anti-p65 antibody. Visualization revealed that p65 nuclear staining decreased markedly in IkkβΔST mice compared to IkkβF/F controls (Figure 1B).
IKKβ deficiency in the gastric epithelium inhibits gastric tumor development
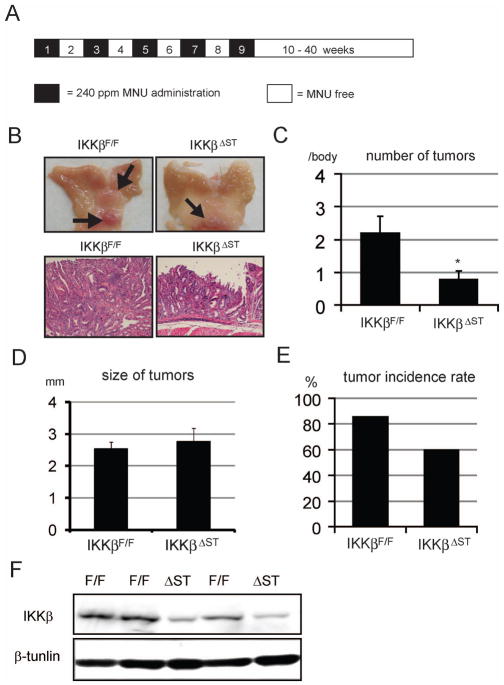
To explore the role of IKKβ in gastric carcinogenesis, we used a mouse model of chemically induced gastric carcinogenesis. In mice, the administration of MNU results in efficient gastric cancer induction (16). IkkβF/F and IkkβΔST mice on the C57BL/6 genetic background were given drinking water containing MNU in light-shielded bottles on alternate weeks (total exposure of 5 weeks), and then normal tap water until week 40 (Figure 2A). The mean number of detectable gastric tumors (± standard error, SE) was significantly lower in IkkβΔST mice (0.80 ± 0.23) compared to IkkβF/F controls (2.21 ± 0.48; Figure 2B and C). In contrast, the average sizes of tumors were similar in both IkkβF/F and IkkβΔST mice (2.75 ± 0.99 mm vs. 2.89 ± 1.12 mm, respectively; Figure 2D). After MNU administration, 86% of the control mice developed gastric tumors, whereas only 60% of the IkkβΔST mice developed tumors (p < 0.05; Figure 2E). These data suggest that IKKβ is involved in MNU-induced gastric tumor development. Finally, we used immunohistochemistry to confirm that tumors in IkkβΔST mice were IKKβ-deficient. Although IKKβ was present in IkkβF/F tumors, it was detectable in fewer than 20% of IkkβΔST tumors. IKKβ present in IkkβΔST samples most likely originated from other types of cells in stomach (Figure 2F).
Figure 2.
Loss of IKKβ in the gastric epithelium inhibits gastric tumor development.
(A) Schematic overview of gastric carcinogenesis model. Black areas indicate MNU (240 ppm) administration periods, and white areas indicate MNU non-administration periods. (B) Representative stomach tumors. Left panels, IkkβF/F tumors; right panels, IkkβΔST tumors. Upper panels show macroscopic views of the tumor-bearing stomachs. Arrows indicate the tumors. Lower panels show H&E staining of tumor parts (×200). (C) Number of tumors in stomachs. Stomachs were observed macroscopically, and tumors > 1 mm in diameter were counted. Values represent the means ± SE for IkkβF/F (n = 14) and IkkβΔST (n = 10) mice (*p < 0.05). (D) Stomach tumor sizes. The stomachs were observed macroscopically, and tumors > 1 mm in diameter were measured. Values represent the means ± SE for IkkβF/F (n = 35) and IkkβΔST (n = 8) mice. (E) Tumor incidence rates in IKKβF/F (85.7%, 12/14) and IKKβΔ ST (60.0%, 6/10) mice. (F) IKKβF/F and IKKβΔST tumors were immunoblotted with antibodies against IKKβ and β-tubulin.
