Abstract
Background. Central nervous system (CNS) human immunodeficiency virus (HIV) infection and immune activation lead to brain injury and neurological impairment. Although HIV enters the nervous system soon after transmission, the magnitude of infection and immunoactivation within the CNS during primary HIV infection (PHI) has not been characterized.
Methods. This cross-sectional study analyzed cerebrospinal fluid (CSF) and blood from 96 participants with PHI and compared them with samples from neuroasymptomatic participants with chronic infection and ≥200 or <200 blood CD4 T cells/μL, and with samples from HIV-seronegative participants with respect to CSF and plasma HIV RNA, CSF to serum albumin ratio, and CSF white blood cell counts (WBC), neopterin levels, and concentrations of chemokines CXCL10 and CCL2.
Results. The PHI participants (median 77 days post transmission) had CSF HIV RNA, WBC, neopterin, and CXCL10 concentrations similar to the chronic infection participants but uniquely high albumin ratios. 18 participants had ≤100 copies/mL CSF HIV RNA, which was associated with low CSF to plasma HIV ratios and levels of CSF inflammation lower than in other PHI participants but higher than in HIV-seronegative controls.
Conclusions. Prominent CNS infection and immune activation is evident during the first months after HIV transmission, though a proportion of PHI patients demonstrate relatively reduced CSF HIV RNA and inflammation during this early period.
Long-term chronic exposure of the central nervous system (CNS) to human immunodeficiency virus (HIV) leads to HIV-associated dementia in 20%–30% of untreated patients, both historically and currently in populations with limited to access to combination antiretroviral therapy (cART) [1, 2]. Less-severe cognitive impairment, which nonetheless may impair quality of life and productive function, is frequently detected in patients on cART, suggesting earlier or ongoing less-severe injury [3, 4]. CNS HIV infection is also associated with a compartmentalized tissue reservoir that allows independent viral replication and selection that may have implications for systemic HIV disease and response to therapy [5]. These CNS effects of HIV have been previously considered significant only after many years of infection, paralleling the slow immunological decline that characterizes systemic disease. However, during the weeks after initial infection, HIV may be detected in both cerebrospinal fluid (CSF) and brain tissue [6–9], and overt neurological disorders manifest in some patients. Moreover, evidence that the intestinal immune repository is permanently depleted within 6 weeks after viral transmission [10, 11] raises the question of whether early “silent” events in the CNS may have similar implications for longer-term neurological integrity.
Systemic primary HIV infection (PHI) is characterized by an early and dramatic peak of HIV RNA in the plasma [12, 13], which is associated with clinical symptoms in at least two-thirds of individuals [14, 15]. A smaller subset of patients develop neurological disorders around seroconversion, including meningitis, encephalopathy, myelopathy, acute mononeuropathy, and polyradiculopathy [16–23]. However, the importance of this early neuroinvasion and related local immune responses for the natural history of CNS infection, establishment of the CNS HIV reservoir, and subsequent development of HIV-related neurological injury has not been systematically studied.
We sought to characterize early events in CNS infection and investigate the hypothesis that very early CNS exposure to HIV may start processes that eventually lead to neurological compromise. In a group of predominantly neuroasymptomatic individuals with PHI, we measured biomarkers associated with CNS inflammation and the development of cognitive and motor dysfunction in later stages of disease. These included: blood and CSF HIV RNA levels; CSF white blood cell (WBC) count and CXCL10 (interferon gamma inducible protein [IP-10]), the principal chemokine associated with CSF lymphocytic pleocytosis, as indices of inflammation; CSF neopterin and CCL2 (monocyte chemoattractant protein-1 [MCP-1]) as measures of CNS macrophage activation and chemotaxis; and CSF to serum albumin ratio as an indicator of blood–brain barrier disruption.
