Abstract
Background. Iron overload can adversely influence the course of infection by increasing microbial replication and suppressing antimicrobial immune effector pathways. Recently, we have shown that the calcium channel blocker nifedipine can mobilize tissue iron in mouse models of iron overload. We therefore investigated whether nifedipine treatment affects the course of infection with intracellular bacteria via modulation of iron homeostasis.
Methods. The effect of nifedipine on intramacrophage replication of bacteria and modulation of cellular iron homeostasis was investigated in the murine macrophage cell line RAW264.7, and the impact of nifedipine treatment on the course of systemic infection was investigated in C57BL/6 mice in vivo.
Results. In RAW264.7 cells, nifedipine treatment significantly reduced intracellular bacterial survival of Salmonella enterica serovar Typhimurium and Chlamydophila pneumoniae. This could be attributed to the induction of the iron exporter ferroportin 1, which limited the availability of iron for intracellular Salmonella. When C57BL/6 mice were infected intraperitoneally with Salmonella and subsequently injected with nifedipine for 3 consecutive days, bacterial counts in livers and spleens were significantly reduced and survival of the mice significantly was prolonged compared with solvent-treated littermates. Nifedipine treatment increased expression of ferroportin 1 in the spleen, whereas splenic levels of the iron storage protein ferritin and serum iron concentrations were reduced.
Conclusions. Our data provide evidence for a novel mechanism whereby nifedipine enhances host resistance to intracellular pathogens via limitation of iron availability.
Iron availability is a central determinant in host-pathogen interactions. On one hand, the metal is essential for metabolic processes in both hosts and microbes. Accordingly, iron availability is linked to the proliferation and pathogenicity of many microorganisms, whereas the restriction of iron from pathogens is an efficient strategy of host defense [1–4]. On the other hand, iron has subtle effects on cell-mediated immune effector pathways. For example, iron inhibits the activity of interferon γ (IFN-γ)–driven effector pathways of macrophages, such as tumor necrosis factor α and nitric oxide formation, resulting in an impaired immune response toward intracellular pathogens [5–9], although a certain amount of iron is important for oxygen radical formation by macrophages via the Fenton reaction [10]. Accordingly, systemic iron overload is associated with unfavorable outcomes of many types of infection [1, 11–13].
Macrophages have multiple pathways for the acquisition of iron [14]. The most important of these are the phagocytosis of senescent erythrocytes with subsequent reutilization of iron, the uptake of transferrin-bound iron via the transferrin receptor 1 (TfR1), and ferrous iron incorporation via the divalent metal transporter 1 (Dmt1, Slc11a2, and Dct1) [15–18]. The major avenue for iron export is accomplished by the transmembrane protein ferroportin 1 (Fpn1 and Slc40a1) [19]. The expression of Fpn1 is regulated at several levels including transcriptional regulation by cytokines [17, 20] as well as posttranscriptional and translational induction by iron [19, 21, 22]. Most importantly, the iron and cytokine inducible peptide hepcidin, which is the master regulator of iron homeostasis [23, 24], posttranslationally reduces the presence of Fpn1 at the cell surface membrane by targeting it for endocytosis. Recent evidence also suggests that M1 and M2 macrophage subtypes differ in their expression of iron metabolism genes and the handling of iron [25], further supporting the idea that effector functions and iron metabolism of macrophages are mutually linked.
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a facultatively intracellular gram-negative bacterium capable of persisting and replicating within host macrophages [26–28]. The intracellular proliferation of Salmonella is highly dependent on a sufficient supply of iron [3]. S. Typhimurium secretes siderophores via IroC and EntS to bind ferric iron, which is subsequently taken up by outer membrane receptors including IronN and FepA [29, 30]. ABC transporters such as FepBCDG are responsible for the transport of siderophores through the cytoplasmic membrane, whereas molecular iron is taken up via Feo-mediated transmembrane transport [31]. Salmonella strains defective in iron transport show reduced virulence as well as an impaired ability to proliferate in vivo [32]. Accordingly, a limited availability of iron within macrophages as observed in classical hemochromatosis is associated with the impaired proliferation of intracellular bacteria, such as salmonellae or mycobacteria, within these cells [33, 34].
The dihydropyridine-like calcium channel blocker nifedipine can reverse tissue iron accumulation in mouse models of primary and secondary iron overload, which has been linked to stimulation of iron transport via Dmt1 [35]. In the present study, we investigated whether nifedipine treatment can modify iron availability for intramacrophage bacteria and thus affect the course of systemic Salmonella infection in mice.
METHODS
Cell Culture
RAW264.7 murine macrophage-like cells were originally isolated from BALB/c mice and obtained from the American Type Culture Collection (ATCC). Cells were grown in low-glucose Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (all obtained from PAA) at 37°C in humidified air containing 5% carbon dioxide.
S. Typhimurium strain ATCC 14028s was grown in Luria-Bertani broth (Sigma-Aldrich) to late logarithmic phase. RAW264.7 cells were seeded into 6-well dishes (1 × 106 cells per well) in 2 mL of DMEM, 10% fetal calf serum, and 2 mmol/L L-glutamine without antibiotics for subsequent infection experiments. After preincubation of S. Typhimurium in complete DMEM at 37°C for 20 minutes, RAW264.7 cells were infected with S. Typhimurium exactly as described elsewhere [3] and washed 3 times with phosphate-buffered saline (PBS; purchased from PAA). Thereafter, cells were replenished with complete DMEM containing 16 μg/mL gentamicin (Gibco) to kill extracellular bacteria. Subsequently, cells were treated with DMEM containing nifedipine (Sigma) or solvent control (ie, dimethyl sulfoxide; obtained from Sigma) and incubated for an additional 16–24 hours. Where indicated, cells were treated with 1 μmol/L synthetic murine hepcidin-1 (PeptaNova), 1 mmol/L ethylene glycol tetraacetic acid (EGTA; obtained from Fluka), or the appropriate solvents. Cells were washed 5 times with PBS, lysed in 0.5% deoxycholic acid (Sigma-Aldrich), and plated in appropriate dilutions onto Luria-Bertani agar plates or prepared for RNA or protein isolation. For certain experiments, RAW264.7 cells were infected with the S. Typhimurium wild-type strain or its isogenic mutant derivatives entC::aph; single mutant and entC::aph sit::bla feo::Tn10 (Tetr); triple mutant. Mutant strains were constructed as described elsewhere [31] and used as detailed above.
