Abstract
We report here the identification of GIDE, a mitochondrially located E3 ubiquitin ligase. GIDE contains a C-terminal Ring finger domain, which is mostly conserved with those of the IAP family members, and which is required for its E3 ligase activity. Overexpression of GIDE induces apoptosis via a pathway involving activation of caspases since the caspase inhibitors, XIAP and an inactive mutant of caspase-9 block GIDE-induced apoptosis. GIDE also activates JNK, and blockade of JNK activation inhibits GIDE-induced release of cytochrome c and Smac and apoptosis, suggesting that JNK activation precedes release of cytochrome c and Smac and is required for GIDE-induced apoptosis. These proapoptotic properties of GIDE require its E3 ligase activity. When somewhat over or underexpressed, GIDE slows or hastens cell growth respectively. These pro-apoptotic or growth rate effects of GIDE may account for its absence from tumor cells.
Keywords: GIDE, apoptosis, E3 ligase, mitochondria, caspase, JNK
Introduction
Apoptosis is required for normal development in all metazoans. Abnormal regulation of apoptosis leads to various diseases, such as cancer, autoimmune and neurodegenerative diseases. Apoptosis is mediated by activation of caspases, which cleave various cellular substrates and lead to characteristic apoptotic morphological changes (1-3). Caspases are synthesized as cytoplasmic zymogen precursors and are activated in a hierarchical pattern in which downstream caspases are activated by upstream caspases (1-3). The upstream caspases are activated through distinct mechanisms. For example, it has been proposed that the death receptor associated caspase-8 is activated by so-called “induced proximity” (4). In most cases, however, activation of the initial caspases involves mitochondria. Upon stimulation by various death signals, the outer membrane of the mitochondria is permeabilized, resulting in the release to the cytosol of molecules including cytochrome c, Smac/DIABLO, Omi/HtrA and GSPT1/eRF3 . Released cytochrome c causes cell death by binding Apaf-1, an event that leads to recruitment and activation of pro-caspase-9 (5). Unlike cytochrome c, mitochondria-released Smac/DIABLO, Omi/HtrA2 and GSPT1/eRF3 induce caspase activation and apoptosis through their interactions with proteins of the IAP family (6-8) .
IAP proteins are universally present in organisms from yeast to humans. All IAPs contain at least one baculovirus IAP repeat (BIR) domain and most also contain a C-terminal RING finger domain, which has E3 ubiquitin ligase activity. Although some IAP proteins have other activities, most IAPs inhibit apoptosis (9) . Among the human IAPs, XIAP is the mostly extensive studied and the most potent inhibitor of apoptosis. XIAP binds to active caspase-9 with high affinity and less well to caspase-3 and -7. Binding of XIAP to active caspases prevents their cleavage of cellular substrates and thus inhibits apoptosis (10). XIAP may also target active caspase-3 for ubiquitination and degradation by the proteasome, thereby inhibiting apoptosis (11). Binding of mitochondria-released Smac/Diablo, Omi/HtrA2 and GSPT1/eRF3 to IAPs causes release of active caspases from the inhibitory IAPs and/or inhibition of ubiquitination and degradation of caspases and therefore promotes apoptosis (9). Recently, it has been shown that XIAP and the baculovirus Op-IAP can ubiquitinate Smac/Diablo and target it for degradation by the proteasome, suggesting IAPs can also inhibit apoptosis through degradation of their antagonists, rather than by directly inhibiting caspases (12). Whatever the mechanisms are, the balance between mitochondria-released death factors and cytosolic IAPs appears to be critically involved in modulation of apoptosis.
Many extrinsic and intrinsic signals can trigger apoptosis. For example, JNK, a mitogen-activated protein kinase (MAPK), activated by stimuli that cause cell stress, such as DNA damage reagents, UV light and proinflammatory cytokines is proapototic under some circumstances in some cell types (13, 14). In Drosophila, the JNK ortholog, DJNK, is required for apoptosis during embryonic patterning of the wing, eye, and gut (15). DJNK phosphorylates DJun, which promotes transcription of Hid and Rpr, two proteins that bind to Drosophila IAP ortholog DIAP1 and prevent DIAP1 from inhibiting the Drosophila caspase, DRONC, thus triggering apoptosis (16).
In mammals, there is evidence for both proapoptotic and antiapoptotic roles of JNK. JNK activity is required for apoptosis of PC12 neuronal cells induced by NGF withdrawal, of MEFs in response to many stimuli, or of thymocytes in response to T cell receptor ligation (17-19). However, knockout of JNK1 and JNK2 in mouse can increase apoptosis in the brain during embryonic development (20).
In this study, we identified a mitochondrial protein, GIDE, that contains a Ring finger domain homologous to those of the IAP family members. GIDE slows the growth of cells and, when overexpressed, induces apoptosis. GIDE has E3 ubiquitin ligase activity, which is required for its ability to induce apoptosis.
Results
Identification and tissue distribution of GIDE
Interested in the anti-apototic functions of the RING finger domains of IAPs, we searched the EST databases for novel proteins that containing C-terminal Ring finger domains like those of IAPs. We thus identified the novel protein, GIDE (for Growth Inhibition and Death E3 Ligase) through alignment of the available cDNA and EST sequences, we obtained a ~2.5 kb human GIDE cDNA sequence which encodes a 352 aa protein. Human GIDE is approximately 90% identical at the amino acid level with its rat and mouse orthologs. The C-terminus of human GIDE contains a Ring finger domain at aa301-343 that resembles those of IAPs (Fig. 1 and data not shown). Except for the Ring finger domain, GIDE is not homologous to any known proteins.