METHODS
Study Design and Participants
Paired plasma and CSF samples were obtained in the context of observational studies in Gothenburg, Sweden; Milan, Italy; Sydney, Australia; and San Francisco, United States. PHI participants (n = 96) were within the first 12 months after HIV-1 transmission as confirmed by a combination of seroconversion, nucleic acid testing, or less-sensitive enzyme-linked immunosorbent assay (ELISA) testing according to the standard serologic testing algorithm for recent HIV seroconversion (STAHRS) methods [24]. We estimated days post–HIV transmission assuming exposure 14 days prior to onset of seroconversion symptoms [25], or, in the absence of symptoms (12 participants), we designated exposure date as the date halfway between the last negative and first positive HIV test [26]. Comparison samples were obtained from HIV-uninfected volunteers (n = 54) and asymptomatic participants with chronic (>3 years duration) HIV-1 infection, who were divided into those with CD4 counts ≥ 200 cells/μL (n = 178) and those with < 200 cells/μL (n = 116), all of whom were either cART naive or off of therapy for ≥3 months. The Institutional Review Board or equivalent body at each institution approved the protocols, and informed consent was obtained from all participants. A portion of the CSF HIV RNA data was previously reported in 2 publications [9, 27].
Specimen Sampling, Processing, and Laboratory Studies
CSF, blood, and general medical and neurological assessments were obtained as previously described [28, 29]. CSF total WBC, protein, albumin, blood albumin, and CD4+ and CD8+ T lymphocyte counts by flow cytometry were measured at each local laboratory on fresh samples. Cell-free CSF and blood plasma were also aliquoted and stored within 6 hours of collection in −70°C freezers monitored for temperature daily using National Institutes of Standards and Technology (NIST)–certified thermometers. Concentrations of CSF and plasma neopterin (BRAHMS Aktiengesellschaft) and CSF CXCL10 and CCL2 (R&D Systems) were measured in previously frozen samples locally or in the laboratory of Dr Fuchs by commercial immunoassays.
Virological Methods
We measured HIV RNA levels in previously frozen cell-free CSF and plasma using the ultrasensitive (50 copies/mL lower limit of detection) Amplicor HIV Monitor (version 1.5; Roche Molecular Diagnostic Systems), Cobas TaqMan RealTime HIV-1 (version 1 or 2; Hoffmann-La Roche), or the Abbott RealTime HIV-1 (Abbot Laboratories) assays. Paired blood and CSF measurements were made using the same assay, typically in the same polymerase chain reaction (PCR) run. A portion of samples with results <50 copies/mL were retested using an ultra-ultrasensitive modification of the Amplicor assay with a lower detection limit of 2.5 copies/mL [30]. CSF to plasma ratio was calculated as (log10 CSF HIV RNA – log10 plasma HIV RNA).
Statistical Analysis
Descriptive statistics were performed using Stata/SE 11.0 (StataCorp LP). The Spearman nonparametric correlation was used for correlation between measured parameters, and simple linear regression was used to assess laboratory measurements according to days after HIV transmission. Group differences were detected through the Mann–Whitney rank sum test and the Kruskal–Wallis test with post hoc testing corrected for multiple comparisons. A multivariate regression model to investigate predictors of CSF HIV RNA included plasma HIV RNA level, CSF WBC count, albumin ratio, CD4 T lymphocyte count, and estimated days post-HIV transmission.
RESULTS
Study Participant Characteristics
HIV-infected PHI participants were studied at a median 77 days (interquartile range [IQR], 45–143 d) after estimated viral transmission (enrollment by site: Gothenburg, 22; Milan, 17; Sydney, 6; and San Francisco, 51). Background characteristics of study participants from all groups are presented in Table 1. Completely asymptomatic seroconversion was present in 12 participants. Neurological symptoms developed during PHI in 17 participants (though not in most at the time of sampling): encephalitis in 3, clinical meningitis in 2, lower motor neuron facial palsy in 2, distal paresthesias in 4, photophobia with intense headache in 4, acute painful polyradiculopathy in 1, and brachial neuritis in 1 participant. PHI participants were presumed to be infected with clade B viruses except those enrolled in Gothenburg who were known to be of more diverse geographic origin. Of 22 participants from Gothenburg, 16 had clade information available; of these, 8 harbored subtype B, 1 subtype A, 1 subtype A/D, 4 subtype C viruses, and 2 subtype CRF01_AE.
Table 1.
Background Demographic and Clinical Characteristics of Study Participants
| HIV uninfected | Primary infection | Chronic HIV CD4 ≥200 | Chronic HIV CD4 <200 | |
| Number in group | 54 | 96 | 178 | 116 |
| Sex, %male | 81% | 89.5% | 78% | 85% |
| Age, y | 44.5a | 36.0 | 36.0 | 37.5 |
| (40–50) | (30–46) | (30–46) | (32–45) | |
| CD4+ T Cells (cells/μL) | 836b | 575b | 421b | 66b |
| (674–1013) | (412–730) | (300–536) | (28–135) | |
| CD8+ T Cells (cells/μL) | 464c | 994d | 1051c | 597c |
| (329–634) | (692–1426) | (735–1357) | (395–805) |
NOTE. Values shown are medians (interquartile ranges) except where noted. Significant differences between groups corrected for post hoc analysis (P < .013) are noted:
Significantly different from all other groups.