Chlamydophila pneumoniae strain CV-6 was propagated as described elsewhere [36]. For experiments, 1 × 106 RAW264.7 cells were seeded in 6-well plates and infected with purified C. pneumoniae at a multiplicity of infection of 2 as described elsewhere [37]. After 1 hour, nifedipine at a concentration 50 μmol/L or solvent was added to the antibiotic-free medium. Twenty-four hours later, RAW264.7 cells were fixed in methanol and stained with fluorescein isothiocyanate–conjugated anti–Chlamydia lipopolysaccharide monoclonal antibody (Oxoid) on coverslips. Chlamydial inclusion bodies within cells were counted by fluorescence microscopy at a magnification of ×100 with a Scope A1 microscope (Zeiss). For the determination of infection efficacy, 100 cells per coverslip were counted, and the number of inclusions per cell was classified as <10, 10–30, and >30 inclusions.
RNA Preparation, Reverse Transcription, and TaqMan Polymerase Chain Reaction
Total RNA was prepared from nitrogen-frozen tissue with the guanidine-thiocyanate/phenol mixture (Peqgold trifast; Peqlab) and prepared according to the manufacturer’s protocol. Reverse transcription was performed with 4 μg of total RNA, random hexamer primers, dNTPs, and Moloney murine leukemia virus reverse transcriptase (all obtained from Invitrogen) in 1× reverse transcription buffer for 1.5 hours at 37°C. The primers and probes that were used for the experiments described here are outlined in Table 1.
Table 1.
Sequences of Primers and Probes for Real-time PCR
| Name | Sequence |
| mu Fpn1 fw | 5′-CTACCATTAGAAGGATTGACCAGCT-3′ |
| mu Fpn1 rv | 5′-CAAATGTCATAATCTGGCCGA-3′ |
| mu Fpn1 probe | 5′-CAACATCCTGGCCCCCATGGC-3′ |
| mu TfR1 fw | 5′-CGCTTTGGGTGCTGGTG-3′ |
| mu TfR1 rv | 5′-GGGCAAGTTTCAACAGAAGACC-3′ |
| mu TfR1 probe | 5′-CCCACACTGGACTTCGCCGCA-3′ |
| mu Dmt1 fw | 5′-GGACTGTGGACGCTCGGTAA-3′ |
| mu Dmt1 rv | 5′-AATGTTGCCACCGCTGGT-3′ |
| mu Dmt1 probe | 5′-CATCTCGAAAGTCCTGCTGAGCGAAGA-3′ |
| mu Hepcidin1 fw | 5′-TGTCTCCTGCTTCTCCCCTTG-3′ |
| mu Hepcidin1 rv | 5′-CAGCCTATGGCCCAGACCCTCA-3′ |
| mu Hepcidin1 probe | 5′-AGCTCTGTAGTCTGTCTCATCTGTTGA-3′ |
| mu Lcn2 fw | 5′-GCCTCAAGGACGACAACATCA-3′ |
| mu Lcn2 rv | 5′-TTCTCTGTCCCCACCGACCAATGC-3′ |
| mu Lcn2 probe | 5′-CACCACCCATTCAGTTGTCAAT-3′ |
| mu LcnR fw | 5′-GGCGATTTCTACAGCGAATGA-3′ |
| mu LcnR rv | 5′-CCTCTTCCTGTTTTATGGCTGGCCTGGT-3′ |
| mu LcnR probe | 5′-CTATCAGCCACCGTGCAGACT-3′ |
| mu Phox47 fw | 5′-GAGGCGGAGGATCCGG-3′ |
| mu Phox47 rv | 5′-TCTTCAACAGCAGCGTACGC-3′ |
| mu Phox47 probe | 5′-CAACTACGCAGGTGAACCGTATGTAACCATCA-3′ |
| iNOS fw | 5′-CAGCTGGGCTGTACAAACCTT-3′ |
| iNOS rv | 5′-CATTGGAAGTGAAGCGTTTCG-3′ |
| iNOS probe | 5′-CGGGCAGCCTGTGAGACCTTTGA-3′ |
| mu TNF-α fw | 5′-TTCTATGGCCCAGACCCTCA-3′ |
| mu TNF-α rv | 5′-TTGCTACGACGTGGGTACA-3′ |
| mu TNF-α probe | 5′-CTCAGATCATCTTCTCTCAAAATTCGAGTGACAAGC-3′ |
| mu IL-6 fw | 5′-TGTTCTCTGGGAAATCGTGGA-3′ |
| mu IL-6 rv | 5′-AAGTGCATCATCGTTGTTCATACA-3′ |
| mu IL-6 probe | 5′-ATGAGAAAAGAGTTGTGCAATGGCAATTCTG-3′ |
| mu IL-10 fw | 5′-CCAGAGCCACATGCTCCTAGA-3′ |
| mu IL-10 rv | 5′-TGGTCCTTTGTTTGAAAGAAAGTCT-3′ |
| mu IL-10 probe | 5′-TGCGGACTGCCTTCAGCCAGG-3′ |
| mu IL-18 fw | 5′-GACTCTTGCGTCAACTTCAAGGA-3′ |
| mu IL-18 rv | 5′-TTGTCTGATTCCAGGTCTCCATT-3′ |
| mu IL-18 probe | 5′-TGATGTTTATTGACAACACGCTTTACTTTATACCTGAAGA-3′ |
| mu IL-23p19 fw | 5′-AGCGGGACATATGAATCTACTAAGAGA-3′ |
| mu IL-23p19 rv | 5′-GTCCTAGTAGGGAGGTGTGAAGTTG-3′ |
| mu IL-23p19 probe | 5′-CCAGTTCTGCTTGCAAAGGATCCGC-3′ |
| mu IL-23p40 fw | 5′-GACCATCACTGTCAAAGAGTTTCTAGAT-3′ |
| mu IL-23p40 rv | 5′-AGGAAAGTCTTGTTTTTGAAATTTTTAA-3′ |