Fig. 1.
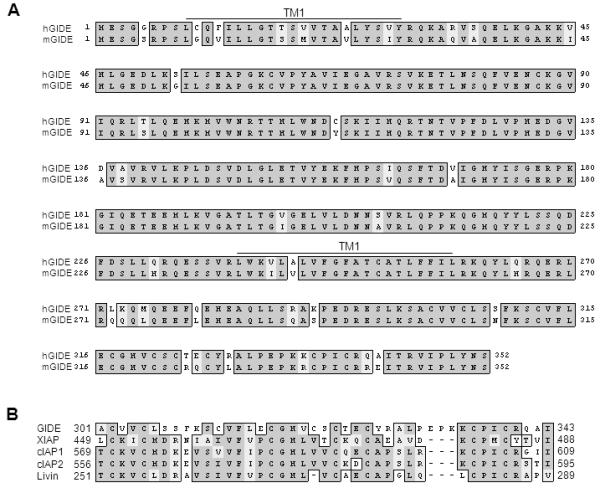
Sequence and structural analysis of GIDE. A. Alignment of human and mouse GIDE amino acid sequences. The putative transmembrane regions (TM) are indicated. B. Alignment of conserved RING-fingers of human GIDE, XIAP, c-IAP1, c-IAP2 and Livin.
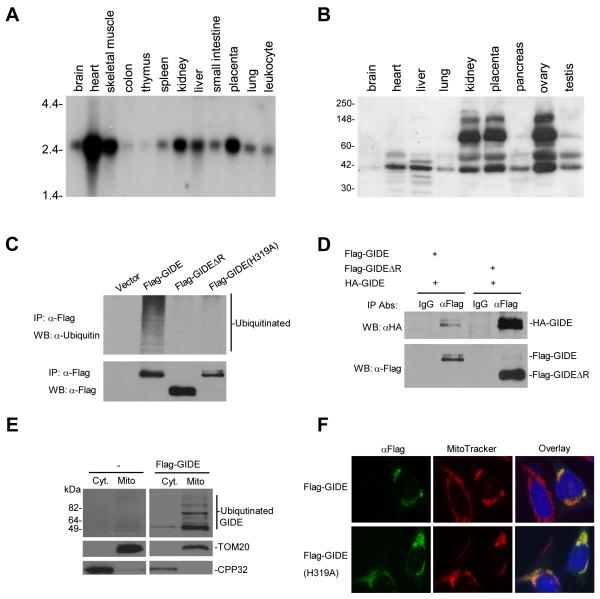
To determine the expression pattern of GIDE, we performed Northern blot analysis with a multiple human tissue mRNA blot. The data suggest that human GIDE mRNA is expressed, as a ~2.5 kb transcript, in most tissues. Expression of GIDE mRNA is relatively abundant in heart, skeletal muscle, placenta, kidney and liver, but barely detectable in colon and thymus (Fig. 2A).
Fig. 2.
Expression, ubiquitination and localization of GIDE. A. Northern blot analysis of human GIDE mRNA expression in various tissues. B. Western blot analysis of human GIDE protein expression in various tissues. C. GIDE is auto-ubiquitinated. 293 cells were transfected with the indicated Flag-tagged plasmids. Cell lysates were immunoprecipitated with anti-Flag antibody, the immunoprecipitates were analyzed by Western blot with anti-ubiquitin (upper panel) and anti-Flag (lower panel) antibodies. D. GIDE forms oligomers. 293 cells were transfected with the indicated GIDE or its mutant plasmids. Cell lysates were immunoprecipitated with anti-Flag antibody or control mouse IgG, the immunoprecipitates were analyzed by Western blot with anti-HA antibody. E. Biochemical study of GIDE localization. 293 cells were transfected with Flag-GIDE or left untransfected for 12 hours. Mitochondrial and cytosolic fractions were isolated and analyzed by Western blots with the indicated antibodies. F. Immunofluorescent staining of GIDE and its mutant GIDE(H319A). HeLa cells were transfected with the indicated plasmids and stained with the indicated antibodies.
We raised a rabbit polyclonal antibody against human GIDE and determined the expression of GIDE protein in human tissues. An ~41 kDa band, the predicted molecular weight of GIDE, was detected in all tissues (Fig. 2B). Interestingly, several other higher molecular weight bands were detected in certain tissues, including kidney, placenta, ovary and testis (Fig. 2B). These bands are probably ubiquitinated GIDE based on our observation that GIDE has E3 ubiquitin ligase activity and is extensively auto-ubiquitinated when overexpressed in mammalian cells (Fig. 2C). These bands are not derived from alternatively spliced GIDE mRNA because only one band was detected in Northern blot analysis (Fig. 2A) and the size of the transcript matches the size of the cDNA we obtained. Those bands do not represent proteins non-specifically recognized by the GIDE antibody because this antibody did not detect any proteins in multiple cancer cell lines (see Discussion).
GIDE has E3 ubiquitin ligase activity and is localized to the mitochondria
Since the Ring finger domains of the IAP family members confer E3 ubiquitin ligase activities, we determined whether GIDE also has this activity. When overexpressed in 293 cells, GIDE was strongly ubiquitinated (Fig. 2C). In these experiments, GIDEΔR, a mutant of GIDE in which the C-terminal Ring finger is deleted, as well as GIDE(H319A), a mutant in which the crucial histidine residue in the Ring finger was mutated to alanine, were not ubiquitinated (Fig. 2C). Interestingly, the expression level of wild-type GIDE was lower than that of GIDE(H319A) or GIDEΔR, suggesting that ubiquitination of GIDE caused its degradation (Figs. 2C and 5C).