Each group comparison significant.
Different from primary infection and chronic HIV CD4 ≥ 200 groups.
Different from HIV uninfected and chronic HIV <200 groups.
Participants with chronic infection were neuroasymptomatic and either cART naive or last treated >3 months earlier. Only 1 PHI participant received 10 days of cART that concluded 4.5 months prior, and another completed a 4-day course of zidovudine/lamivudine for postexposure prophylaxis 36 days before the visit. The groups were similar with respect to proportion of women, and the HIV-seronegative group was older than the HIV-infected groups. Despite a median duration of only approximately 2.5 months of infection, the PHI group was characterized by a significant reduction in blood CD4+ and elevation in CD8+ cells compared with the HIV-seronegative group, indicating the rapid effect on systemic T lymphocytes during this early period of infection.
HIV RNA Levels in CSF Relative to Plasma
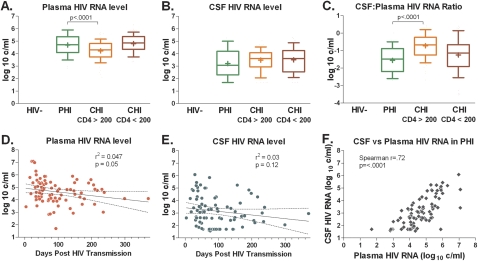
Individuals with PHI, as expected, had high HIV RNA levels in plasma that were similar to those in untreated participants with advanced immunosuppression (chronic HIV CD4 < 200), and higher compared with levels of the group of patients with chronic HIV CD4 and ≥200 (P < .0001, Figure 1A). Despite their high plasma HIV RNA levels, participants with PHI had median CSF HIV RNA levels that were similar to those of the other groups (with a trend for lowest levels in PHI: 3.07 log10 copies/mL, IQR, 2.31–4.18 log10 copies/mL in PHI participants; 3.52 log10 copies/mL, IQR, 2.76–4.06 log10 copies/mL in patients with chronic HIV and CD4 counts ≥200; and 3.62 log10 copies/mL, IQR, 2.55–4.24 log10 copies/mL in patients with chronic HIV and CD4 counts <200; P = .064 for overall comparison, Figure 1B). Thus, the median CSF to plasma RNA ratio in PHI patients (–1.48 log10) was at least three-fold lower than the ratios measured in either chronic HIV group (−0.64 log10 and −1.14 log10, Figure 1C).This low CSF relative to plasma HIV burden during PHI indicates a clear distinction between the 2 compartments during this early period, as well as a fundamental difference in the relationship between the CSF and blood compartments during PHI compared with chronic infection, when CSF levels are typically 1 log reduced relative to plasma levels.
Figure 1.
Human immunodeficiency virus (HIV) RNA levels in the blood and cerebrospinal fluid (CSF) compartments and their relationship in each HIV-infected group (A–C); simple linear regression between HIV RNA levels in each compartment and number of days posttransmission (D, E); and univariate Spearman correlation between CSF and plasma HIV RNA levels in primary HIV infection (PHI) participants (F). Upper plots indicate median (middle line), interquartile range (extent of boxes), and 10–90% range (whiskers). Means are denoted by ‘+.’ In D and E regression lines (solid) and 95% confidence intervals (dotted) are indicated. Significant differences between PHI participants and other groups are shown.
Determinants of CSF Viral Burden During PHI
Although plasma HIV RNA was found in higher levels during the earliest period of infection as expected (P = .05, Figure 1D), CSF HIV RNA did not show a clear association with time from HIV exposure (P = .12, Figure 1E). In the PHI group, plasma and CSF HIV RNA showed a close association (P < .0001, Figure 1F) to an extent similar to that seen in chronic infection (not shown) but reflecting lower CSF relative to plasma levels. Plasma HIV RNA level was greater than CSF level in 95 of 96 participants; 1 participant had equivalent levels, with undetectable HIV RNA level in both compartments. Our multivariate linear regression model to identify independent predictors of CSF viral burden in PHI participants revealed that CSF HIV RNA independently correlated with plasma HIV RNA (increasing by 0.64 log10 for each 1 log10 increase in plasma, P < .0001), but not significantly with CSF WBC count, albumin ratio, days posttransmission, or blood CD4 count.
