Concomitant antibiotic (CA) use compromised initial response to Clostridium difficile infection therapy and durability of that response. Fidaxomicin was significantly more effective than vancomycin in achieving clinical cure in the presence of CAs and preventing recurrence regardless of CA use.
Abstract
Background. Treatment guidelines recommend stopping all implicated antibiotics at the onset of Clostridium difficile infection (CDI), but many individuals have persistent or new infections necessitating the use of concomitant antibiotics (CAs). We used data from 2 phase 3 trials to study effects of CAs on response to fidaxomicin or vancomycin.
Methods. Subjects with CDI were treated for 10 days with fidaxomicin 200 mg every 12 hours or vancomycin 125 mg every 6 hours, assessed for resolution of symptoms, and followed up for an additional 4 weeks for evidence of recurrence. Rates of cure, recurrence, and global cure (cure without recurrence) were determined for subgroups of subjects defined by CA use and treatment group.
Results. CAs were prescribed for 27.5% of subjects during study participation. The use of CAs concurrent with CDI treatment was associated with a lower cure rate (84.4% vs 92.6%; P < .001) and an extended time to resolution of diarrhea (97 vs 54 hours; P < .001). CA use during the follow-up was associated with more recurrences (24.8% vs 17.7%; not significant), and CA administration at any time was associated with a lower global cure rate (65.8% vs 74.7%; P = .005). When subjects received CAs concurrent with CDI treatment, the cure rate was 90.0% for fidaxomicin and 79.4% for vancomycin (P = .04). In subjects receiving CAs during treatment and/or follow-up, treatment with fidaxomicin compared with vancomycin was associated with 12.3% fewer recurrences (16.9% vs 29.2%; P = .048).
Conclusions. Treatment with CAs compromised initial response to CDI therapy and durability of response. Fidaxomicin was significantly more effective than vancomycin in achieving clinical cure in the presence of CA therapy and in preventing recurrence regardless of CA use.
Antibiotic treatment is often associated with diarrhea and symptoms ranging from mild abdominal discomfort to watery diarrhea, severe colitis, and even death. Although antibiotic-associated diarrhea may result directly from altered gastrointestinal motility or from disruption of normal fecal flora, the major cause of antibiotic-associated diarrhea is Clostridium difficile [1–3]. C. difficile is a ubiquitous, gram-positive, anaerobic spore-forming bacillus implicated in 20%–30% of cases of antibiotic-associated diarrhea, in 50%–70% of cases of antibiotic-associated colitis, and in >90% of cases of antibiotic-associated pseudomembranous colitis. Asymptomatic carriage of C. difficile is found in 1%–3% of healthy adults [1–3]. After recent exposure to the hospital environment, 15%–25% of individuals are colonized, and asymptomatic fecal carriage rates vary from 15% to 50% in long-term care facilities [4, 5].
C. difficile infection (CDI) results from a perfect storm created by disruption of the normal gut microflora, which forms a protective barrier known as “colonization resistance” to intrusion of pathogenic organisms, along with overgrowth of native or newly acquired C. difficile [6–9]. Although the mechanisms by which antibiotics induce CDI are not clearly established, the purported high rate of success of fecal enemas in resolving CDI suggests that antibiotic alteration of fecal flora is an important causative factor.
The initial management of CDI involves discontinuation of antibiotics to allow the normal bowel microflora to restore itself. Although there are no controlled clinical trials demonstrating that this improves the clinical outcome of CDI, 3 small studies illustrated that solely discontinuing clindamycin was successful in resolving active symptoms of CDI [10–12]. Recurrent disease, however, was seen in 25% of cases.
Systemic infections requiring concomitant antibiotics (CAs) often occur during the course of CDI treatment. Although eminently logical, because antibiotics can initiate CDI, to our knowledge, adverse effects of CAs on CDI outcomes have heretofore not been reported. During the course of 2 phase 3 trials comparing fidaxomicin, formerly OPT-80, with vancomycin in 1164 CDI subjects, it was noted that more than one-quarter of subjects received CAs for adventitious infections during the CDI treatment period or during the immediate 4-week follow-up. We report here the adverse effects on clinical outcomes of CDI associated with CA therapy and the ability of fidaxomicin therapy to mitigate some of those effects in comparison with vancomycin treatment.