Fig. 5.
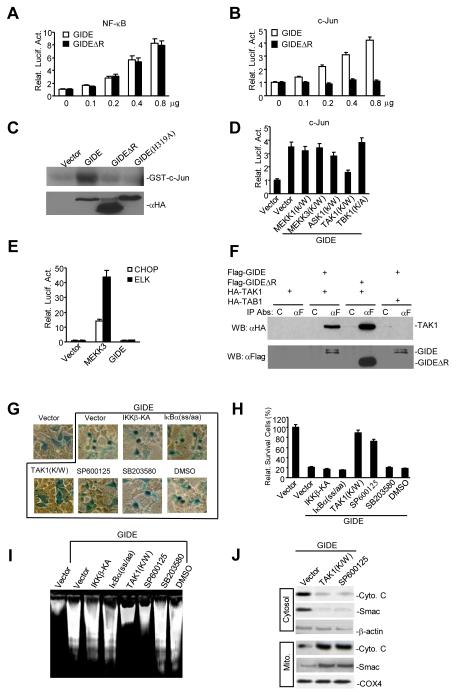
GIDE induces apoptosis through a JNK-dependent pathway. A. Effects of GIDE and GIDEΔR on NF-κB activation. 293 cells (~1×105) were transfected with 0.1 μg of NF-κB-luciferase reporter plasmid and the indicated amounts (in μg) of expression plasmids. Reporter assays were performed sixteen hours after transfection. B&C. Effects of GIDE and its mutants on JNK activation. 293 cells were transfected with GIDE and its mutants as indicated. C-Jun reporter assays (B) or in vitro kinase assays with GST-c-Jun as substrate (C) were performed as described (31). D. GIDE-induced JNK activation is inhibited by TAK1 kinase inactive mutant. 293 cells were transfected with GIDE and the indicated kinase inactive mutants. C-Jun reporter assays were performed as described (47). E. GIDE does not activate CHOP or Elk-1. 293 cells were transfected with GIDE or MEKK3. CHOP and Elk-1 reporter assays were performed as described (31). F. GIDE interacts with TAK1. 293 cells were transfected with the indicated plasmids. Cell lysates were immunoprecipitated with anti-FLAG or control mouse IgG. The immunoprecipitates were analyzed by Western blot with anti-HA (upper panel) and anti-Flag (lower panel) antibodies. G-I. Effects of various mutants or small molecule inhibitors on GIDE-induced apoptosis. 293 cells were transfected with the indicated plasmids, treated with SP600125 (20 μM), SB203580 (20 μM) or left untreated as indicated for 16 hours. The cells were stained with X-gal (G), the number of survived blue cells were counted (H), or DNA fragmentation experiments were performed (I). J. JNK is essential for GIDE-induced cytochrome c and Smac release. 293 cells were transfected with GIDE plus control vector or TAK1(K/W), or treated with SP600125 (20 μM) for 16 hours. The cells were fractionated and analyzed by Western blots with the indicated antibodies.
Consistent with its ability to ubiquitinate itself, GIDE formed homo-oligomers (Fig. 2D). A GIDE mutant lacking its Ring finger could still interact with wild-type GIDE (Fig. 2D), suggesting the N-terminal domain of GIDE mediates its oligomerization. Taken together, these data indicate that the Ring finger domain and the N-terminal domain of GIDE are required for its E3 ligase activity and oligomerization, respectively.
Structural analysis indicated that GIDE contains two transmembrane regions (Fig. 1), suggesting that GIDE is probably localized to membrane organelles. To test this, we performed cellular fractionation and immunofluorescent staining experiments. GIDE was mostly localized to mitochondria (Fig. 2E&F). The ligase inactive mutant of GIDE (H319A) was also localized to mitochondria (Fig. 2F), suggesting that ubiquitination of GIDE does not affect its localization.
GIDE induces apoptosis through the intrinsic mitochondria-dependent apoptotic pathways
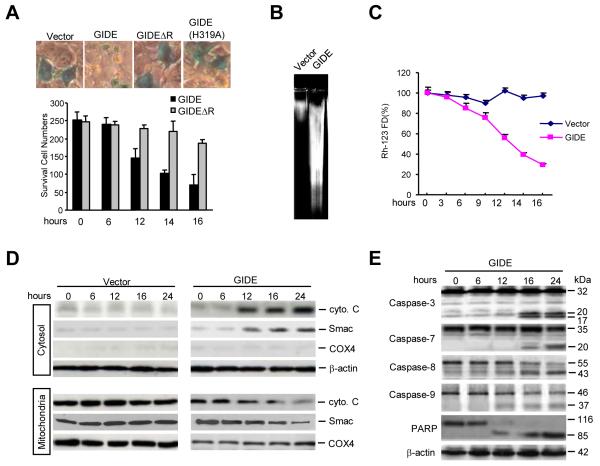
Since GIDE contains a C-terminal Ring finger domain that is conserved with those of IAP family proteins, we determined whether GIDE plays a role in apoptosis. Surprisingly, overexpression of GIDE caused massive cellular condensation, round-up, detachment from the dish and DNA fragmentation, phenotypes typical of apoptotic cells (Fig. 3A). These phenotypes were observed 12 hours after transfection (Fig. 3A) and were dose-dependent (data not shown). Overexpression of GIDEΔR or GIDE(H319A) had no significant apoptotic effects (Fig. 3A). These data indicate that GIDE can induce rapid apoptosis and the E3 ligase activity of GIDE is important for this activity.