IKKβ deficiency potentiates MNU-mediated apoptosis
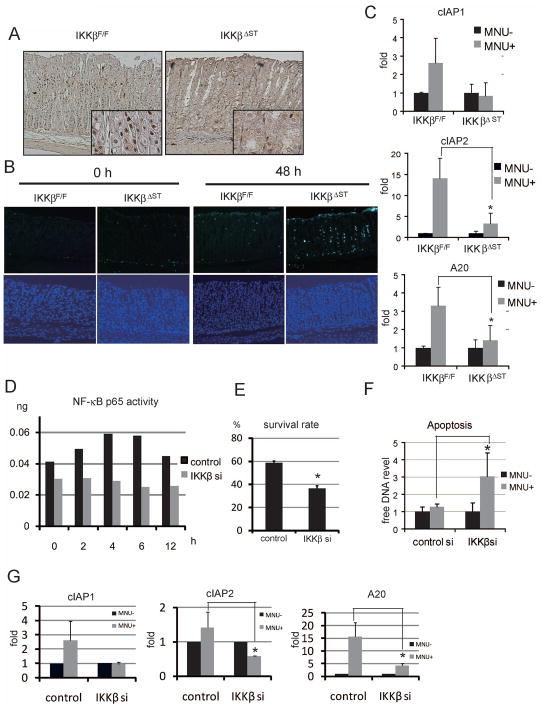
A single oral dose of MNU activated NF-κB, as determined by p65 nuclear staining in gastric epithelia from IkkβF/F mice at 4 h (Figure 3A). As expected, NF-κB activation was not observed in IkkβΔST mice (Figure 3A).
Figure 3.
IKKβ knockdown potentiates MNU-mediated apoptosis.
(A) Four hours after the oral administration of MNU (1000 ppm), stomachs were stained immunohistochemically with anti-phospho-p65 antibody (×200, ×800). (B) MNU (1000 ppm) was administered orally and apoptotic cells were identified using the TUNEL assay (upper panels; ×100). Lower panels show counterstaining with DAPI (×100). (C) Total mRNA was isolated 6 h after a single oral dose of MNU (1000 ppm) to IkkβF/F and IkkβΔST mice. The expression levels of each gene were measured using real-time PCR. (D) Vector carrying IKKβ siRNA or control siRNA was transfected into SH101 cells, followed by treatment with 250 μM MNU after 48 h. The cells were corrected at the indicated times, and NF-μB/p65 activity was quantified using ELISA. The y-axis shows p65 immunoreactivity compared to that of recombinant p65. (E) Cells transfected with IKKβ siRNA (IKKβsi) or control siRNA (control) were treated with MNU for 48 h, after which the numbers of living cells were counted. The survival rates of treated vs. untreated cells are shown (*p < 0.05). (F) Levels of DNA released into the culture medium 48 h after MNU stimulation were measured (*p < 0.05). (G) Cells transfected with IKKβsi or control siRNA were treated with MNU for 6 h, after which the expression levels of the indicated genes were measured using real-time PCR (*p < 0.05).
Cellular injury is strongly linked to carcinogenesis, and IKKβ is reportedly required for apoptosis associated with DNA damage (17). We therefore examined whether MNU potentiates apoptotic induction in IkkβΔST mice. As expected, oral MNU administration induced gastric apoptosis to a greater degree in IkkβΔST mice than in IkkβF/F mice, whereas we found no difference between IkkβF/F and IkkβΔST mice without MNU treatment (Figure 3B and Supplemental figure 2).
Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis of anti-apoptotic genes showed a marked induction of cIAP2 and A20 in IkkβF/F mice that was nearly absent in IkkβΔST mice (Figure 3C). Compensatory proliferation is reportedly linked to carcinogenesis (8). We therefore analyzed cell proliferation using proliferating cell nuclear antigen (PCNA) immunostaining, but found no obvious difference between IkkβF/F and IkkβΔST mice, indicating that increased apoptosis is not associated with stimulation of the proliferative response (Supplemental Figure 3). In vitro, we decreased IKKβ expression in SH101 human gastric cancer cells using a specific small interfering RNA (siRNA), which showed no detectable induction of NF-κB activation (Figure 3D). After MNU treatment, cells expressing IKKβsi showed higher levels of cell death and apoptosis than cells transfected with control siRNA (Figure 3E and F). We analyzed the expression of anti-apoptotic genes that are putatively regulated by NF-κB after MNU stimulation and found that the expression of cIAP2 and A20 increased in control cells; in contrast, these genes showed only very slight up-regulation in IKKβsi-expressing cells (Figure 3G).