Inflammatory and Blood Brain Barrier Markers in PHI
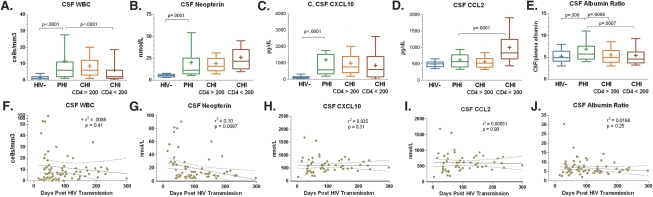
Median levels of CSF WBC (6 cells/mm3 [IQR, 2–11 cells/mm3] vs 1 cell/mm3, IQR, 0–2 cells/mm3; P < 0.0001), neopterin (10.6 nmol/L [IQR, 7.0–24.5 nmol/L] vs 5.2 nmol/L [IQR, 3.8–6.5]; P < .0001), CXCL10 (560 pg/dL [IQR, 330–1503 pg/dL] vs 98 pg/dL [IQR, 52–143 pg/dL]; P < .0001), and albumin ratio (5.74 [IQR, 4.64–7.50] vs 4.91 [IQR, 4.12–6.27]; P = .009) in the PHI participants were elevated as compared with HIV-seronegative controls (Figure 2A–C, E). CSF CCL2 was not significantly elevated in PHI compared with HIV-seronegative controls (588 pg/dL [IQR, 423–786 pg/dL] vs 494 pg/dL [IQR, 411–552 pg/dL] P = .08, Figure 2D). Levels of these markers in PHI participants were similar to those detected in chronic HIV patients with CD4 counts >200, except for albumin ratio, which was elevated compared with that found in both groups with chronic infection (P < .001 for each), and plasma neopterin, which was higher in PHI than in HIV-seronegative and chronic HIV (CD4 >200) (P < .02 for each, data not shown). Only CSF neopterin changed significantly (P = .01) in correlation with days post–HIV transmission in the PHI group, with higher levels in participants sampled in the earliest stages (Figure 2F–J).
Figure 2.
Comparison of levels of immune and inflammatory markers within the cerebrospinal fluis (CSF) in 4 groups of participants: HIV-uninfected (HIV-), primary HIV infection (PHI), chronic infection with CD4 counts ≥200 cells/μL (CHI CD4 ≥200), and chronic infection with CD4 counts <200 cells/μL (CHI CD4 <200). Upper plots indicate median (middle line), interquartile range (extent of boxes), and 10%–90% range (whiskers). Means are denoted with the ‘+.’ Significant differences between PHI participants and other groups are shown. On lower plots, simple linear regression between each CSF marker and days post transmission of sampling are shown with regression lines (solid) and 95% confidence intervals (dotted).
Minimal CSF HIV RNA Group
Eighteen PHI participants (18.8%) had markedly low (<100 copies/mL) HIV RNA levels in the CSF (Figure 3A); 14 of these 18 had undetectable levels by a standard ultrasensitive assay with a lower limit of detection of 50 copies/mL. When measurements were repeated with the ultra-ultrasensitive assay in 6 of these samples with <50 copies/mL (based on availability of specimens), HIV RNA was detected in all specimens (range, 3.7–46.5 copies/mL). The CSF HIV RNA ≤ 100 copies/mL, or “minimal CSF HIV RNA,” group was similar in age and days post HIV transmission in comparison with the PHI participants with CSF HIV RNA >100 copies/mL, but had higher median blood CD4 counts (727.5 cells/ul vs 527.5 cell/ul, P = .0001), and was 100% male. 1 unique participant with minimal CSF HIV RNA had undetectable levels of HIV RNA in plasma as well as CSF, and in follow-up proved to be a systemic “elite” or “HIV controller” (maintaining undetectable viral loads in the absence of therapy). Minimal CSF HIV RNA participants had lower plasma HIV RNA levels than participants with CSF HIV RNA >100 copies/mL (3.93 log10 copies/mL [IQR, 2.98–4.26 log10 copies/mL] versus 4.86 log10 copies/mL [IQR, 4.26–5.41 log10 copies/mL]; P = .0001). However, a lower ratio of CSF relative to plasma HIV RNA ratio in minimal CSF HIV RNA participants (−2.24 log10 copies/mL [IQR, 1.29–2.40 log10 copies/mL]) compared with PHI participants with CSF HIV RNA >100 copies/mL (−1.40 log10 copies/mL [IQR, 0.84–2.08 log10 copies/mL]; P = .007 f) suggests that the reduced CSF viral burden in this group is not fully accounted for by reduced plasma HIV RNA levels (Figure 3B and C).