Fig. 3.
GIDE induces apoptosis in 293 cells. A. The effects of GIDE and its mutants on cell survival. 293 cells (~1×105) were transfected with 0.1 μg of CMV-β-gal plasmid and 0.5 μg of the indicated expression plasmids. Sixteen hours after transfection, cells were stained with X-gal and photographed (upper panels), or survived blue cells were counted (lower panel). B. Overexpression of GIDE causes DNA fragmentation. 293 cells (~1×106) were transfected with 10 μg of the indicated plasmids. Sixteen hours after transfection, DNA was isolated from the transfected cells and analyzed by 2% agarose gel. C. Overexpression causes loss of mitochondrial membrane potential. 293 cells (~2×105) were transfected with 1 μg of the indicated expression plasmids. Sixteen hours after transfection, cells were stained with Rh123 (10 μg/ml) at 37 0C for 30 min and then washed with PBS (+) for three times. The mitochondrial membrane potential was analyzed by flow cytometry. D. Overexpression of GIDE causes release of cytochrome C and Smac from mitochondria to cytosol. 293 cells were transfected with GIDE or control plasmid for the indicated times. The cell lysates were fractionated and analyzed by Western blots with the indicated antibodies. E. Overexpression of GIDE causes activation of caspases. 293 cells were transfected with GIDE for the indicated times. Cell lysates were analyzed by Western blots with the indicated antibodies.
To determine the mechanisms responsible for GIDE-induced apoptosis, we examined the effects of GIDE on mitochondrial membrane potential, cytochrome c and Smac release, and caspase activation. Overexpression of GIDE caused dramatic loss of mitochondrial membrane potential (Figure 3C) and release of mitochondrial cytochrome c and Smac into the cytosol (Fig. 3D). As controls, the mitochondrial protein COX4, which is not released from damaged mitochondria, was not released into the cytosol (Fig. 3D). Consistent with these observations, overexpression of GIDE caused processing of pro-caspase-3, -7, -8, and -9, as well as the classic caspase substrate PARP (Fig. 3E). In these experiments, it seems that caspase-9 and -8 were processed earlier than caspase-3 and -7 (12 vs 16 hours after transfection), suggesting that caspase-3 and -7 function downstream of caspase-9 and/or -8. These data indicate that GIDE activates the intrinsic apoptotic pathways.
GIDE-induced apoptosis is blocked by caspase inhibitors and IAPs
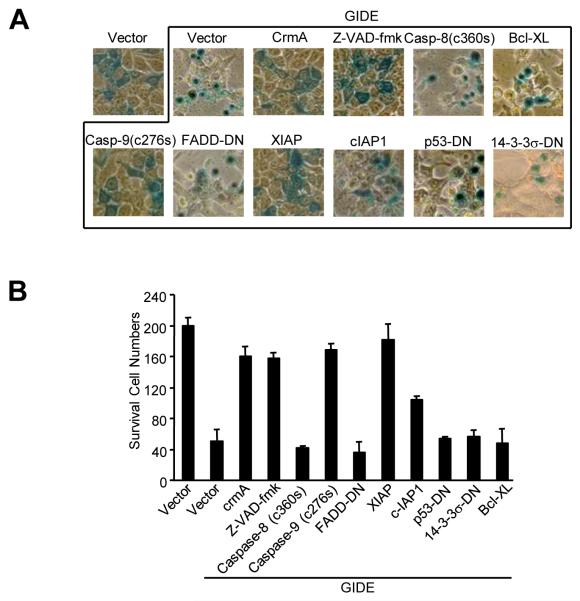
Various apoptosis inhibitors were used to determine whether cytochrome c and Smac release and caspase activation are required for GIDE-induced apoptosis. In transient transfection experiments, the caspase inhibitory protein crmA and small molecular pan-caspase inhibitor z-VAD-fmk potently inhibited GIDE-induced apoptosis and DNA fragmentation (Fig. 4). Interestingly, a caspase-inactive caspase-9 mutant had the same effects. In these experiments, a caspase-inactive caspase-8 mutant and FADD dominant negative mutant inhibited TNF-R1- (data not shown) but not GIDE-induced apoptosis (Fig. 4). Since overexpression of GIDE activated both caspase-9 and -8, it is likely that caspase-9 functions upstream of caspase-8 and other caspases, and caspase-9 but not one of its downstream caspases is required for GIDE-induced apoptosis. Alternatively, caspase-8 activation may be a secondary event in GIDE-mediated apoptosis. In these experiments, XIAP also completely inhibited GIDE-induced apoptosis, while cIAP1 had a weaker inhibitory role (Fig. 4). These results suggest that GIDE induces apoptosis through caspase-9 activation and this process is inhibited by IAPs.
Fig.4.
Effects of various apoptosis inhibitors on GIDE-induced apoptosis. 293 cells were transfected with GIDE and the indicated plasmids or treated with caspase inhibitor z-VAD-fmk. Sixteen hours after transfection, cells were stained with X-gal and photographed (A), or survived blue cells were counted (B).