METHODS
Study Population and Design
Subjects from 2 prospective double-blinded, randomized, parallel-group, noninferiority studies were pooled for these analyses (www.clinicaltrials.gov: study NCT00314951, May 2006 through August 2008, United States, Canada; study NCT00468728, April 2007 through December 2009, United States, Belgium, Canada, France, Germany, Italy, Spain, Sweden, United Kingdom). Eligible subjects were 16 years of age or older, had received a diagnosis of a first episode of CDI or a first recurrence of CDI within the previous 3 months, and had received no more than 24 hours of pretreatment with vancomycin or metronidazole (up to 4 doses). Subjects treated for ≥3 days with metronidazole without improvement of symptoms were also eligible. Treatment with other potentially effective therapies for CDI, eg, oral bacitracin, fusidic acid, and rifaximin, was not allowed. CDI was defined by a change in bowel habits, with >3 unformed bowel movements (or >200 mL unformed stool for subjects with rectal collection devices) during the 24 hours before randomization, and the presence of either C. difficile toxin A or B in the stool within 48 hours before randomization. Informed consent was obtained from all study participants.
Study Conduct
Participants were randomized to receive oral fidaxomicin (200 mg twice daily) or oral vancomycin (125 mg 4 times daily) for 10 days. Participants were evaluated daily during the 10-day treatment period for cure or treatment failure on the basis of symptoms of fever, nausea, vomiting, abdominal pain, flatus, and the number of daily bowel movements. If subjects were cured, recurrence was assessed by means of weekly phone calls during 4 weeks of follow-up. Fecal samples were collected before the first dose of study drug, at end of treatment, and at recurrence of symptoms and assayed for toxins A and B. All concomitant medications were recorded. Subjects were considered to have taken CAs if they received 1 or more oral or intravenous doses of antibiotic(s) during the treatment or follow-up periods.
Clinical cure was defined as resolution of diarrhea (≤3 unformed stools for 2 consecutive days) maintained until the end of therapy and for 2 days afterward. Clinical failure was defined as persistent diarrhea, the need for additional CDI therapy, or both. Recurrence was defined as the reappearance of symptoms of CDI within 4 weeks after completing treatment, the presence of C. difficile toxin A, B, or both in stool, and the need for retreatment. Subjects with clinical cure were followed for evidence of recurrence and had an end-of-study visit between days 36 and 40. Global cure was defined as clinical cure with no recurrence. The evaluable population for cure consisted of subjects who received ≥3 days of treatment and who were considered to have experienced clinical failure and also subjects who received ≥8 days of treatment and who were evaluated for cure at an end-of-treatment visit. Subjects were evaluable for recurrence if they were cured at the end of treatment, had recurrent symptoms within 28 days or were evaluated at a follow-up visit 28 ± 2 days following the last dose of study drug, and received no other antibiotics for CDI and no medication that might confound analysis of recurrence.
Antibiotics with high risk of contributing to symptomatic CDI were identified, and CA use was also categorized by number of classes received by each subject (Table 1; online only; [13, 14], D. Gerding, MD, written communication, 2010; E. Goldstein, MD, written communication, 2010; M. Miller, MD, written communication, 2010). Topical antibiotics, treatments for CDI, and antifungal and antiviral agents with no antibacterial activity were not included as CAs. Antineoplastic and immunomodulating agents were taken by 67 (11.9%) of 564 subjects in the fidaxomicin treatment group and 48 (8.2%) of 583 subjects in the vancomycin group (safety population). Effects of these agents were not considered or controlled for in the analyses reported here.
Study Drugs
Study drugs were overencapsulated so that all capsules were identical in appearance. Subjects randomized to receive fidaxomicin received 2 capsules containing 125 mg fidaxomicin and 2 placebo capsules, alternating every 6 hours, each day. Subjects in the vancomycin treatment group received 4 capsules containing 125 mg vancomycin every 6 hours each day. The investigator, sponsor, site personnel, and subjects were blinded to treatment assignment.
Statistical Analysis
The effect of CAs used during the treatment phase (days 1–10) was analyzed for the outcomes of clinical cure and time to resolution of diarrhea (TTROD), and CA use at any time during treatment and/or follow-up (days 1–40) was analyzed for global cure. Analysis of recurrence was performed separately for CA use during the treatment period (days 1–10), during follow-up (days 11–40), and at any time (days 1–40). On an individual subject basis, treatment and follow-up periods were defined by the dates of the first and last dose of study drug and the follow-up visit.