IKKβ deficiency decreases MNU-induced IL-1α expression
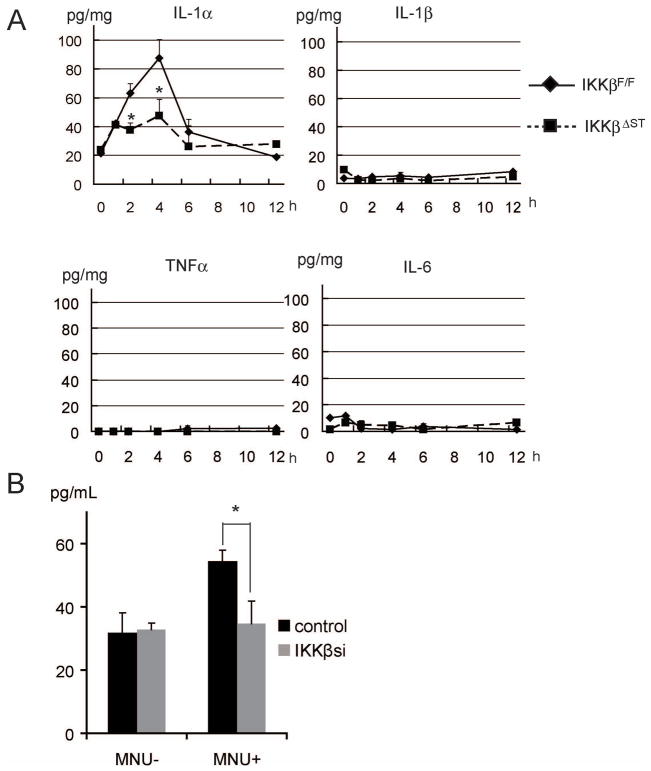
Pro-inflammatory cytokines, chemokines, and adhesion molecules, which regulate the inflammatory responses, are frequently observed in tumor microenvironments, and many cytokines released during the inflammatory response have critical roles in cancer cell survival. We therefore analyzed the expression of inflammatory cytokines, using a cytokine array, and found that, among more than 40 cytokines, MNU induced only IL-1α protein expression in the stomachs of IkkβF/F mice; notably, this response was lacking in IkkβΔST mice. IL-1β, TNF-α, and IL-6 were not induced in either IkkβF/F or IkkβΔST mice (Figure 4A and Supplemental Figure 4).
Figure 4.
Loss of IKKβ suppresses MNU-induced IL-1α expression.
(A) MNU (1000 ppm) was administered orally to IkkβF/F and IkkβΔST mice, and stomachs were collected and lysed at the indicated times. Cytokine levels were measured using ELISA. (B) SH101 cells transfected with IKKβ siRNA (IKKβsi) or control siRNA (control) were treated with or without 250 μM MNU. IL-1α levels in the culture medium were measured 12 h after MNU stimulation (*p < 0.05).
In vitro, we assessed the level of IL-1α in the culture medium after MNU stimulation of SH101 cells. The IL-1α level increased in medium collected from MNU-treated control cells, but not in medium collected from IKKβsi-expressing cells (Figure 4B). These results suggest that MNU-induced IL-1α expression is dependent upon IKKβ.
IL-1α increases cell proliferation and inhibits apoptosis
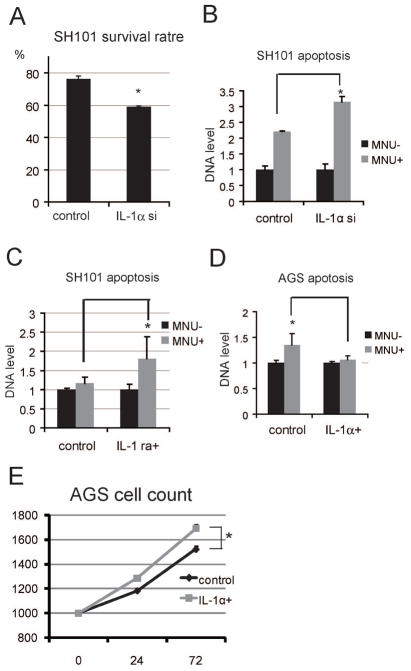
IL-1α modulates various growth-promoting pathways, including anti-apoptotic signaling and cellular proliferation. Therefore, we analyzed the roles of IL-1α in tumor cell growth in vitro. To investigate the anti-apoptotic function of this cytokine, we used a specific siRNA to reduce IL-1α expression in SH101 gastric cancer cells, which constitutively express IL-1α. The survival rate of MNU-treated cells expressing IL-1αsi was lower than that of cells transfected with control siRNA (Figure 5A). We also examined the degree of apoptotic induction, as determined by DNA release, and found that IL-1αsi-expressing cells showed increased apoptosis relative to the control (Figure 5B).