A dominant negative mutant of p53 and Bcl-XL did not inhibit GIDE-induced apoptosis (Fig. 4), but inhibited p53RFP- and Bax-mediated apoptosis respectively (data not shown). Consistently, 14-3-3σ, a cytoplasmic sequester of Bax, did not block GIDE-induced apoptosis (Fig. 4). Moreover, GIDE could induce apoptosis in Bax−/−Bak−/− deficient mouse embryonic fibroblasts (data not shown). Taken together, these data suggest that GIDE-induced apoptosis is not mediated by the classical Bax/Bak-dependent pathways.
Recently, it has been shown that the E3 ubiquitin ligase Itch, upon phosphorylation by JNK, promotes degradation of c-FLIP(L) and apoptosis. In our experiments, we found that GIDE could not ubiquitinate c-FLIP or cause its degradation. In addition, c-FLIP(L) or its short alternative splice form could not inhibit GIDE-induced apoptosis (data not shown). These data indicate that GIDE-induced apoptosis is not mediated by degradation of c-FLIP.
GIDE activates JNK through TAK1-dependent pathways
In the course of our study, another group identified GIDE (referred as clone 266N) in a large-scale screen for NF-κB activating proteins but did not report a detailed functional characterization of the protein (21). We therefore determined whether GIDE can indeed activate NF-κB. Both GIDE and GIDEΔR weakly activated NF-κB in a dose dependent manner (Fig. 5A). Since IAPs can activate both NF-κB and JNK (22, 23) and GIDE is related to IAPs, we determined whether GIDE can also activate JNK. Overexpression of GIDE activated JNK in a dose-dependent manner (Fig. 5B). Overexpression of GIDE also activated JNK in kinase assays using GST-c-Jun as a substrate (Fig. 5C). Interestingly, deletion of the Ring finger domain of GIDE abolished its ability to activate JNK (Fig. 5B&C), suggesting that GIDE’s E3 ligase activity is required for GIDE-induced JNK but not NF-κB activation. Previously, it has been shown that many Ring finger domain-containing proteins including TRAF2, TRAF6 and XIAP activate JNK through MAPKKK (MEKK) family members (23, 24), we examined whether kinase-inactive mutants of MEKK1, MEKK3, ASK1, TAK1 and TBK1 could inhibit GIDE-induced JNK activation. In these experiments, only the kinase inactive mutant of TAK1, TAK1(K/W), inhibited GIDE-induced JNK activation (Fig. 5D). Interestingly, it has been shown that XIAP activates JNK through TAK1 (23). In reporter assays, GIDE did not activate p38 and ERK as indicated by CHOP and Elk1 reporter gene assays respectively (Fig. 5E).
Since GIDE activates JNK through TAK1, we examined whether GIDE could interact with TAK1 and found, in transient transfection and co-immunoprecipitation experiments, that it could. Deletion of GIDE’s C-terminal Ring finger domain did not affect its ability to interact with TAK1 (Fig. 5F).
JNK activation is required for GIDE-induced apoptosis
Since GIDE can activate both NF-κB and JNK, we determined which event is required for GIDE-induced apoptosis. In apoptosis assays, overexpression of IKKβ-KA and IκBα(SS/AA), mutants that inhibit GIDE-induced NF-κB (data not shown), did not inhibit GIDE-induced apoptosis and DNA fragmentation (Fig. 5G). In contrast, overexpression of the TAK1 kinase-inactive mutant, TAK1(K/W), dramatically inhibited GIDE-induced apoptosis and DNA fragmentation (Fig. 5G-I). Moreover, the JNK specific small molecular inhibitor SP600125, but not the p38 inhibitor SB203590 or carrier DMSO, also inhibited GIDE-induced apoptosis and DNA fragmentation (Fig. 5G-I). Consistently, TAK1(K/W) and SP600125 also blocked GIDE-induced release of cytochrome c and Smac into the cytosol (Fig. 5J). These data suggest that JNK, but not NF-κB activation is required for GIDE-induced release of cytochrome c and Smac into the cytosol and subsequent apoptosis.
Over or underexpression of GIDE inhibits or stimulates cell growth respectively
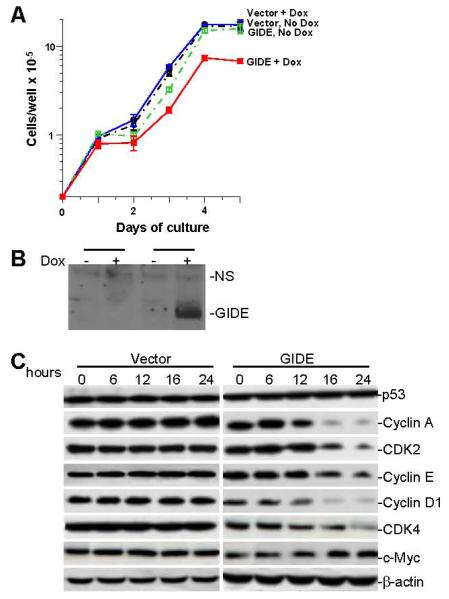
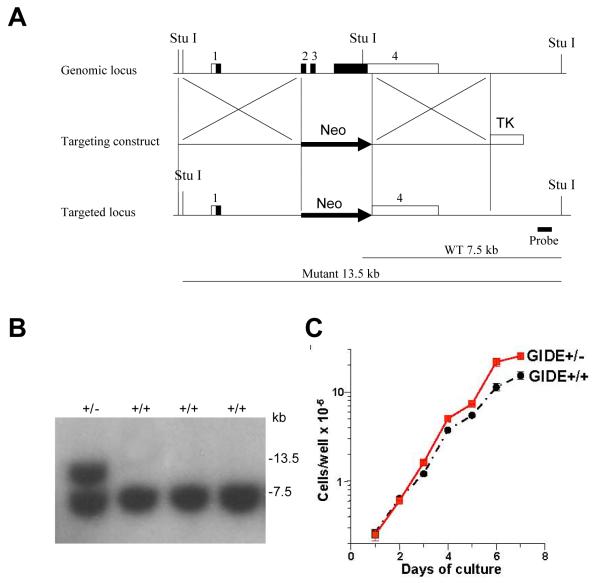
As described above, GIDE potently induces apoptosis when overexpressed in 293 or other cells. To determine the effect of GIDE when expressed at relatively lower levels, we made GIDE inducible NIH3T3 cell lines. Inducible expression of GIDE in NIH3T3 cells caused apoptosis of only a small fraction (1-5%) of cells as suggested by annexin 5 staining (data not shown). Instead, ectopic induction of GIDE expression by doxcycline inhibited the growth of NIH3T3 cells, increasing their cycle time from about 15.4 hours to 19.2 hours (Fig. 6A&B). In accord with this result, overexpression of GIDE caused degradation of cyclin A, E, D1, as well as CDK2 and CDK4, but not p53 and c-Myc (Fig. 6C). To find out if the opposite result applied in cells underexpressing GIDE, we measured the growth rates of embryonic stem (ES) cells heterozygous for deletion of the GIDE gene (Fig. 7A). The GIDE+/− cells grew faster than GIDE+/+ cells, with cycle times of 18.6 hours versus 21.8 hours (Figure 7B).