Figure 3.
Comparisons between laboratory parameters in primary human immunodeficiency virus (HIV) infection (PHI) participants with minimal (≤100 copies/mL) cerebrospinal fluid (CSF) HIV RNA (denoted CSF VL≤ 100) and other PHI participants (denoted CSF VL >100). On scatter plots, bars indicate medians. Nonparametric statistical comparisons are shown.
The minimal CSF HIV RNA group had significantly lower median levels of CSF WBC, CSF neopterin, and CSF CXCL10 than those of PHI participants with HIV RNA >100 copies/mL (Figure 3D–F), whereas the albumin ratios and CCL2 levels did not differ between these groups (not shown). However, despite similar CD4 counts, the minimal CSF HIV RNA group had elevated CSF WBC (P = .0002), neopterin (P = .0025), and CXCL10 (P = .008) compared with the HIV-seronegative group, suggesting that immune activation in the CNS may be present even with an extremely low copy number of HIV RNA in the CSF in PHI participants.
DISCUSSION
Early entry of HIV into the CNS may be a seminal event, leading to rapid induction of neuroimmune activation that in later-stage disease is associated with neuronal injury and cognitive impairment. We have investigated the virological and inflammatory correlates of predominantly asymptomatic CNS infection during PHI through a systematic evaluation of HIV RNA levels and biomarkers of immune activation and blood–brain barrier disruption in the CSF. Whereas in some regards the PHI participants resemble those with more chronic infection and relative CD4+ T cell preservation, in certain aspects they also differ, indicating both a rapid onset and ongoing evolutionary change during this early period of infection. Our findings indicate that PHI is characterized by a higher ratio between plasma and CSF HIV RNA levels, suggesting a differential distribution of infection between these compartments during this early stage. Despite a relatively low CSF viral burden in relation to plasma, PHI is characterized by blood–brain barrier disruption as well as levels of CNS inflammation equal to those found in chronic established infection.
In contrast to earlier assumptions that HIV was primarily latent in early infection with only gradual early effects on the immune system that were marked by blood CD4+ T-cell levels, recent findings have changed the focus to the first days and months of infection as having major impact on the immune system and viral pathogenesis [11]. Thus, the rate of CD4 T-cell decline is greatest during PHI, critically impairing subsequent host immune control of HIV; levels of plasma HIV RNA, early CD4 nadir, and systemic T-cell activation measured during PHI have been found to be robust predictors of the course of immunological decline and disease progression in HIV [31–34]. Small-scale human studies have previously suggested that PHI importantly also “sets the stage” for subsequent CNS impairment and is associated with CNS entry of HIV. In a retrospective study, participants with any systemic or neurological symptoms during seroconversion had a more rapid progression of neurocognitive impairment than those without symptoms [35], which suggests that systemic factors associated with more pronounced clinical disease during acute infection may be drivers of neurological injury. Detection of HIV in CSF during PHI was documented very early in the HIV epidemic in isolated individuals [36, 37], HIV was identified in brain tissue in an iatrogenic case of acute infection [6], and subsequent studies have demonstrated varying levels of HIV RNA in the CSF in PHI participants [7–9, 27]. The concept that CNS inflammation and injury might be started during PHI has been suggested by the finding of elevated CSF neopterin and beta-2-microglobulin in 3 patients (two without neurological symptoms) during acute infection [38], and the detection of elevated neurofilament protein light chain (NFL), a marker of axonal injury, in some CSF samples (also included in this study) obtained from participants during PHI [27].