Response rates were determined for clinical cure, recurrence, and global cure, and 2-sided 95% confidence intervals (CIs) were constructed around point estimates. To compare subgroups of subjects determined by CA exposure and CDI treatment, 2-sided CIs were constructed around the differences, and a χ2 test determined the significance of differences in proportions. TTROD was compared by means of Kaplan-Meier analysis using log rank and Wilcoxon tests for determination of significance. A P value of <.05 was considered to reveal a significant difference.
Results are presented for the per protocol population. Results for the intent-to-treat population were similar for all outcomes.
RESULTS
Subject Population and Concomitant Antibiotic Use
A total of 1164 subjects were enrolled and 999 subjects were evaluable for clinical and global cure (481 treated with fidaxomicin and 518 treated with vancomycin). Age of subjects ranged from 18 to 94 years (mean = 62 years; SD = 18 years), 59% (584/999) were female, and 61% (606/999) were inpatients; baseline characteristics were similar between treatment groups. For analysis of recurrence, 794 subjects were evaluable (391 treated with fidaxomicin and 403 treated with vancomycin). Median time receiving study drug was 11 days for each treatment group. Subjects who received either drug for <8 days were by definition those whose therapy failed after at least 3 days of treatment.
Table 1 summarizes exposure to CAs used to treat coincident infections by treatment period. CAs were categorized by antibiotic class and risk of contributing to the incidence or progression of CDI. In the combined population of 999 subjects, 275 (27.5%) received CA(s) at some time during the study and 192 (19.2%) received CA(s) concurrently with study drug (days 1–10). Among the 794 subjects evaluable for analysis of recurrence following clinical cure, 129 (16.2%) received CAs during follow-up (days 11–40) and 185 subjects (23.3%) received antibiotics at any time during the study (days 1–40). Among subjects who received CAs, 101 (36.7%) of 275 received >1 class of CA during study participation; 6 classes was the maximum taken by a single subject. Overall, 15.5% of subjects were exposed to ≥1 dose of a high-risk antibiotic; 10.1% received high-risk CAs concurrently with CDI treatment, and 7.9% of subjects evaluable for recurrence received CAs during the follow-up period. CA use was similar between the fidaxomicin and vancomycin treatment groups.
Table 1.
Participants Receiving Concomitant Antibiotics (CAs) by Study Period
| Parameter | Treatment (days 1–10)a | Treatment or follow-up (days 1–40) | ||||
| Fidaxomicin (n = 481) | Vancomycin (n = 518) | All (n = 999) | Fidaxomicin (n = 481) | Vancomycin (n = 518) | All (n = 999) | |
| Subjects evaluable for clinical cure and global cure | ||||||
| No CAs used | 391 (81.3) | 416 (80.3) | 807 (80.8) | 349 (72.6) | 375 (72.4) | 724 (72.5) |
| ≥1 CA used | 90 (18.7) | 102 (19.7) | 192 (19.2) | 132 (27.4) | 143 (27.6) | 275 (27.5) |
| CA use by CDI risk | ||||||
| Highb | 44 (9.1) | 57 (11.0) | 101 (10.1) | 75 (15.6) | 80 (15.4) | 155 (15.5) |
| Mediumc | 36 (7.5) | 40 (7.7) | 76 (7.6) | 61 (12.7) | 64 (12.4) | 125 (12.5) |