Fig. 6.
Inducible expression of GIDE inhibits cell growth. A. NIH3T3 tet-on cells were transfected with empty vector (Vector) or GIDE (GIDE), together with pTK-Hyg. The cells were selected with hygromycin (500 μg/ml) for 15 days. Stable GIDE expressing clones were induced with doxcycline or left untreated and cell growth was monitored by counting every day for 5 days. Results shown are the means and standard errors. Similar results were obtained with an independent clone of 3T3 cells overexpressing GIDE. B. Induction of GIDE expression in the GIDE transfected 3T3 cells was confirmed by Western blot analysis with anti-GIDE antibody. C. 293 cells were transfected with GIDE or control vector for the indicated times. Levels of the indicated cell cycle related proteins were examined by Western blots. D. GIDE +/+ and +/− ES cells were plated on irradiated feeder cells. The cells in triplicate cultures were counted daily thereafter, with the feeder cells excluded by their different cell size. Results shown are the means and standard errors of one of 4 experiments.
Fig. 7.
Under expression of GIDE stimulates cell growth. A. Targeting strategy for the GIDE locus. A. Boxes represent exons 1-4 of the mouse GIDE gene. Empty boxes represent 5′ untranslated region and filled boxes represent coding region. Following homologous recombination, The exons 2, 3 and 4 containing coding region were replaced with the neomycin resistance gene (Neo) on the targeted allele. Thymidine kinase (TK) from herpes simplex virus was used for negative selection. The GIDE gene was isolated from genomic DNA extracted from embryonic stem cells (TC1) by PCR. The targeting vector was constructed by replacing a 2.1-kb fragment encoding the GIDE open reading frame (exons 2, 3 and 4) with a neomycin-resistance gene cassette (Neo), and a herpes simplex virus thymidine kinase (HSV-TK) was inserted into the genomic fragment for negative selection. The targeting construct was linearized by Sal I restriction digestion and electroporated into TC1 ES cells (drived from 129Sv mouse strain). G418 and gancyclovir doubly resistant colonies were selected and screened by Southern blot analysis. One of these colonies was found to contain the correctly targeted allele. B. Genotyping of GIDE-deficient TC1 ES cell clones. Genomic DNA from TC1 ES cell clones was digested with Stu I and hybridized to the probe. Results for wild type (+/+) and herterozygous (+/−) GIDE-deficient TC1 ES cell clones are shown. C. GIDE +/+ and +/− ES cells were plated on irradiated feeder cells. The cells in triplicate cultures were counted daily thereafter, with the feeder cells excluded by their different cell size. Results shown are the means and standard errors of one of 4 experiments.
These data suggest that GIDE can cause growth arrest, probably by promoting degradation of cyclins and CDKs.
Discussion
We report here the identification of GIDE, an E3 ubiquitin ligase that, when overexpressed, induces apoptosis through novel caspase-9 and JNK activation dependent pathways. When less overexpressed, GIDE slows cell growth rates, and, when underexpressed, speeds the cell cycle. This phenomenon may be related to the fact that in spite of the fact that we have chimeric founders containing GIDE+/− ES cells, we have been unable to obtain heterozygous progeny from these mice (data not shown). The aberrant growth rate caused by heterozygous deletion of GIDE may be lethal at an early stage of embryonic development.
GIDE contains a C-terminal Ring finger domain, which is mostly conserved with those of the IAP family members (Fig. 1). The Ring finger domain of GIDE is required for its E3 ligase activity and auto-ubiquitination (Fig. 2). Other than the Ring finger domain, GIDE is not conserved with known proteins, suggesting that GIDE may function through novel mechanisms. GIDE contains two transmembrane regions and is localized to the mitochondria. Overexpression of GIDE activates JNK, an event that precedes release of cytochrome c and smac and apoptosis. These phenomena require GIDE’s E3 ligase domain. In addition, caspase inhibitors crmA and z-VAD-fmk, as well as XIAP and an inactive mutant of caspase-9 block GIDE-induced apoptosis (Fig. 4). These results suggest that GIDE induces caspase-dependent apoptotic pathways. Thus our findings suggest that the E3 ubiquitin ligase, GIDE, induces apoptosis through activation of the protein kinase JNK and subsequent activation of caspases. The processes by which GIDE slows cell growth are currently unknown.