| mu IL-23p40 probe | 5′-CCACTCACATCTGCTGCTCCACAAGAAG-3′ |
| mu TGF-β fw | 5′-TGACGTCACTGGAGTTGTACGG-3′ |
| mu TGF-β rv | 5′-TTCAGCGCTCACTGCTCTTGTGACAG-3′ |
| mu TGF-β probe | 5′-GGTTCATGTCATGGATGGTGC-3′ |
| mu IL-4 fw | 5′-ACAGGAGAAGGGACGCCAT-3′ |
| mu IL-4 rv | 5′-TCCTCACAGCAACGAAGAACACCACA-3′ |
| mu IL-4 probe | 5′-GAAGCCCTACAGACGAGCTCA-3′ |
| mu IL-1β fw | 5′-GATGAGGACATGAGCACCTTCTT-3′ |
| mu IL-1β rv | 5′-CATCTTTGAAGAAGAGCCCATCCTCTGTGA-3′ |
| mu IL-1β probe | 5′-GCAGGTTATCATCATCATCCCA-3′ |
| mu IL-12/23p40 fw | 5′-GACCATCACTGTCAAAGAGTTTCTAGA-3′ |
| mu IL-12/23p40 rv | 5′-CCACTCACATCTGCTGCTCCACAAGAAG-3′ |
| mu IL-12/23p40 probe | 5′-AGGAAAGTCTTGTTTTTGAAATTTTTTAA -3′ |
| mu IL-17A fw | 5′-GCTCCAGAAGGCCCTCAG-3′ |
| mu IL-17A rv | 5′-ACCTCAACCGTTCCACGTCACCCTG-3′ |
| mu IL-17A probe | 5′-CTTTCCCTCCGCATTGACA-3′ |
Animals
Male C57BL/6 mice (age, 8–12 weeks) were kept according to guidelines of the Medical University of Innsbruck (Innsbruck, Austria) and the Austrian Ministry for Science and Education (based on the Austrian Animal Testing act of 1989; BMWF-66.011/77-II/10b/2008) in the animal quarter at the Medical University of Innsbruck. Mice were fed a standard diet (iron, 180 mg/kg; Altromin C 1000). For infection experiments, mice were injected intraperitoneally with 500 colony-forming units (CFUs) of S. Typhimurium suspended in 200 μL of PBS. Twenty-four hours later, mice were injected intraperitoneally with nifedipine at a dose of 5 mg/kg body weight or solvent control (0.1% ethanol), respectively, for 3 consecutive days. Mice were observed twice daily for signs of illness.
For further investigations, animals were killed 96 hours after infection. Parts of livers and spleens were homogenized in sterile 0.1% sodium dodecyl sulfate (Sigma-Aldrich) and plated onto agar plates to enumerate bacterial CFUs. Paraffin-embedded tissue sections were analyzed by means of hematoxylin-eosin staining, which was performed according to a standard protocol. Serum iron concentrations were determined by a QuantiChrome assay (BioAssay System). Organs were frozen in liquid nitrogen for further RNA and protein preparation.
Radioactive Iron Uptake Experiments
RAW264.7 cells were seeded in complete DMEM and infected with S. Typhimurium as described above. Following 3 washing cycles with serum-free, HEPES-buffered DMEM, cells were incubated therein. For the investigation of bacterial radioactive iron uptake, 10 μmol/L 59Fe-citrate was added and incubated for 4 hours and intramacrophage bacteria were harvested as described elsewhere [18]. Radioactivity uptake was expressed as femtomoles of iron taken up per hour and per 1 × 106 Salmonella.
Western Blot Analysis
Protein extracts from nitrogen-frozen tissue were prepared and homogenized in cytoplasmic lysis buffer (25 mmol/L Tris-HCl [pH, 7.4], 40 mmol/L KCl, and 1% Triton X-100) containing 1 μg/mL aprotinin and leupeptin (Sigma). Thirty micrograms of protein was loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel. Proteins were blotted onto nylon membranes (Hybond P; Amersham Pharmacia) and blocked in 1× Tris-buffered saline, 2% dry milk, and 0.1% Tween. The membrane was incubated with either rabbit antiferritin antibody (2.5 μg/mL; Sigma), rabbit antiferroportin antibody (2.5 μg/mL; Eurogentec), or rabbit antiactin antibody (2 μg/mL; Sigma). Horseradish peroxidase–conjugated secondary antirabbit immunoglobulin G antibody (Dako) was used to detect cross-reactivity with SuperSignal West Dura Extended Duration substrate (Thermo Fisher Scientific).