Despite the fact that our participant pool was largely neuroasymptomatic, most our PHI participants had detectable CSF HIV RNA levels, similar to those found in participants with chronic HIV. Importantly, we also detected evidence of intrathecal inflammation as indicated by elevations in total CSF WBCs, CXCL10, the major chemokine associated with CSF lymphocytosis, and neopterin, an indicator of intrathecal macrophage activation [39, 40]. What was most surprising in our findings was that the extent of these abnormalities in cellular immune responses at a very early stage of HIV infection, approximately 2.5 months after exposure, is similar to that measured in participants with established chronic infection. Furthermore, CNS inflammation does not appear to be a transient effect evident only in the earliest stages of infection, but instead is detected in participants throughout the first year of infection. The role of a proinflammatory immune milieu in initiation and progression of neuronal dysfunction in HIV infection is well established, though the exact mechanisms underlying these processes are still only partially understood (see [41, 42] for reviews). During late-stage chronic infection, elevations of CSF neopterin [39, 43] and CSF CXCL10 [40] levels are associated with the presence of HIV-associated neuronal injury and dementia. In the asymptomatic chronic stage, HIV-associated CSF pleocytosis presumably reflects a substrate for HIV replication within the CNS, and correlates with levels of CSF CXCL10 [44]. During chronic infection, degree of CSF pleocytosis and percentage of activated CD8 T lymphocytes within the CSF modulates the relationship between plasma and CSF HIV [28, 45, 46] such that higher levels of CSF immune activation markers are associated with a higher CSF to plasma HIV ratio.
In PHI participants, we found a low CSF to plasma HIV ratio despite local immune responses comparable to those of patients with chronic infection, suggesting that the inflammation witnessed during this period plays a different role in CNS HIV infection than that observed during later stages of infection. One possibility is that CNS immune activation during this early period is due primarily to a “spillover” from systemic immune activation, rather than to signals released by infected cells and replicating virus within the CNS. The low relative levels of CCL2 in the CSF compared with elevated CSF neopterin levels suggests that the activation of macrophages in the periphery rather than local CNS chemokine production drives mononuclear cell transport across the blood–CNS barriers. It is also possible that a portion of the immune activation detected in the CSF during PHI reflects a partially effective immune response that during this period reduces the CSF viral burden relative to plasma, though in a balance such that suppressed virus in some is associated with low levels of inflammation. Finally, this distinction might be explained by as yet unknown differences in the mechanisms and profiles of host immune responses between the 2 stages. For example, the differential elevation of CSF CXCL10 versus CCL2 levels during PHI may indicate a distinct role of interferon gamma in the early recruitment of activated target cells and amplification of CNS HIV infection. In any case, this early initiation of CNS immune activation is likely critical to the establishment of local CNS infection, whereby signals leading to immune cell entry feed a cascade of recruitment of infected and infection-vulnerable activated cells into the CNS. Furthermore, a modest elevation in the CSF to serum albumin ratio indicates disruption of the normal blood–brain barrier during this early period of infection. This blood–brain barrier compromise does not appear to be principally responsible for the detection of CSF HIV during PHI, however, given a relatively smaller CSF to plasma HIV RNA ratio than that in later stages of infection, lack of an independent association between albumin ratio and CSF HIV RNA, and our recent finding of CNS compartmentalization of HIV species in some of these participants [47].
Our observation of a phenomenon of strikingly low CSF HIV RNA levels in a proportion of PHI participants further highlights the distinction between CNS HIV infection during PHI compared with chronic infection. Almost 20% of participants during this early period had <100 copies/mL CSF HIV RNA levels in the setting of elevated plasma HIV RNA levels, in marked contrast with levels in participants with untreated chronic infection who have ubiquitous measurable CSF infection with HIV RNA levels typically between 1000 and 10000 copies/mL, approximately 1 log lower than plasma levels. An isolated low CSF viral burden was associated with reduced, but not absent, CNS immune responses as compared with the remaining PHI participants. Whether these extremely low CSF HIV RNA levels result from a successful targeted CNS immune response during early infection or from a relatively reduced viral neuroinvasion during acute infection is at this point unclear. Further investigation of host immune parameters, characterization of CSF-derived viruses, and sampling of individuals in the earliest stages after HIV exposure is necessary to understand determinants of the extent of CSF infection during PHI. Additionally, follow-up observation of this cohort will reveal whether an early reduced HIV RNA level in CSF has consequences for the long-term trajectory of CNS HIV.