Other than itself, the physiological substrates for GIDE are unknown. We examined whether GIDE could ubiquitinate several candidate proteins, including XIAP, Survivin, an IAP family member which is highly expressed in cancer cells but not in normal cells and has an anti-apoptotic role, cFLIP, an anti-apoptotic protein which is also a substrate of the E3 ligase Itch and TAK1, a kinase activating JNK. The results indicated that none of these proteins was a GIDE substrate (data not shown).
In co-immunoprecipitation experiments, GIDE interacted with the MAP3K family member TAK1 (Fig. 5F). A kinase inactive mutant of TAK1, but not mutants of MEKK1, MEKK3, ASK1 and TBK1, inhibited GIDE-induced JNK activation (Fig. 5D). These results suggest that TAK1 is specifically involved in GIDE-induced JNK activation. GIDE-induced TAK1 and JNK activation requires its C-terminal Ring finger domain that confers its autoubiquitination (Fig. 5). Although the exact mechanisms are unclear, GIDE-mediated TAK1 activation may be similar to how TAK1 is activated by TRAF6. TRAF6 is an E3 ubiquitin ligase that is critically involved in IL-1- and Toll-like receptor-induced NF-κB and JNK activation pathways (25). In these pathways, TRAF6 signals through TAK1 and the auto-ubiquitination of TRAF6 is sufficient to trigger the activation of TAK1 (25). XIAP also interacts with TAK1 and may activate TAK1 through a similar mechanism.
JNK may promote apoptosis by engaging the mitochondria-dependent intrinsic pathway. It has been shown that UV-induced Bax activation and cytochrome c release are absent in JNK1/JNK2-deficient MEFs, and Bax and Bak double deficient cells are resistant to apoptosis induced by UV and JNK activation (26-28). It has also been shown that JNK-dependent phosphorylation of the BH3 proteins Bim and Bmf causes their dissociation from dynein and myosin motor complexes and subsequently these dissociated proteins induce apoptosis through Bax and Bak (28). Recently, it has been shown that JNK activation leads to phosphorylation of 14-3-3 proteins, which causes dissociation of 14-3-3 proteins with Bax. The released Bax is translocated to mitochondria where it triggers the intrinsic apoptotic pathway (29). Moreover, in RelA-deficient MEFs or human HeLa cells expressing an IκBα mutant that blocks NF-κB activation, activation of JNK causes processing of Bid into jBid by an unknown mechanism. The processed jBid is translocated to the mitochondria, where it selectively triggers release of Smac but not cytochrome c. The released Smac causes activation of caspase-8 and promotes apoptosis (13). Based on these previous studies, it is possible that GIDE-induced apoptosis is also mediated by the 14-3-3 and Bcl2 family members. However, when overexpressed in Bax and Bak double knockout fibroblasts, GIDE could still induce apoptosis (data not shown), which is consistent with the observation that Bcl-XL and 14-3-3 could not block GIDE-induced apoptosis (Fig. 3). In addition, although XIAP could block GIDE-induced apoptosis, GIDE could not ubiquitinate XIAP or cause XIAP degradation (data not shown), indicating XIAP is not a direct target of GIDE. Thus our findings suggest that GIDE induces a novel, non-classic apoptotic pathway.
Northern blot and Western blot analysis suggest that GIDE is expressed in most normal human tissues at relatively high levels (Fig. 2). However, we failed to detect GIDE expression in ten examined cancer cell lines, including seven lung-derived (H549, H441, H290, H157, and 16HBE), one each of liver- (Hep3B), colon- (H1299) and kidney- (293) derived cancer cell lines (data not shown). Together with our observations that GIDE induces apoptosis or growth arrest when ectopically expressed in immortal cell lines, these observations suggest the possibility that GIDE is a tumor suppressor gene that is down-regulated in cancer cells.
Materials and Methods
Reagents
Antibodies against Flag and HA epitopes (Sigma), ubiquitin, PARP, cyclin A, cyclin D1, cyclin E, CDK2,4, β-actin (Santa Cruz Biotechnology), cytochrome c, Smac, caspase-7,8,9, p53β (Cell Signaling Technology) , and Cox4 (Molecular Probes), z-VAD-fmk (R&D systems), Rh-123 (Sigma), SP600125 (Sigma), SB203580 (Sigma), and human embryonic kidney 293 cells (ATCC, Manassas, VA) were purchased from the indicated manufactures. NIH3T3 Tet-on cell line was provided by Dr. James Hagman (National Jewish Medical and Research Center).
Constructs
Mammalian expression plasmids for IκBα (SS/AA), FADD-DN, crmA, TAK1(K/W), TBK1(K/W), ASK1(K/W), MEKK1(K/W), MEKK3, MEKK3(K/W), HA-TAK1, HA-TAB1, cIAP1, p53-DN, Bcl-XL, Caspase 8(c360s), Caspase 9(c276s) were previously described (30-32).
Mammalian expression plasmids for HA- or FLAG-tagged GIDE, GIDEΔR, GIDE(H319A), XIAP, 14-3-3σ-DN, caspase-9(C276S) were constructed by PCR amplification of the corresponding cDNA fragments and subsequently cloning into a CMV promoter-based vector containing an N-terminal HA or FLAG tag.