Atomic Absorption Spectrometry
Intracellular iron levels were determined by means of atomic absorption spectrometry as described elsewhere [38].
Statistical Analysis
Statistical analysis was performed using the Statistics package for the Social Science software package (version 11.5; SAS Institute). Calculations for statistical differences between the various groups were performed by use of the analysis of variance technique and Bonferroni correction for multiple variables, the Student t test, or the Mann--Whitney U test. Differences in survival were compared with the log-rank test and Fisher exact test.
RESULTS
Effects of Nifedipine Treatment on S. Typhimurium Infection of Murine Macrophages
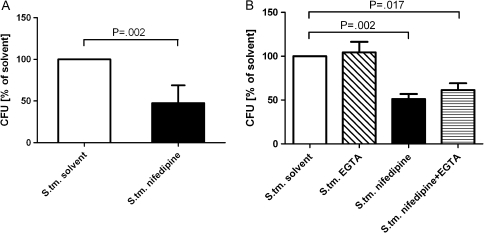
The effects of nifedipine treatment on intracellular bacterial counts were assessed in RAW264.7 cells infected with S. Typhimurium. After treatment with 50 μmol/L nifedipine for 24 hours, the number of S. Typhimurium CFUs recovered from macrophages was significantly reduced compared with that recovered from solvent-treated control cells (Figure 1).
Figure 1.
Effects of nifedipine on intracellular bacterial load. RAW264.7 cells were infected with Salmonella enterica serovar Typhimurium (S. tm.) at a multiplicity of infection of 10 and stimulated with solvent, nifedipine (50 μmol/L), hepcidin (1 μmol/L), and/or ethylene glycol tetraacetic acid (EGTA; 1 mmol/L) for 24 hours. Cell lysates were plated onto agar plates to determine the number of colony-forming units (CFUs) of Salmonella. Graphs show means (± SD). Calculations for statistical differences between various groups were performed by means of the Student’s t test or multivariate analysis with the Bonferroni correction (n = 3–6).
To see whether these effects of nifedipine are concentration dependent, we performed dose-response experiments using nifedipine at final concentrations of 0.1–100 μmol/L. Thereby, we could demonstrate that nifedipine dose-dependently and significantly reduced intracellular survival of Salmonella within RAW264.7 macrophages at concentrations of 0.25–100 μmol/L, whereas lower concentrations had no effect (Supplemental Figure 1).
Given the known pharmacological action of nifedipine as a calcium channel blocker, the possible role of extracellular calcium influx was investigated using the impermeable calcium chelator EGTA. No effect of extracellular calcium chelation on the intracellular growth of Salmonella was observed in either the presence or absence of nifedipine (Figure 1).
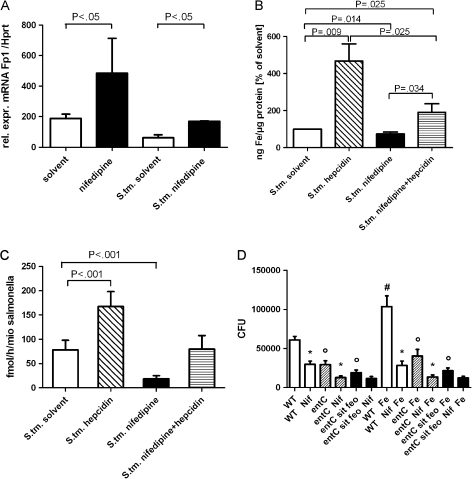
Because macrophages exhibit enhanced expression of the only known iron exporter Fpn1 upon Salmonella infection [3] and because nifedipine affects transmembrane iron transport [35], we next determined whether the effect of nifedipine on intracellular Salmonella growth is related to the modulation of macrophage iron transport and intracellular iron availability. The messenger RNA (mRNA) expression of the iron uptake genes Dmt1 and TfR1 was modified by S. Typhimurium infection [33] but not by the addition of nifedipine (details not shown). However, mRNA levels of the iron export Fpn1 were significantly increased in S. Typhimurium–infected macrophages following treatment with nifedipine, in comparison with application of solvent (Figure 2).
Figure 2.
Effects of nifedipine (Nif) on iron gene regulation, intracellular iron content, and iron acquisition in Salmonella enterica serovar Typhimurium (S. tm.) infection. Experiments were performed as described in the text. Fpn1 messenger RNA (mRNA) levels of macrophages were determined by quantitative reverse-transcription polymerase chain reaction. Values were normalized to the levels of housekeeping gene hypoxanthine phosphoribosyl transferase (Hprt) mRNA (A). The intracellular iron content of infected and treated RAW264.7 cells was determined by means of atomic absorption spectrometry. Iron content was normalized to micrograms of protein of cell pellets (B ). Iron uptake by S. Typhimurium was determined by measuring 59Fe incorporation of bacteria in fmol per hour per one million (mio) Salmonella (C ). RAW264.7 cells were infected with different strains of S. Typhimurium: wild type (WT) (white bars), entC single mutant (shaded bars), and entC sit feo triple mutant (black bars). Differences between the entC or entC sit feo strains and the WT solvent control with or without iron are indicated by circles (P < .001). Differences in the number of colony-forming units (CFUs) following nifedipine treatment of macrophages infected with WT and entC mutant bacteria were compared with respective solvent controls, as indicated by asterisks (P < .001). The difference in the number of CFUs upon iron sulphate addition is indicated by a hash mark (P < .001) (D). Bars represent means (± SD) of 3–6 independent experiments. Calculations for statistical differences between various groups were performed by means of the Student’s t test, Mann--Whitney U test, or multivariate analysis with the Bonferroni correction where appropriate. Rel. expr., relative expression.