Increasing evidence suggests that despite the ability of cART to suppress systemic and CSF HIV RNA levels to undetectable levels, a significant proportion of treated patients with chronic HIV infection display mild but distinct neurocognitive impairment [4, 48, 49]. A potential explanation for this disquieting phenomenon is that treatment started during the chronic stage of HIV infection fails to prevent or interrupt injurious CNS processes, including viral invasion, cellular trafficking, and immune activation, which are established early during infection. Our data suggest that initial seeding of the CNS leads almost universally to a process of cellular immune activation detected in the CSF. Identification of PHI as a crucial period for establishment of CNS infection and immune activation, and potentially for intervention to prevent the start of CNS injury, may provide a new rationale for cART or immunomodulatory therapy in early HIV infection.
Funding
This work was supported by the National Institutes of Health (grants R01 MH081772, R01 MH62701, R01 NS37660, R01 NS43103, K23 MH074466, P30 AI027763, P01 AI071713, and M01 RR0008336); Sahlgrenska Academy at University of Gothenburg (project ALFGBG-11067); and the Swedish Research Council (project 2007-7092. 3).
Acknowledgments
We sincerely thank the participants who volunteered for these studies. We also thank the San Francisco General Hospital (SFGH)/University of California–San Francisco (UCSF) Clinical Research Center, staff at the ARI-UCSF Laboratory of Clinical Virology, and Lauren Poole, Ed Diaz, and Lisa Harms for their invaluable help.
References
- 1.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–24. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 2.Wong MH, Robertson K, Nakasujja N, et al. Frequency of and risk factors for HIV dementia in an HIV clinic in sub-Saharan Africa. Neurology. 2007;68:350–5. doi: 10.1212/01.wnl.0000252811.48891.6d. [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–42. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 4.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18:S75–8. [PubMed] [Google Scholar]
- 5.Venturi G, Catucci M, Romano L, et al. Antiretroviral resistance mutations in human immunodeficiency virus type 1 reverse transcriptase and protease from paired cerebrospinal fluid and plasma samples. J Infect Dis. 2000;181:740–5. doi: 10.1086/315249. [DOI] [PubMed] [Google Scholar]
- 6.Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 7.Enting RH, Prins JM, Jurriaans S, Brinkman K, Portegies P, Lange JM. Concentrations of human immunodeficiency virus type 1 (HIV-1) RNA in cerebrospinal fluid after antiretroviral treatment initiated during primary HIV-1 infection. Clin Infect Dis. 2001;32:1095–9. doi: 10.1086/319602. [DOI] [PubMed] [Google Scholar]
- 8.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: Implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Tambussi G, Gori A, Capiluppi B, et al. Neurological symptoms during primary human immunodeficiency virus (HIV) infection correlate with high levels of HIV RNA in cerebrospinal fluid. Clin Infect Dis. 2002;30:962–5. doi: 10.1086/313810. [DOI] [PubMed] [Google Scholar]
- 10.Centlivre M, Sala M, Wain-Hobson S, Berkhout B. In HIV-1 pathogenesis the die is cast during primary infection. AIDS. 2007;21:1–11. doi: 10.1097/QAD.0b013e3280117f7f. [DOI] [PubMed] [Google Scholar]
- 11.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Lindbäck S, Karlsson AC, Mittler J, et al. Karolinska Institutet Primary HIV Infection Study Group. Viral dynamics in primary HIV-1 infection. AIDS. 2000;14:2283–91. doi: 10.1097/00002030-200010200-00009. [DOI] [PubMed] [Google Scholar]
- 13.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 14.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 16.Carne CA, Tedder RS, Smith A, et al. Acute encephalopathy coincide with seroconversion for anti-HTLV-III. Lancet. 1985;326:1206–8. doi: 10.1016/s0140-6736(85)90740-8. [DOI] [PubMed] [Google Scholar]
- 17.Ho DD, Rota TR, Schooley RT, et al. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. New Engl J Med. 1985;313:1493–7. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 18.Denning DW. The neurological features of acute HIV infection. Biomed Pharmacother. 1988;42:11–4. [PubMed] [Google Scholar]
- 19.Hagberg L, Malmvall BE, Svennerholm L, Alestig K, Norkrans G. Guillain-Barré syndrome as an early manifestation of HIV central nervous system infection. Scand J Infect Dis. 1986;18:591–2. doi: 10.3109/00365548609021668. [DOI] [PubMed] [Google Scholar]
- 20.Piette AM, Tusseau F, Vignon D, et al. Acute neuropathy coincident with seroconversion for anti-LAV/HTLV-III. Lancet. 1986;1:852. doi: 10.1016/s0140-6736(86)90956-6. [DOI] [PubMed] [Google Scholar]