Northern blot
Human multiple tissue mRNA blots were purchased from Clontech (Palo Alto, CA). The blots were hybridized with 32P-labeled GIDE cDNA in the Rapid Hybridization Buffer (Clontech, Palo Alto, CA) under high-stringency conditions.
Transfection, co-immunoprecipitation, reporter gene, immunofluorescence, in vitro kinase, apoptosis assays and production of heterozygous ES cells
DNA fragmentation assays
Transfected cells were washed twice with cold PBS and lysed with 2% NP-40 containing 0.2 mg/ml Proteinase K. DNA in the lysate was precipitated with 2 volumes of ethanol. The pellets were dissolved in H2O and analyzed by electrophoresis with 2% gel.
Mitochondrial membrane potential assays
293 cells (~2×105) were transfected with 1 μg of expression plasmids for the indicated time. The transfected cells were harvested and washed with PBS(+) for three times and stained with Rh123 (10 μg/ml) at 37 0C for 30 min, then washed with PBS (+) for three times. The mitochondrial membrane potential was analyzed by flow cytometry.
Induced expression of GIDE in NIH3T3 tet-on cells
NIH3T3 tet-on cells were transfected with pTRE-HA-GIDE (or pTRE) and pTK-Hyg in a ratio of 10:1 by Lipofectamine 2000 following the manufacturer’s instructions (Invitrogen, Carlsbad, CA). One day after transfection, cells were selected with hygromycin (500 μg/ml) for 15 days. Individual clones were induced with doxcycline (1 μg/ml) and induction of GIDE expression was confirmed by Western blot.
Growth curves
The growth rate of stable clones of transfected NIH3T3 tet-on cells was measured by counting the number of cells. Cells were subcultured into 6-well plates at a density of 1.5×104 cells/well in medium with or without doxcycline (1 μg/ml). The medium was replaced with fresh medium every two days. The cells were trypsinized and counted every 24 hours for 5 days. Similar assays were performed for GIDE+/+ and +/− ES cells, except doxycyclin was not added and the cells were grown on irradiated feeder cells.
Acknowledgements
The authors thank Saiphone Webb for her help in production and analysis of the GIDE+/− ES cells. This work was supported by the Chinese 863 program (#2006AA02A306), the 111 project #B06018, and USPHS CA108771.
References
- 1.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 4.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill MM, Adrain C, Martin SJ. Portrait of a killer: the mitochondrial apoptosome emerges from the shadows. Mol Interv. 2003;3:19–26. doi: 10.1124/mi.3.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 7.Olson MR, Holley CL, Yoo SJ, Huh JR, Hay BA, Kornbluth S. Reaper is regulated by IAP-mediated ubiquitination. J Biol Chem. 2003;278:4028–4034. doi: 10.1074/jbc.M209734200. [DOI] [PubMed] [Google Scholar]
- 8.Hegde R, Srinivasula SM, Datta P, Madesh M, Wassell R, Zhang Z, Cheong N, Nejmeh J, Fernandes-Alnemri T, Hoshino S, Alnemri ES. The polypeptide chain-releasing factor GSPT1/eRF3 is proteolytically processed into an IAP-binding protein. J Biol Chem. 2003;278:38699–38706. doi: 10.1074/jbc.M303179200. [DOI] [PubMed] [Google Scholar]
- 9.Duckett CS. IAP proteins: sticking it to Smac. Biochem J. 2005;385:e1–2. doi: 10.1042/BJ20041800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS. Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J Biol Chem. 2004;279:51082–51090. doi: 10.1074/jbc.M408655200. [DOI] [PubMed] [Google Scholar]
- 13.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 14.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 15.Kockel L, Homsy JG, Bohmann D. Drosophila AP-1: lessons from an invertebrate. Oncogene. 2001;20:2347–2364. doi: 10.1038/sj.onc.1204300. [DOI] [PubMed] [Google Scholar]
- 16.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 17.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 18.Hochedlinger K, Wagner EF, Sabapathy K. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene. 2002;21:2441–2445. doi: 10.1038/sj.onc.1205348. [DOI] [PubMed] [Google Scholar]
- 19.Sabapathy K, Kallunki T, David JP, Graef I, Karin M, Wagner EF. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J Exp Med. 2001;193:317–328. doi: 10.1084/jem.193.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, Hayashi H, Sugano S. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- 22.Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R. Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275:22064–22068. doi: 10.1074/jbc.M910346199. [DOI] [PubMed] [Google Scholar]
- 23.Sanna MG, da Silva Correia J, Ducrey O, Lee J, Nomoto K, Schrantz N, Deveraux QL, Ulevitch RJ. IAP suppression of apoptosis involves distinct mechanisms: the TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol. 2002;22:1754–1766. doi: 10.1128/MCB.22.6.1754-1766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 26.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. Embo J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu WH, Johnson H, Shu HB. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem. 1999;274:30603–30610. doi: 10.1074/jbc.274.43.30603. [DOI] [PubMed] [Google Scholar]
- 31.Xu LG, Li LY, Shu HB. TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J Biol Chem. 2004;279:17278–17282. doi: 10.1074/jbc.C400063200. [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Liu T, Xu LG, Chen D, Zhai Z, Shu HB. SIKE is an IKK epsilon/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. Embo J. 2005;24:4018–4028. doi: 10.1038/sj.emboj.7600863. [DOI] [PMC free article] [PubMed] [Google Scholar]