To study the metabolic consequence of increased Fpn1 expression, we next measured intracellular iron levels and iron acquisition by intracellular Salmonella (Figure 2). Nifedipine treatment of Salmonella-infected macrophages resulted in both a significant reduction in intracellular iron levels and a reduced amount of iron acquisition by the intracellular bacteria.
To determine whether this effect was related to Fpn1-mediated iron export, the experiments were repeated in the presence of synthetic hepcidin, which binds to Fpn1, thereby resulting in its internalization and degradation with subsequent impairment of iron export [23]. The addition of hepcidin significantly increased intracellular iron levels in Salmonella-infected macrophages, both in the presence and in the absence of nifedipine, consequently increasing Salmonella iron acquisition (Figure 2) and intracellular proliferation of bacteria [33, 39]. However, direct exposure of Salmonella cultures to nifedipine in vitro at concentrations of 1–1000 μmol/L had no observable growth inhibitory effect (data not shown), which rules out a direct toxic or iron-chelating effect of the drug toward bacteria.
Strains of S. Typhimurium that are deficient in iron acquisition were then used to compare the antimicrobial actions of nifedipine with the effects of genetic microbial iron limitation. Salmonella lacking the entC (siderophore uptake) or entC, sit, and feo (both siderophore and elemental iron uptake) genes exhibited deficient intracellular replication in RAW264.7 cells in comparison with wild-type bacteria (Figure 2), mimicking the effects of nifedipine treatment. Nifedipine had no significant effect on bacterial replication in cells infected with the triple mutant strain deficient in both siderophore and elemental iron uptake. Moreover, the addition of 50 μmol/L iron sulfate significantly increased the growth of intracellular wild-type Salmonella, but bacteria in nifedipine-treated cells were unable to exhibit higher levels of growth in the presence of iron supplementation, which suggests that nifedipine limits iron availability for intracellular bacteria.
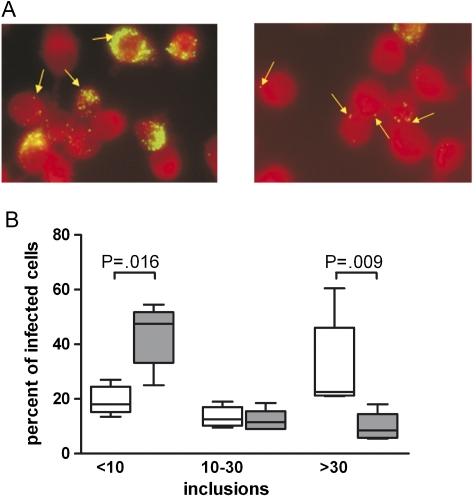
To study whether the antimicrobial activity of nifedipine may also apply to other intracellular pathogens, we next investigated the effects of nifedipine toward the intracellular pathogen C. pneumoniae. Therefore, we infected RAW264.7 cells with C. pneumoniae and treated the infected cells with nifedipine (50 μmol/L) or solvent for 24 hours. Nifedipine treatment resulted in a significant reduction of bacteria counts in RAW264.7 cells and a decrease in the number of inclusion bodies per cell (Figure 3).
Figure 3.
Effect of nifedipine treatment on multiplication of Chlamydophila pneumoniae in macrophages. RAW264.7 cells were infected with C. pneumoniae at a multiplicity of infection of 2 and then treated with 50 μmol/L nifedipine or solvent (control) and evaluated 24 hours later as described in the text. A, Representative slides for control (left) and nifedipine-treated RAW264.7 cells (right). Fluorescein isothiocyanate–marked C. pneumoniae inclusions are indicated by arrows. Original magnification ×630. B, Relative number of infected cells with differences in the quantities of inclusions. The number of inclusions was categorized as <10, 10–30, or >30 inclusions per cell. The y-axis shows the relative percentage of infected cells with different numbers of inclusion bodies. White boxes represent solvent-treated control cells, and gray boxes represent nifedipine-treated cells. Data are shown for 5 independent experiments performed in duplicate or triplicate. Calculations for statistical significance were performed by means of the Mann--Whitney U test.
Effects of Nifedipine Treatment on S. Typhimurium Infection In Vivo
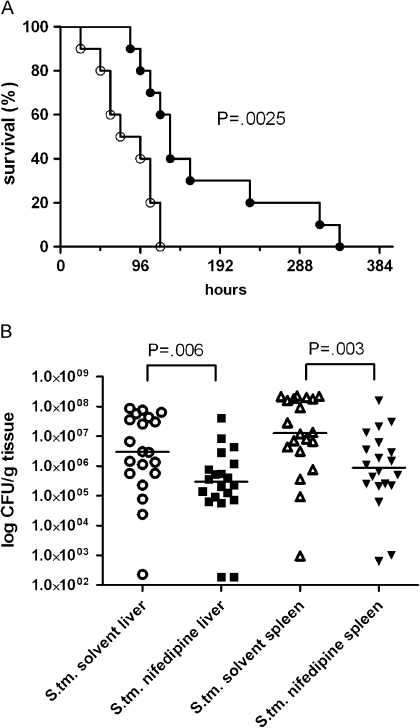
To examine the relevance of the antibacterial effect of nifedipine seen in RAW264.7 cells in vivo, C57BL/6 mice were inoculated intraperitoneally with S. Typhimurium and treated with nifedipine or a solvent control. The administration of nifedipine or solvent for 3 consecutive days was initiated 24 hours after bacterial inoculation and resulted in significantly prolonged survival of the mice receiving nifedipine (P = .003; log-rank test). The mean (± SD) survival time in the solvent control group was 82 (± 11) hours, compared with 170 (± 29) hours in the nifedipine-treated group (Figure 4). Interestingly, no mouse died during and within 24 hours after the termination of nifedipine treatment (total infection time, 96 hours), whereas by this time point >50% of the control mice succumbed to infection, reflecting a significant survival benefit for nifedipine-treated animals after the third injection (72 hours; P = .033; Fisher exact test).