| Lowd | 23 (4.8) | 31 (6.0) | 54 (5.4) | 45 (9.4) | 53 (10.2) | 98 (9.8) |
| CA use by no. of classese | (n = 90) | (n = 102) | (n = 192) | (n = 132) | (n = 143) | (n = 275) |
| 1 | 73 (81.1) | 73 (71.6) | 146 (76.0) | 85 (64.4) | 89 (62.2) | 174 (63.3) |
| 2 | 13 (14.4) | 25 (24.5) | 38 (19.8) | 31 (23.5) | 39 (27.3) | 70 (25.5) |
| 3 | 4 (4.4) | 1 (1.0) | 5 (2.6) | 7 (5.3) | 9 (6.3) | 16 (5.8) |
| 4–6 | 0 | 3 (2.9) | 3 (1.6) | 9 (6.8) | 6 (4.2) | 15 (5.5) |
| Subjects evaluable for recurrence | ||||||
| Follow-up (days 11–40)f | Treatment or follow-up | |||||
| Fidaxomicin (n = 391) | Vancomycin (n = 403) | All (n = 794) | Fidaxomicin N = 391 | Vancomycin N = 403 | All N = 794 | |
| No CAs used | 330 (84.4) | 335 (83.1) | 665 (83.8) | 302 (77.2) | 307 (76.2) | 609 (76.7) |
| ≥1 CA used | 61 (15.6) | 68 (16.9) | 129 (16.2) | 89 (22.8) | 96 (23.8) | 185 (23.3) |
| CA use by CDI risk | ||||||
| Highc | 28 (7.2) | 35 (8.7) | 63 (7.9) | 46 (11.8) | 51 (12.7) | 97 (12.2) |
| Mediumd | 25 (6.4) | 24 (6.0) | 49 (6.2) | 35 (9.0) | 40 (9.9) | 75 (9.4) |
| Lowe | 31 (7.9) | 30 (7.4) | 61 (7.7) | 36 (9.2) | 36 (8.9) | 72 (9.1) |
| CA use by no. of classesf | (n = 61) | (n = 68) | (n = 129) | (n = 89) | (n = 96) | (n = 185) |
| 1 | 42 (68.9) | 45 (66.2) | 87 (67.4) | 62 (69.7) | 62 (64.6) | 124 (67.0) |
| 2 | 11 (18.0) | 16 (23.5) | 27 (20.9) | 17 (19.1) | 25 (26.0) | 42 (22.7) |
| 3 | 3 (4.9) | 5 (7.4) | 8 (6.2) | 3 (3.4) | 4 (4.2) | 7 (3.8) |
| 4–6 | 5 (8.2) | 2 (2.9) | 7 (5.4) | 7 (7.9) | 5 (5.2) | 12 (6.5) |
NOTE. Data are no. (%) of subjects.
Or from first dose of study drug to last dose.
Subject received ≥1 dose of high-risk antibiotic (carbapenem; 2nd-, 3rd-, or 4th-generation cephalosporin, fluoroquinoline, lincosamide, or pivampicillin or temocillin).
Subject received ≥1 dose of medium-risk antibiotic (penicillin, penicillin combination, 1st-generation cephalosporin, macrolide, monobactam, or streptogramin).
Subject received ≥1 dose of low-risk antibiotic (all other systemic antibiotics).
There were 19 antibiotic classes (see Supplementary table). All cephalosporins were combined into 1 class, and all penicillins and penicillin combinations were combined into 1 class.
Or from 1 day after last dose of study drug until follow-up visit.
Effect of Concomitant Antibiotic Use on Clinical Outcomes
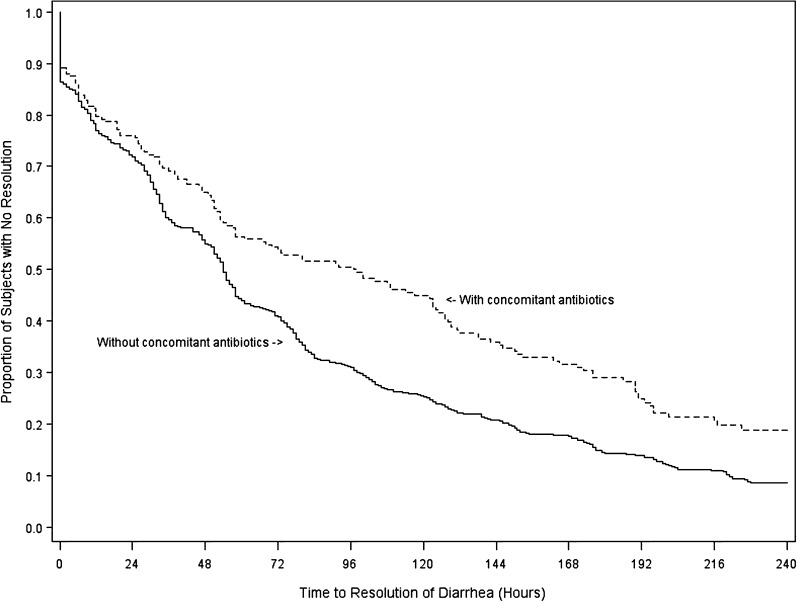
Clinical cure was achieved in 909 (91%) of 999 subjects in the per protocol set. For the combined fidaxomicin and vancomycin treatment groups (Table 2), clinical cure was achieved by 92.57% of subjects who did not receive CAs, compared with 84.38% of those who received CAs concurrently with study drug (8.2% difference [95% CI, 3.0%–13.9%]; P < .001). Global cure was observed in 74.72% of subjects who did not receive CAs but in only 65.82% of subjects receiving CAs at any time during the study (8.9% difference [95% CI, 2.54%–15.4%]; P = .005). TTROD was lengthened by use of CAs concurrently with CDI treatment (Figure 1). Median TTROD was 54 hours (95% CI, 51–57 hours) for subjects receiving no CAs and 97 hours (95% CI, 58–123 hours) for those receiving CAs during the treatment period.