Figure 5.
IL-1α promotes cell proliferation and inhibits apoptosis.
(A) SH101 cells transfected with IL-1α siRNA (IL-1αsi) or control siRNA (control) were treated with 250 μM MNU for 48 h, after which the number of living cells was counted. The survival rates of treated vs. untreated cells are shown (*p < 0.05). (B) Cells were treated as above. The degree of apoptotic induction was determined based on the amount of released DNA (*p < 0.05). (C) Cells were stimulated with or without 250 μM MNU and/or 50 ng/mL of IL-1 receptor antagonist (IL-1ra) for 48 h, and the degree of apoptotic induction was determined as above (*p < 0.05). (D) AGS cells were stimulated with or without 250 μM MNU and/or 50 ng/mL IL-1α for 48 h, and the degree of apoptotic induction was determined as above (*p < 0.05). (E) AGS cells were treated with or without IL-1α, and cell numbers were determined at the indicated times (*p < 0.05).
To determine whether these effects were autocrine and paracrine, we pre-treated cells with an IL-1 receptor antagonist (IL-1ra), followed by MNU stimulation. IL-1ra treatment increased the degree of apoptotic induction compared to untreated cells (Figure 5C). These results suggest that IL-1α acts as an anti-apoptotic agent in SH101 cells via autocrine and paracrine signaling.
To confirm our results, we examined the effect of IL-1α pre-treatment on MNU-induced apoptosis in a second gastric cancer cell line (AGS) that does not express IL-1α in the resting condition. IL-1α pre-treatment decreased MNU-induced apoptosis (Figure 5D), supporting the notion that IL-1α plays a role in anti-apoptotic signaling.
Next, to investigate the effect of IL-1α on cell proliferation, we stimulated AGS cells with IL-1α and evaluated cell growth. IL-1α treatment increased cell numbers in a time-dependent fashion compared to untreated controls (Figure 5E).
IL-1R deficiency inhibits MNU-induced gastric tumor development
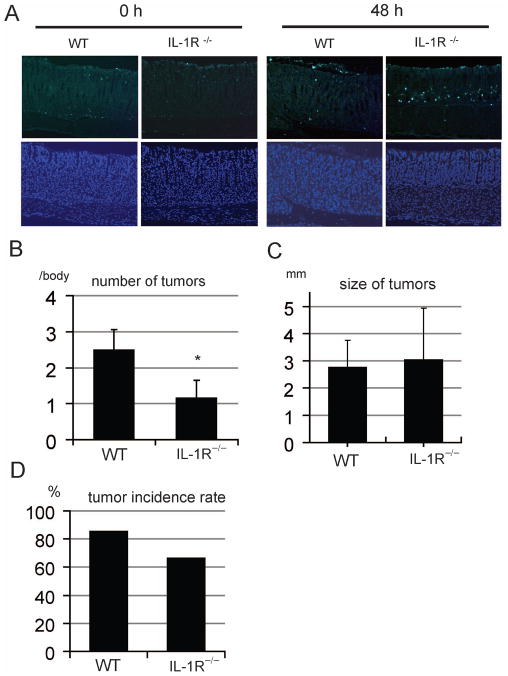
To investigate the anti-apoptotic function of IL-1α in vivo, wild-type (WT) and IL-1 receptor null mice (Il-1r−/−) were administered an oral dose of MNU, and apoptosis was analyzed using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay. As in the case of IkkβΔST mice, MNU induced a greater degree of gastric apoptosis in Il-1r−/− mice than in WT mice (Figure 6A and Supplementary figure 5).
Figure 6.
IL-1R knockout inhibits MNU-induced gastric tumor development.