Figure 4.
Effect of nifedipine treatment on the course of Salmonella enterica serovar Typhimurium (S. tm.) septicemia in mice. Mice were intraperitoneally injected with S. Typhimurium and 24 hours later treated with either nifedipine (filled circles) or solvent (open circles) for 3 days as detailed in the text (n = 10 mice per group; P = .003; log-rank test) (A). In another set of experiments with the same treatment regimen, bacterial counts in spleens and livers were determined 96 hours after initiation of infection. White circles and black rectangles represent colony-forming units (CFUs) in the livers, white triangles and black inverse triangles represent bacterial load in the spleens, and the horizontal bars show median values (B). Differences were calculated by means of the Mann–Whitney U test (P = .006 and P = .003 for livers and spleens, respectively).
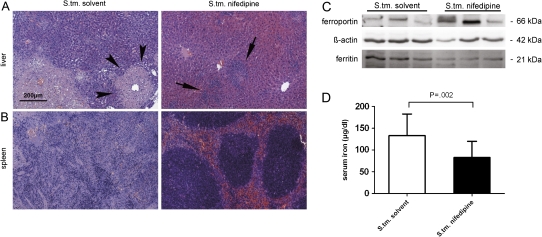
To further characterize the mechanisms responsible for the prolonged survival of mice receiving nifedipine treatment, we found that nifedipine treatment resulted in a significant reduction of bacterial counts in livers (P = .006) and spleens (P = .003) at 96 hours after the onset of infection (Figure 4). This was paralleled by a significant reduction in abscess size and preservation of organ architecture in both livers and spleens. Although the organs of solvent-treated mice contained extensive hepatic abscesses, mice treated with nifedipine exhibited only immune cell infiltration without frank abscess formation. In addition, effacement of splenic and hepatic architecture was observed in control mice after 96 hours of infection but not in mice receiving nifedipine (Figure 5).
Figure 5.
Histopathological changes and differences in protein expression in organs of Salmonella enterica serovar Typhimurium (S. tm.)–infected mice upon nifedipine treatment. Shown are representative hematoxylin-eosin-stained sections of livers (A) and spleens (B) of mice in 1 of 3 independent experiments treated with solvent (n = 9) or/and nifedipine (n = 10), as described in the text, obtained 96 hours after onset of infection. The black bar represents a scale of 200 μm. Arrowheads highlight abscess formation in the liver (solvent treatment group), whereas arrows illustrate immune cell infiltration (nifedipine treatment group). Western blot analysis of spleen samples was performed exactly as described in the text. Equal loading of protein extracts was confirmed by incubation with anti–ß-actin antibody (middle). Results for 3 representative mice in each group are shown (C ). Serum iron levels from 3 independent experiments of solvent-treated mice (n = 19) (white bar) and nifedipine-treated mice (n = 17) (black bar) were determined by a colorimetric assay. Differences between groups were calculated with the Student’s t test (P = .002) (D).
To determine whether nifedipine affects systemic iron homeostasis during Salmonella infection in vivo, expression of Fpn1 and ferritin was measured in the livers and spleens of infected animals. Splenic expression of Fpn1 protein was increased in nifedipine-treated animals compared with controls. In contrast, expression of the iron storage protein ferritin was reduced upon nifedipine treatment of S. Typhimurium–infected mice (Figure 5). In parallel with the decreased expression of ferritin in spleens, nifedipine treatment resulted in a significant reduction in serum iron levels (P = .002) (Figure 5).
Because iron availability can affect immune effector pathways, the mRNA levels for cytokine genes (iNos, phox p47, IFN-γ, IL-4, IL-17A, TNF-α, IL-1β, IL-6, IL-12p35, IL-12/23p40, IL-23p19, IL-18, IL-10, and TGF-β) were measured in the spleens of infected animals by quantitative reverse-transcription polymerase chain reaction. However, after 96 hours of infection, no significant differences in the relative mRNA expression of these cytokine genes (related to the expression of the housekeeping gene hypoxanthine phosphoribosyl transferase) were observed between Salmonella-infected mice treated with solvent and those treated with nifedipine (data not shown).
DISCUSSION
This study provides evidence that the calcium channel blocker nifedipine can reduce mortality during systemic S. Typhimurium infection in mice. The improved survival is attributable to the ability of nifedipine to reduce iron availability for intramacrophage Salmonella, which has an essential requirement for the nutrient iron when residing within macrophages. The limitation of intracellular iron availability can effectively inhibit the growth and proliferation of this and other intracellular pathogens [3, 33, 40–45].
A cause-effect relationship between nifedipine-mediated iron restriction and the protective effect of this drug, both in vitro and in vivo, is supported by our demonstration of reduced cellular iron content of infected macrophages along with impaired iron uptake by intracellular Salmonella in the presence of nifedipine. Furthermore, a significant reduction in intramacrophage Salmonella replication following the addition of nifedipine was not seen when macrophages were infected with an entC sit feo triple mutant Salmonella strain [31] that is unable to utilize either elemental or siderophore-bound iron. In addition, we could demonstrate that the antimicrobial effect of nifedipine is not restricted to S. Typhimurium but was also observed upon infection of macrophages with another intracellular pathogen, C. pneumoniae. This result suggests that targeting microbial iron acquisition systems and reducing the access of microbial pathogens to iron within the host is a promising avenue for the treatment of certain infections [1–3, 14, 31, 46, 47].