Table 2.
Effect of Concomitant Antibiotic (CA) Therapy During Treatment and/or Follow-up Periods
| Endpoint study period | No CA | ≥1 CA | Difference, % (95% CI) | P |
| Clinical cure (n = 999) | ||||
| Treatment (days 1–10) | 92.57 (747/807) | 84.38 (162/192) | 8.19 (2.98–13.89) | <.001 |
| Recurrence (n = 794) | ||||
| Treatment (days 1–10) | 17.88 (118/660) | 23.88 (32/134) | −6.00 (−14.04 to 1.46) | .11 |
| Follow-up (days 11–40) | 17.74 (118/665) | 24.81 (32/129) | −7.06 (−15.3 to 0.60) | .06 |
| At any time (days 1–40) | 17.57 (107/609) | 23.24 (43/185) | −5.67 (−12.63 to 0.92) | .08 |
| Global cure (n = 999) | ||||
| At any time (days 1–40) | 74.72 (541/724) | 65.82 (181/275) | 8.91 (2.54–15.37) | .005 |
NOTE. Data are % (proportion) of subjects unless otherwise specified.
Figure 1.
Kaplan-Meier analysis of time to resolution of diarrhea (TTROD). Median TTROD was 97 hours (95% confidence interval [CI], 58–123 hours) for those who received concomitant antibiotics during the treatment period and 54 hours (95% CI, 51–57 hours) for subjects receiving no concomitant antibiotics with treatment. The difference was significant by log rank and Wilcoxon tests (P < .001 for each).
Subjects who achieved clinical cure were analyzed for recurrence within 28 days of completing treatment. Use of CAs tended to increase recurrence but did not reach significance (Table 2). Antibiotic use during follow-up most influenced the risk of recurrence: 24.81% of subjects taking CAs after completing treatment experienced recurrence within 4 weeks. In contrast, only 17.74% of those who received no CAs after completing treatment experienced recurrence, and the difference was marginally significant (−7.1% difference [95% CI, −15.3% to 0.60%]; P = .06).
Clinical cure rates were compared for subjects receiving ≥1 dose of 1 high-risk antibiotic and those receiving only low-risk antibiotic(s) during the same time period (Table 3). Use of high-risk CAs decreased the rate of clinical cure by 15.48% from 96.67% to 81.19% (P = .04). Use of high-risk CAs tended to increase the risk of recurrence, but the differences were not significant (data not shown). Overall, 101 (10%) of 999 subjects received >1 class of antibiotic during study participation. Among subjects taking antibiotics concurrently with study drug treatment, use of ≥2 classes was associated with a 71.74% cure rate, compared with 88.36% for subjects who received ≤1 class (difference, 16.62% [95% CI, −30.8% to −3.24%]; P = .007). There were no statistically significant differences in recurrence rates according to number of CA classes (data not shown).
Table 3.