(A) MNU (1000 ppm) was administered orally to wild-type mice (WT) and IL-1 receptor knockout mice (IL-1R−/−). After 4 h, the stomachs were removed and apoptotic levels were determined via the TUNEL assay (upper panel; ×100). Lower panels show counter-staining with DAPI (×100). (B) The numbers of tumors in WT and IL-1R−/− stomachs are shown. The stomachs were observed macroscopically, and tumors > 1 mm of diameter were counted. Values represent the means ± SE in WT (n = 14) and IL-1R−/− (n = 6) mice (*p < 0.05). (C) WT and IL-1R−/− stomach tumor sizes are shown. The stomachs were observed macroscopically, and tumors > 1 mm in diameter were measured. Values represent the means ± SE in WT (n = 31) and IL-1R−/− (n = 7) mice (*p < 0.05). (D) The tumor incidence rate in each group is shown.
Finally, to explore the role of IL-1 in gastric cancer development, we subjected Il-1r−/− mice to MNU-induced gastric carcinogenesis. The number of detectable gastric tumors decreased significantly in Il-1r−/− mice compared to WT controls (Figure 6B and C). In contrast, the mean sizes of tumors were similar in WT and Il-1r−/− mice (Figure 6D). Among the MNU-treated control mice, 82% developed gastric tumors, whereas only 67% of Il-1r−/− mice developed tumors (p < 0.05; Figure 6D). These data suggest that IL-1 signaling, as well as IKKβ, is required for efficient gastric tumor induction in response to MNU administration.
Discussion
In this study, we showed that IKKβ is oncogenic in the gastric epithelium. This result is consistent with the CAC model, in which the deletion of IKKβ in intestinal epithelial cells led to an increase in epithelial apoptosis and a dramatic decrease in tumor incidence, without affecting tumor size (6). In our model, MNU treatment caused more severe apoptosis in the stomachs of IkkβΔST mice than in IkkβF/F mice, as in the CAC model. We also showed that the expression of several anti-apoptotic genes regulated by NF-κB, including cIAP2 and A20, decreased in the stomachs of IkkβΔST mice compared to IkkβF/F mice. Alkylating agents, such as MNU, induce rapid p53-dependent apoptosis in gastric epithelial cells (18). However, increased apoptosis in IkkβΔST mice appeared to be independent of p53 activation, as p53 activation was unchanged in the epithelia of both IkkβF/F and IkkβΔST mice (data not shown). Another possible mechanism for the observed increase in apoptosis in IKKβ-deficient epithelial cells is enhanced c-Jun N-terminal kinase (JNK) activation (19, 20). However, the lack of change in JNK activation in the two genotypes suggests that enhanced JNK activation is not responsible for increased epithelial apoptosis in response to MNU treatment (Supplemental Figure 6).
On the other hand, liver-specific IKKβ knockout mice treated with the chemical carcinogen diethylnitrosamine (DEN) developed hepatocellular carcinoma (HCC) to a more severe degree than did WT mice (8). In these mice, excessive HCC development was linked to the increased compensatory proliferation of hepatocytes, which is a consequence of elevated hepatocyte death in the absence of IKKβ. Based on PCNA staining, MNU treatment did not affect the proliferative response in IkkβF/F vs. IkkβΔST mice, suggesting that MNU-induced apoptosis does not stimulate a proliferative response in gastric epithelial cells (Supplemental Figure 3). One possible explanation for this discrepancy between our study and that of Maeda et al. (8) is that the turnover of gastric epithelial cells is very rapid compared to that of hepatocytes. Numerous PCNA-positive nuclei are observed in gastric epithelia, even without stimulation, whereas normal livers exhibit very few PCNA-positive hepatocytes and hepatocytes rapidly begin to proliferate after liver injury.
In this study, we identified IL-1α as a key downstream molecule in gastric cancer development. However, other major inflammatory cytokines regulated by NF-κB, including IL-1β, TNFα, and IL-6, did not appear to be involved in this model, as we did not observe the expression of these cytokines after MNU treatment. IL-1 signaling pathways are reported to play an important role in gastric ulcer and cancer development. For example, IL-1 is reported to potently inhibit gastric acid secretion and ulcer formation (21). Our results also suggest that IL-l acts as an autocrine stimulator of human gastric cancer cells as reported (22). IL-1 polymorphisms have also been associated with an increased risk of gastric cancer (23). In addition to gastric cancer, IL-1α expression is induced by the potent carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and is associated with skin carcinogenesis in mice (24). In this study, we have provided in vivo evidence that IL-1α is produced by gastric epithelial cells and our data support the idea that IL-1 signaling is important in gastric cancer development.