This study also provides evidence of the mechanism by which nifedipine limits iron availability for intracellular Salmonella. We show here that nifedipine induces up-regulation of the iron export protein Fpn1, leading to cellular iron egress and reduced levels of intracellular iron. Nifedipine shows dose-dependent effects. The extracellular calcium chelator EGTA had no effect on intracellular bacterial growth in the presence or absence of nifedipine, which suggests that the calcium channel blocker is not acting by inhibiting the influx of extracellular calcium but might impede the mobilization of membrane-associated stores. Further studies will be required to determine whether nifedipine-mediated inhibition of calcium signaling is responsible for increased Fpn1 expression. Alternatively, nifedipine induction of Dmt1-mediated iron transport [35] could lead to a transient increase in intracellular iron level that subsequently induces the expression of Fpn1 [19, 21, 22, 40]. Increased Fpn1 expression leads to iron egress [3] and a reduction in intracellular iron concentrations. The importance of this pathway for the control of iron availability to intracellular pathogens is further supported by the finding that addition of the Fpn1 antagonist hepcidin increases intracellular iron levels [23] and promotes the proliferation of Salmonella within RAW264.7 macrophages [39, 41, 43]. Accordingly, the ability of nifedipine to limit bacterial iron availability is attenuated by the addition of hepcidin. These observations are in agreement with other evidence that induction of Fpn1 expression and cellular iron export is an effective defensive strategy for limiting iron availability for intracellular pathogens [3]. Moreover, cells with a gain- of-function mutation of Fpn1 can reduce the growth of intracellular Salmonella [41], whereas loss-of-function mutations as seen in flatiron mice are associated with an increased growth of intracellular bacteria [43]. No significant effects of nifedipine on cytokine expression were seen. The alternative possibility that nifedipine might impair phagocytosis of Salmonella by macrophages was excluded by the lack of a measurable effect of nifedipine on Salmonella uptake by RAW264.7 cells compared with solvent-treated controls (data not shown). Furthermore, in vitro and in vivo infections were initiated 1 and 24 hours, respectively, prior to nifedipine treatment, to avoid possible effects of nifedipine on early interactions between Salmonella and host cells or direct toxic effects of nifedipine on the bacteria.
In clinical settings, severe infection or septicemia may be associated with hypotension, and the therapeutic use of an antihypertensive drug, such as nifedipine, might be problematic. A cost-benefit ratio of nifedipine in sepsis will need to be prospectively evaluated. However, in the murine model, none of the mice infected with S. Typhimurium died during treatment with nifedipine, indicating a net benefit of nifedipine treatment. Other pharmacological agents with more selective iron modulatory effects may be of greater utility in systemic infections complicated by hypotension.
Nevertheless, calcium channel blockers are pleiotropic, and nifedipine may exert ambivalent effects in infection. A previous study has shown that long-term oral administration of nifedipine over a period of 6 months resulted in impaired neutrophil function along with reduced killing of engulfed Salmonella and reduced survival following a lethal dose of S. Typhimurium [48]. Thus, although short-term administration of nifedipine is beneficial in murine salmonellosis, long-term administration may have deleterious effects on innate immunity, including impaired neutrophil signaling [49]. Our study provides evidence that nifedipine favorably affects the course of systemic infection with Salmonella as a result of macrophage iron mobilization, which restricts availability of the metal to intracellular pathogens.
Supplementary Data
Supplementary Data are available at The Journal of Infectious Diseases online.
Funding
This work was supported by Fonds zur Foerderung der Wissenschaftlichen Forschung (grant 19664); and the National Institutes of Health (grants AI39557 and AI77629 to F. C. F.).
Acknowledgments
We thank Susanna Desole and Teresa Fritz for help in preparing histopathological images, and Sabine Engl, Markus Seifert, and Sylvia Berger for excellent technical assistance.
References
- 1.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg ED. Modulation of intramacrophage iron metabolism during microbial cell invasion. Microbes Infect. 2000;2:85–9. doi: 10.1016/s1286-4579(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 3.Nairz M, Theurl I, Ludwiczek S, et al. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella Typhimurium. Cell Microbiol. 2007;9:2126–40. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 4.Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron—a metal at the host-pathogen interface. Cell Microbiol. 2010;12:1691–702. doi: 10.1111/j.1462-5822.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham-Rundles S, Giardina PJ, Grady RW, Califano C, McKenzie P, De Sousa M. Effect of transfusional iron overload on immune response. J Infect Dis. 2000;182(suppl 1):S115–21. doi: 10.1086/315919. [DOI] [PubMed] [Google Scholar]
- 6.Oexle H, Kaser A, Most J, et al. Pathways for the regulation of interferon-gamma-inducible genes by iron in human monocytic cells. J Leukoc Biol. 2003;74:287–94. doi: 10.1189/jlb.0802420. [DOI] [PubMed] [Google Scholar]
- 7.Recalcati S, Taramelli D, Conte D, Cairo G. Nitric oxide-mediated induction of ferritin synthesis in J774 macrophages by inflammatory cytokines: role of selective iron regulatory protein-2 downregulation. Blood. 1998;91:1059–66. [PubMed] [Google Scholar]
- 8.Weiss G, Werner-Felmayer G, Werner ER, Grunewald K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–76. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105–22. doi: 10.1146/annurev.nutr.012809.104804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins HL, Kaufmann SH, Schaible UE. Iron chelation via deferoxamine exacerbates experimental salmonellosis via inhibition of the nicotinamide adenine dinucleotide phosphate oxidase-dependent respiratory burst. J Immunol. 2002;168:3458–63. doi: 10.4049/jimmunol.168.7.3458. [DOI] [PubMed] [Google Scholar]