Effect of Concomitant Antibiotics (CAs) by Risk and Number of Classes on Clinical Cure Rates
| Clinical cure | CA use during treatment phase (days 1–10) |
|||
| CA risk categorya |
No. of CA antibiotic classesb |
|||
| Low | High | 1 | ≥2 | |
| % (proportion) of subjects | 96.67 (29/30) | 81.19 (82/101) | 88.36 (129/146) | 71.74 (33/46) |
| Difference (95% CI) | −15.48 (−1.94 to −24.39) | −16.62 (−30.77 to −3.24) | ||
| P | .04 | .007 | ||
NOTE. CI, confidence interval.
See Table 1 for explanation of risk categories. There were no significant differences by risk category or number of classes for endpoints of recurrence and global cure. There were no significant differences for high risk vs low/medium risk combined for any endpoint.
There were 19 antibiotic classes (see Supplementary table). All cephalosporins were combined into 1 class, and all penicillins and penicillin combinations were combined into 1 class.
Effect of Fidaxomicin or Vancomycin Treatment With or Without Concomitant Antibiotics
In the absence of CA use, fidaxomicin and vancomycin were equivalent in achievement of clinical cure by the end of treatment (92.3% vs 92.8%, respectively; P = .80). When subjects received 1 or more CAs concurrently with study drug, fidaxomicin was superior to vancomycin in achieving clinical cure (Table 4): 90.0% versus 79.4%, respectively (10.6% difference [95% CI, 0.23%–20.3%]; P = .04). When subjects received no additional antibiotics at any time during the study, the global cure rate was 80.8% for fidaxomicin-treated subjects and 69.1% for vancomycin-treated subjects (11.7% difference [95% CI, 5.43%–17.9%]; P < .001). Global cure rates were substantially reduced in both treatment groups when subjects received CAs at any time, but significantly more fidaxomicin-treated subjects than vancomcyin-treated subjects were cured with no recurrence of CDI. The proportion of subjects achieving global cure was 72.7% for fidaxomicin and 59.4% for vancomycin (13.3% difference [95% CI, 2.1%–24.1%]; P = .02). There was no significant difference in TTROD between the fidaxomicin and vancomycin treatment groups in the presence or absence of CAs.
Table 4.
Comparison of Fidaxomicin and Vancomycin Treatment in the Absence or Presence of Concomitant Antibiotics (CAs)
| Endpoint | % (proportion) of subjects |
|||
| study period | Fidaxomicin | Vancomycin | Difference (95% CI) | P |
| No CAa | ||||
| Clinical cure | ||||
| Treatment | 92.33 (361/391) | 92.79 (386/416) | –0.46 (–4.13 to 3.19) | .80 |
| Recurrence | ||||
| Treatment | 12.23 (40/327) | 23.42 (78/333) | −11.19 (−16.89 to −5.35) | <.001 |
| Follow-up | 11.52 (38/330) | 23.88 (80/335) | −12.37 (−18.01 to −6.57) | <.001 |
| At any time | 11.92 (36/302) | 23.13 (71/307) | −11.21 (−17.10 to −5.16) | <.001 |
| Global cure | ||||
| At any time | 80.80 (282/349) | 69.07 (259/375) | 11.74 (5.43–17.89) | <.001 |
| Any CA | ||||
| Clinical cure | ||||
| Treatment | 90.00 (81/90) | 79.41 (81/102) | 10.59 (0.23–20.34) | .04 |
| Recurrence | ||||
| Treatment | 17.19 (11/64) | 30.00 (21/70) | −12.81 (−26.41 to 1.66) | .08 |
| Follow-up | 21.31 (13/61) | 27.94 (19/68) | −6.63 (−20.98 to 8.29) | .38 |
| At any time | 16.85 (15/89) | 29.17 (28/96) | −12.31 (−23.90 to −0.12) | .048 |
| Global Cure | ||||
| At any time | 72.73 (96/132) | 59.44 (85/143) | 13.29 (2.11–24.05) | .02 |
| No high-risk CAb | ||||
| Clinical cure | ||||
| Treatment | 92.22 (403/437) | 91.97 (424/461) | 0.25 (−3.32 to 3.79) | .89 |
| Recurrence | ||||
| Treatment | 12.22 (44/360) | 24.25 (89/367) | −12.03 (−17.50 to −6.42) | <.001 |
| Follow-up | 11.85 (43/363) | 23.64 (87/368) | −11.80 (−17.20 to −6.26) | <.001 |
| At any time | 11.59 (40/345) | 23.86 (84/352) | −12.27 (−17.79 to −6.61) | <.001 |
| Global cure | ||||
| At any time | 80.79 (328/406) | 68.26 (299/438) | 12.52 (6.66–18.25) | <.001 |
| Any high-risk CA | ||||
| Clinical cure | ||||
| Treatment | 88.64 (39/44) | 75.44 (43/57) | 13.20 (−2.25 to 27.01) | .09 |
| Recurrence | ||||
| Treatment | 22.58 (7/31) | 27.78 (10/36) | −5.20 (−24.96 to 15.55) | .63 |
| Follow-up | 28.57 (8/28) | 34.29 (12/35) | −5.71 (−27.29 to 17.01) | .63 |
| At any time | 23.91 (11/46) | 29.41 (15/51) | −5.50 (−22.42 to 12.04) | .54 |
| Global cure | ||||
| At any time | 66.67 (50/75) | 56.25 (45/80) | 10.42 (−4.83 to 25.11) | .18 |
NOTE. CI, confidence interval.