Recently, hepatocyte-specific IKKβ ablation was reported to enhance liver damage with the release of IL-1α, whereas the inhibition of IL-1α action or the ablation of its receptor inhibited carcinogen-induced compensatory proliferation and liver tumorigenesis (25). In this case, IL-1α from necrotic cells functions as a damage-associated molecular pattern (DAMP) molecule. In contrast, in our study, IL-1α gene expression was higher in IkkβF/F than in IkkβΔST mice, indicating that the mechanism of enhanced IL-1α protein expression in gastric and liver tumorigenesis may differ.
We have shown that IL-1α plays roles in tumor growth and anti-apoptotic signaling, which are the hallmarks of cancer (26). IL-1α activates several signaling pathways, including those of NF-κB, JNK, p38, and extracellular signal-regulated kinase (ERK), and may be involved in growth and anti-apoptotic signaling (27). Recently, Tu et al. (27) reported that the stomach-specific expression of human IL-1β in transgenic mice led to spontaneous gastric inflammation and cancer. They also suggested that the phenotype is correlated with the early recruitment of myeloid-derived suppressor cells to the stomach, but they did not analyze the function of IL-1β in tumor cell growth or anti-apoptotic signaling (28). Here, we analyzed the recruitment of myeloid cells after MNU injection and found that very few inflammatory cells accumulated in either IkkβF/F or IkkβΔST mice (data not shown).
H. pylori infection is the most important etiological agent of gastric cancer. We have previously shown that H. pylori activates IKKβ/NF-κB signaling both in vitro and in vivo and may be critical for gastric inflammation, ulcer formation, and cancer development (29–31). IL-1α expression has also been reported in gastric epithelia infected with H. pylori (32). These observations suggest that the IKKβ/NF-κB–IL1α axis in gastric epithelial cells is a critical pathway for gastric carcinogenesis. However, H. pylori infection in conventional mice, such as C57/BL6, caused such slight inflammation that it is impossible to evaluate the relationship between inflammation and gastric carcinogenesis.
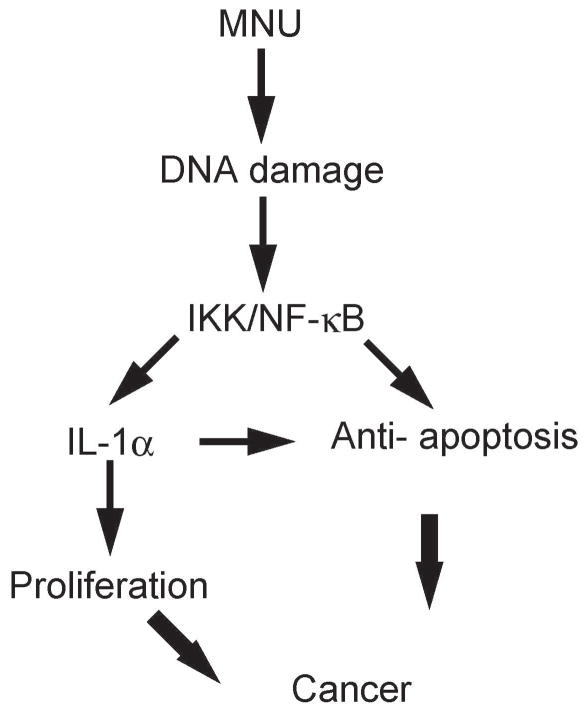
In conclusion, we established a conditional IKKβ gastric mucosal epithelium knockout mouse and used it in a MNU-mediated gastric cancer model. Mice lacking IKKβ in the gastric epithelium exhibited a marked decrease in gastric carcinogenesis relative to the controls. Moreover, impaired tumor development was correlated with the decreased expression of IL-1α signaling, which is associated with anti-apoptotic signaling and cell proliferation (Figure 7). Thus, IKKβ/IL-1α may be an attractive target for the prevention and treatment of gastric cancer.
Figure 7.