- 21.Brew BJ, Perdices M, Darveniza P, et al. The neurological features of early and 'latent' human immunodeficiency virus infection. Aust N Z J Med. 1989;19:700–5. doi: 10.1111/j.1445-5994.1989.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 22.Scarpini E, Sacilotto G, Lazzarin A, Geremia L, Doronzo R, Scarlato G. Acute ataxia coincident with seroconversion for anti-HIV. J Neurol. 1991;238:356–7. doi: 10.1007/BF00315340. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos F, Mallada J, Ricart C, Zabala JA. Ataxic neuropathy associated with human immunodeficiency virus seroconversion. Arch Neurol. 1994;51:236. doi: 10.1001/archneur.1994.00540150022010. [DOI] [PubMed] [Google Scholar]
- 24.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007;21:19–48, vii. doi: 10.1016/j.idc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Lindbäck S, Thorstensson R, Karlsson AC, et al. Karolinska Institute Primary HIV Infection Study Group. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. AIDS. 2000;14:2333–9. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 26.Little SJ, Frost SD, Wong JK, et al. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J Virol. 2008;82:5510–18. doi: 10.1128/JVI.02579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdulle S, Mellgren A, Brew BJ, et al. CSF neurofilament protein (NFL)—A marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–32. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 28.Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: Relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–8. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 30.Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001;286:171–9. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 31.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 32.Davenport MP, Zhang L, Shiver JW, Casmiro DR, Ribeiro RM, Perelson AS. Influence of peak viral load on the extent of CD4+ T-cell depletion in simian HIV infection. J Acquir Immune Defic Syndr. 2006;41:259–65. doi: 10.1097/01.qai.0000199232.31340.d3. [DOI] [PubMed] [Google Scholar]
- 33.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 34.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–9. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 35.Wallace MR, Nelson JA, McCutchan JA, Wolfson T, Grant I. Symptomatic HIV seroconverting illness is associated with more rapid neurological impairment. Sex Transm Infect. 2001;77:199–201. doi: 10.1136/sti.77.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho DD, Sarngadharan MG, Resnick L, Dimarzoveronese F, Rota TR, Hirsch MS. Primary human T-lymphotropic virus type III infection. Ann Intern Med. 1985;103:880–3. doi: 10.7326/0003-4819-103-6-880. [DOI] [PubMed] [Google Scholar]
- 37.Chiodi F, Sönnerborg A, Albert J, et al. Human immunodeficiency virus infection of the brain. I. Virus isolation and detection of HIV specific antibodies in the cerebrospinal fluid of patients with varying clinical conditions. J Neurol Sci. 1988;85:245–57. doi: 10.1016/0022-510x(88)90184-0. [DOI] [PubMed] [Google Scholar]
- 38.Sönnerborg AB, von Stedingk LV, Hansson LO, Strannegård OO. Elevated neopterin and beta 2-microglobulin levels in blood and cerebrospinal fluid occur early in HIV-1 infection. AIDS. 1989;3:277–83. doi: 10.1097/00002030-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Hagberg L, Dotevall L, Norkrans G, Larsson M, Wachter H, Fuchs D. Cerebrospinal fluid neopterin concentrations in central nervous system infection. J Infect Dis. 1993;168:1285–8. doi: 10.1093/infdis/168.5.1285. [DOI] [PubMed] [Google Scholar]
- 40.Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–63. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.González-Scarano F, Martín-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 42.Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: Neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–45. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffin DE, McArthur JC, Cornblath DR. Neopterin and interferon-gamma in serum and cerebrospinal fluid of patients with HIV-associated neurologic disease. Neurology. 1991;41:69–74. doi: 10.1212/wnl.41.1.69. [DOI] [PubMed] [Google Scholar]
- 44.Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister H, Fontana A. Identification of a T cell chemotactic factor in cerevrospinal fluid of HIV-1-infected individuals as interferon-γ inducible protein 10. J Neuroimmunol. 1999;93:172–81. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 45.Marra CM, Maxwell CL, Collier AC, Robertson KR, Imrie A. Interpreting cerebrospinal fluid pleocytosis in HIV in the era of potent antiretroviral therapy. BMC Infect Dis. 2007;7:37. doi: 10.1186/1471-2334-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–52. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–21. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 49.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]