See Table 1 for explanation of high risk.
CA use increased recurrence rates for both the fidaxomicin and vancomycin treatment groups, but recurrence was consistently less frequent following fidaxomicin treatment whether subjects received CAs or not (Table 4). Differences reached significance (P < .001) for subjects receiving no CA during treatment, follow-up, or at any time during the study, and recurrence was approximately doubled for all comparisons of vancomycin with fidaxomicin. For example, if subjects were cured with fidaxomicin and received no CAs during the follow-up period, 11.5% experienced recurrence of CDI whereas 23.9% of subjects cured with vancomycin had recurrences (difference, −12.4% [95% CI, −18.0% to −6.57%]; P < .001). Likewise, for subjects who received CAs during follow-up after fidaxomicin cure, 21.3% had a recurrence of CDI, compared with 27.9% of vancomycin-cured subjects (P = .38). When all subjects taking CAs at any time from the first dose of study drug to the end-of-study follow-up visit were compared by treatment group, fidaxomicin had a 12.3% advantage over vancomycin (16.9% vs 29.2%), and the difference was significant (95% CI, −23.9% to −0.12%; P = .048). In subjects receiving high-risk antibiotics, recurrence tended to be less frequent (by ≥5%) following fidaxomicin than vancomycin treatment, but none of the differences was significant.
DISCUSSION
Guidelines recommend discontinuation of CA therapy in individuals with CDI (AII recommendation [15]), but individuals frequently require antibiotic treatment during CDI therapy to manage concurrent systemic infections. In this study, 28% of subjects were treated with antibiotics for other infections at the same time as CDI treatment or during 4 weeks of follow-up. Because subjects with immediately life-threatening CDI were excluded from the study, this is likely an underestimate of antibiotic use by all subjects treated for CDI. In this study population, receipt of any antibiotic concurrently with treatment (either fidaxomicin or vancomycin) reduced the cure rate from 92.6% to 84.4% (P <.001) and prolonged the median time to resolution by 43 hours.
Receipt of CA concurrent with vancomycin reduced the cure rate from 92.8% overall to 79.4% (13.4% difference; P = .04), but the response to fidaxomicin treatment was relatively unaffected by CA use (92.3% overall and 90.0% in the presence of CA). We observed that treatment with antibiotics associated with a high risk of developing CDI de novo had further negative effects on response to therapy; however, the impact was blunted by fidaxomicin but not by vancomycin.
The risk of recurrence increased by 50% when subjects in this study received CA(s) during the period following completion of therapy for CDI (24.8% vs 17.8%). In a target group of subjects who were treated successfully for CDI and who then received CAs for another infection during the subsequent month, an estimate of 25% relapse may be low. In this study, a subject in whom CA therapy was initiated on day 1 after completing CDI treatment was followed for recurrence for 4 weeks; however a subject initiated on CA therapy on day 14 after completing CDI treatment was reassessed after only 2 weeks (study day 28) and therefore may have had a recurrence after completing the study.