Proposed model of gastric carcinogenesis in MNU-treated mice.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, see: http://www.textcheck.com/certificate/sxLM4j
Materials and Methods
Mice
This study was approved by the Ethics Committee of the Institute for Adult Diseases, Asahi Life Foundation, and animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the same institution. Ikkβ F/F mice (14), which are aphenotypic, were crossed with Foxa3-Cre transgenic mice (15) to eventually generate IkkβF/F:Foxa3-Cre (referred to as IkkβST) mice. All mice were maintained under pathogen-free conditions and a 12/12-h light/dark cycle.
Gastric cancer model
N-methyl-N-nitrosourea (MNU; Sigma Chemical) was dissolved in distilled water at a concentration of 240 ppm and was freshly prepared twice a week for administration in drinking water in light-shielded bottles. Six-week-old wild-type and Jnk1−/− mice were given drinking water containing 240 ppm MNU on alternate weeks for a total of 10 weeks of exposure, according to a protocol described in a previous report (33). The MNU-treated mice were sacrificed 40 weeks after MNU administration began.
Preparation of tissue samples for tumor counting and histological analysis
Each mouse stomach was removed and opened along the greater curvature. The number and long diameter of tumors in the stomach were measured under a dissecting microscope (5× magnification). Tumors > 0.5 mm in diameter were mapped and counted. After counting, all of the excised stomachs were fixed for 24 h in neutral-buffered 10% formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E).
Immunohistochemistry and TUNEL assay
Sections were deparaffinized, rehydrated, treated with 3% H2O2 in phosphate-buffered saline (PBS), and incubated overnight at 4°C with anti-p65 and anti-PCNA (Santa Cruz Biotechnology) antibodies. The binding of the primary antibody was detected using biotin-labeled anti-rabbit IgG or anti-rat IgG antibodies (1:500 dilution; Vector Laboratories), followed by a streptavidin-horseradish peroxidase (HRP) reaction, visualization using 3,3′-diaminobenzidine (Sigma-Aldrich), and counterstaining with hematoxylin. No specific staining was observed in negative control slides prepared without the primary antibody. To evaluate epithelial cell apoptosis, the TUNEL assay was performed according to the manufacturer’s instructions (ApopAlert DNA Fragmentation Assay Kit, TAKARA Bio Inc.).
Cells and MNU treatment
The human gastric cancer cell lines AGS and SH101 were maintained in RPMI-1640 or Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), L-glutamine, 100 units penicillin G, and 100 μg/mL streptomycin. For transformation analysis, cells were exposed to MNU (100 μg/mL) for 1 h, once a week for up to 6 weeks. Cell numbers were determined using a cell counting kit, according to the manufacturer’s protocol (Dojindo Laboratories).
Immunoblotting and cytokine array
Culture supernatants were assayed using a Human Chemokine Antibody Array according to the manufacturer’s instructions (RayBiotech, Inc.). Protein lysates were prepared from cultured mouse embryonic fibroblasts (MEFs), separated by SDS-PAGE, transferred to polyvinylidene difluoride membrane (Millipore), and analyzed by immunoblotting with anti-IKKβ, anti-JNK1, anti-phospho-JNK, and anti-tubulin antibodies (Cell Signaling).
Quantitative real-time RT-PCR
Gastric tissues from MNU-treated mice were examined for mRNA expression using quantitative real-time RT-PCR. RNA was extracted using ISOGEN reagents (Nippon Gene), according to the manufacturer’s protocol. First-strand cDNA was generated using SuperScript II (Invitrogen Life Technologies) and mRNA expression was measured via real-time RT-PCR, using GAPDH mRNA for normalization. Primer sequences are available upon request.
Statistical analysis
Data are expressed as the means ± standard error (SE). Differences were analyzed using Student’s t-test. Values of P ≤ 0.05 were deemed statistically significant.
Supplementary Material
Acknowledgments
This study was supported by Grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (nos. 17209026 and 19390205). We thank Drs. Michael Karin for providing IkkβF/F.