- 11.McDonald CJ, Jones MK, Wallace DF, Summerville L, Nawaratna S, Subramaniam VN. Increased iron stores correlate with worse disease outcomes in a mouse model of schistosomiasis infection. PLoS One. 2010;5:e9594. doi: 10.1371/journal.pone.0009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195:1745–53. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 13.Weiss G, Umlauft F, Urbanek M, et al. Associations between cellular immune effector function, iron metabolism, and disease activity in patients with chronic hepatitis C virus infection. J Infect Dis. 1999;180:1452–8. doi: 10.1086/315052. [DOI] [PubMed] [Google Scholar]
- 14.Weiss G. Iron metabolism in the anemia of chronic disease. Biochim Biophys Acta. 2009;1790:682–93. doi: 10.1016/j.bbagen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Delaby C, Pilard N, Puy H, Canonne-Hergaux F. Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J. 2008;411:123–31. doi: 10.1042/BJ20071474. [DOI] [PubMed] [Google Scholar]
- 16.Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- 17.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–54. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 18.Olakanmi O, Rasmussen GT, Lewis TS, Stokes JB, Kemp JD, Britigan BE. Multivalent metal-induced iron acquisition from transferrin and lactoferrin by myeloid cells. J Immunol. 2002;169:2076–84. doi: 10.4049/jimmunol.169.4.2076. [DOI] [PubMed] [Google Scholar]
- 19.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J Biol Chem. 2002;277:39786–1. doi: 10.1074/jbc.M201485200. [DOI] [PubMed] [Google Scholar]
- 21.Zoller H, Theurl I, Koch R, Kaser A, Weiss G. Mechanisms of iron mediated regulation of the duodenal iron transporters divalent metal transporter 1 and ferroportin 1. Blood Cells Mol Dis. 2002;29:488–97. doi: 10.1006/bcmd.2002.0587. [DOI] [PubMed] [Google Scholar]
- 22.Tchernitchko D, Bourgeois M, Martin ME, Beaumont C. Expression of the two mRNA isoforms of the iron transporter Nramp2/DMTI in mice and function of the iron responsive element. Biochem J. 2002;363:449–55. doi: 10.1042/0264-6021:3630449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 24.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Recalcati S, Locati M, Marini A, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol. 2010;40:824–35. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 26.Lahiri A, Lahiri A, Iyer N, Das P, Chakravortty D. Visiting the cell biology of Salmonella infection. Microbes Infect. 2010;12:809–18. doi: 10.1016/j.micinf.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Leung KY, Finlay BB. Intracellular replication is essential for the virulence of Salmonella Typhimurium. Proc Natl Acad Sci U S A. 1991;88:11470–4. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews-Polymenis HL, Baumler AJ, McCormick BA, Fang FC. Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect Immun. 2010;78:2356–69. doi: 10.1128/IAI.00096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100:3677–82. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabsch W, Methner U, Voigt W, Tschape H, Reissbrodt R, Williams PH. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect Immun. 2003;71:6953–61. doi: 10.1128/IAI.71.12.6953-6961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crouch ML, Castor M, Karlinsey JE, Kalhorn T, Fang FC. Biosynthesis and IroC-dependent export of the siderophore salmochelin are essential for virulence of Salmonella enterica serovar Typhimurium. Mol Microbiol. 2008;67:971–83. doi: 10.1111/j.1365-2958.2007.06089.x. [DOI] [PubMed] [Google Scholar]
- 32.Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella Typhimurium. Mol Microbiol. 2000;35:1146–55. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 33.Nairz M, Theurl I, Schroll A, et al. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–51. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J Leukoc Biol. 2007;81:195–204. doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- 35.Ludwiczek S, Theurl I, Muckenthaler MU, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007;13:448–54. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- 36.Cles LD, Stamm WE. Use of HL cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1990;28:938–40. doi: 10.1128/jcm.28.5.938-940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellmann-Weiler R, Martinz V, Kurz K, et al. Divergent modulation of Chlamydia pneumoniae infection cycle in human monocytic and endothelial cells by iron, tryptophan availability and interferon gamma. Immunobiology. 2010;215:842–8. doi: 10.1016/j.imbio.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–86. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 39.Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11:1365–81. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461–73. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chlosta S, Fishman DS, Harrington L, et al. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect Immun. 2006;74:3065–7. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella Typhimurium. Eur J Immunol. 2008;38:1923–36. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- 43.Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–74. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrd TF, Horwitz MA. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes: coordinate upregulation by iron transferrin and downregulation by interferon gamma. J Clin Invest. 1993;91:969–76. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fritsche G, Nairz M, Werner ER, Barton HC, Weiss G. Nramp1-functionality increases iNOS expression via repression of IL-10 formation. Eur J Immunol. 2008;38:3060–7. doi: 10.1002/eji.200838449. [DOI] [PubMed] [Google Scholar]
- 46.Gordeuk V, Thuma P, Brittenham G, et al. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N Engl J Med. 1992;327:1473–7. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- 47.Schrettl M, Bignell E, Kragl C, et al. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200:1213–9. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nalini K, Andrabi KI, Ganguly NK, Wahi PL. Nifedipine administration impairs natural resistance of mice to Salmonella Typhimurium. Mol Cell Biochem. 1990;95:133–7. doi: 10.1007/BF00219971. [DOI] [PubMed] [Google Scholar]
- 49.Shima E, Katsube M, Kato T, et al. Calcium channel blockers suppress cytokine-induced activation of human neutrophils. Am J Hypertens. 2008;21:78–84. doi: 10.1038/ajh.2007.13. [DOI] [PubMed] [Google Scholar]