Abbreviations used in this paper
- ERK
extracellular signal-regulated kinase
- IKK
IkappaB kinase
- IL
interleukin
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MNU
N-methyl-N-nitrosourea
- NF-κB
nuclear factor kappa B
- TNF
tumor necrosis factor
References
- 1.Maeda S, Omata M. Inflammation and cancer: Role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2 :301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 5.Li Z-W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for NF-κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 8.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121 :977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Dajee M, Lazarov M, Zhang JY, Cai T, Green CL, Russell AJ, Marinkovich MP, Tao S, Lin Q, Kubo Y, et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature. 2003;421:639–643. doi: 10.1038/nature01283. [DOI] [PubMed] [Google Scholar]
- 10.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10 :75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 11.Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, Boeing H, Del Giudice G, Palli D, Saieva C, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–1501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 12.Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 13.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 14.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170:4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 15.Lee CS, Sund NJ, Behr R, Herrera PL, Kaestner KH. Foxa2 is required for the differentiation of pancreatic alpha-cells. Dev Biol. 2005;278:484–495. doi: 10.1016/j.ydbio.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Tatematsu M, Yamamoto M, Iwata H, Fukami H, Yuasa H, Tezuka N, Masui T, Nakanishi H. Induction of glandular stomach cancers in C3H mice treated with N-methyl-N-nitrosourea in the drinking water. Jpn J Cancer Res. 1993;84:1258–1264. doi: 10.1111/j.1349-7006.1993.tb02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habraken Y, Piette J. NF-kappaB activation by double-strand breaks. Biochem Pharmacol. 2006;72:1132–1141. doi: 10.1016/j.bcp.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Shibata W, Maeda S, Hikiba Y, Yanai A, Sakamoto K, Nakagawa H, Ogura K, Karin M, Omata M. c-Jun NH2-terminal kinase 1 is a critical regulator for the development of gastric cancer in mice. Cancer Res. 2008;68:5031–5039. doi: 10.1158/0008-5472.CAN-07-6332. [DOI] [PubMed] [Google Scholar]
- 19.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 20.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120 :649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Tache Y, Saperas E. Potent inhibition of gastric acid secretion and ulcer formation by centrally and peripherally administered interleukin-1. Ann N Y Acad Sci. 1992;664:353–368. doi: 10.1111/j.1749-6632.1992.tb39774.x. [DOI] [PubMed] [Google Scholar]
- 22.Ito R, Kitadai Y, Kyo E, Yokozaki H, Yasui W, Yamashita U, Nikai H, Tahara E. Interleukin 1 alpha acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 1993;53:4102–4106. [PubMed] [Google Scholar]
- 23.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Eckard J, Shah R, Malluck C, Frenkel K. Interleukin-1alpha up-regulation in vivo by a potent carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) and control of DMBA-induced inflammatory responses. Cancer Res. 2002;62:417–423. [PubMed] [Google Scholar]
- 25.Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill L. The Toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–563. doi: 10.1042/bst0280557. [DOI] [PubMed] [Google Scholar]
- 28.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14 :408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda S, Yoshida H, Ogura K, Mitsuno Y, Hirata Y, Yamaji Y, Akanuma M, Shiratori Y, Omata M. H. pylori activates NF-kappaB through a signaling pathway involving IkappaB kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology. 2000;119:97–108. doi: 10.1053/gast.2000.8540. [DOI] [PubMed] [Google Scholar]
- 30.Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanai A, Maeda S, Shibata W, Hikiba Y, Sakamoto K, Nakagawa H, Ohmae T, Hirata Y, Ogura K, Muto S, Itai A, Omata M. Activation of IkappaB kinase and NF-kappaB is essential for Helicobacter pylori-induced chronic gastritis in Mongolian gerbils. Infect Immun. 2008;76:781–787. doi: 10.1128/IAI.01046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maekawa T, Kinoshita Y, Matsushima Y, Okada A, Fukui H, Waki S, Kishi K, Kawanami C, Nakata H, Hassan S, Wakatsuki Y, Ota H, Amano K, Nakao M, Chiba T. Helicobacter pylori induces proinflammatory cytokines and major histocompatibility complex class II antigen in mouse gastric epithelial cells. J Lab Clin Med. 1997;130:442–449. doi: 10.1016/s0022-2143(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 33.Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Shimizu N, Kobayashi K, Fukushima S, Tatematsu M. N-methyl-N-nitrosourea concentration-dependent, rather than total intake-dependent, induction of adenocarcinomas in the glandular stomach of BALB/c mice. Jpn J Cancer Res. 1998;89:385–391. doi: 10.1111/j.1349-7006.1998.tb00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.