Recurrence rates were consistently lower for fidaxomicin-treated subjects than for their vancomycin-treated counterparts. Some of the protection against recurrence afforded by treatment with fidaxomicin was lost when subjects received high-risk CAs during follow-up, supporting the model that fidaxomicin spares the commensal flora and thereby reduces the risk of regrowth of residual C. difficile. Vancomycin itself can contribute to the acquisition of CDI and is known to reduce fecal counts of several commensal bacterial species [16–18], so that treatment with a second high-risk antibiotic has a lesser effect on the already high incidence of recurrence after vancomycin treatment (99 [24.6%] of 403 overall in this study). The overall recurrence rate following fidaxomicin treatment was only 51 (13.0%) of 391 but was more than doubled (29.0%) among subjects who received high-risk CAs during follow-up. This is still lower than the recurrence rate (34.3%) for subjects treated with vancomycin who then received high-risk CAs during follow-up.
To our knowledge, response to discontinuation of antibiotics as primary treatment of CDI has been documented in few studies and none since the advent of widespread BI/NAP1/027 C. difficile. In only 2 studies is it explicitly stated that all initial antibiotics were stopped [19, 20]. In 3 studies, antibiotics were discontinued unless they were deemed “essential to the patient’s clinical treatment” [21, 22]. Other studies either report stopping or switching antibiotics or make no reference to the initial antibiotic [23–29].
In summary, individuals being treated for CDI will often need other antibiotics to treat concurrent infections, which can undermine response to treatment and increase the risk of relapse or reinfection. Compared with vancomycin therapy, treatment with fidaxomicin appears to blunt the deleterious effects of concurrent antibiotics on initial response and the risk of recurrence.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
K. M. M. wrote first and final drafts with contributions from all coauthors. Yin Kean performed the statistical analyses. Sharon Dana, PhD, a medical writer, assisted in preparation and editing. Primary data and analyses were made available to all coauthors.
Financial support. This work was supported by Optimer Pharmaceuticals, Inc.
Potential conflicts of interests K. W. has received payment for consulting from Iroko Pharmaceuticals and grant support from Optimer Pharma, Abbott, and Merck. P. S. S. is an employee of Optimer Pharma and has received additional fees for travel support and for participation in review activities. Y.-K. S. is an employee of Optimer Pharma and has received additional fees for travel support and for participation in review activities. Y. G. has received payment for travel support from Optimer Pharma. S. L. G. has received payment from Optimer Pharmaceuticals as an employee and is the editor in chief of Clinical Infectious Diseases. T. J. L. has received payment for consulting and advisory board activities from Optimer pharma and has also received grant support through his institution from Optimer. M. A. M. has received payment for consulting and advisory board activities from Optimer, grant support through his institution from Optimer, and consulting fees from Iroko. A. L. has received grant support through his institution through Wellstar Health System. K. M. M. has received support for travel to meetings and fees for participation in an Endpoint committee meeting from Optimer Pharmaceuticals and has also received grant support through her institution from Optimer.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Bartlett JG. Clinical practice: antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–9. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.Gerding DN, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults: a prospective case-controlled epidemiologic study. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 3.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–62. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 4.Marciniak C, Chen D, Stein AC, Semik PE. Prevalence of Clostridium difficile colonization at admission to rehabilitation. Arch Phys Med Rehabil. 2006;87:1086–90. doi: 10.1016/j.apmr.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RL, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis. 2007;45:992–8. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 6.Barc MC, Depitre C, Corthier G, Collignon A, Su WJ, Bourlioux P. Effects of antibiotics and other drugs on toxin production in Clostridium difficile in vitro and in vivo. Antimicrob Agents Chemother. 1992;36:1332–5. doi: 10.1128/aac.36.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA. 1993;269:71–5. [PubMed] [Google Scholar]
- 8.Rafii F, Sutherland JB, Cerniglia CE. Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag. 2008;4:1343–58. doi: 10.2147/tcrm.s4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starr JM, Impallomeni M. Risk of diarrhoea, Clostridium difficile and cefotaxime in the elderly. Biomed Pharmacother. 1997;51:63–7. doi: 10.1016/s0753-3322(97)87728-1. [DOI] [PubMed] [Google Scholar]
- 10.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16:459–77. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 11.Tedesco FJ. Clindamycin-associated colitis: review of the clinical spectrum of 47 cases. Am J Dig Dis. 1976;21:26–32. doi: 10.1007/BF01074135. [DOI] [PubMed] [Google Scholar]
- 12.Tedesco FJ, Barton RW, Alpers DH. Clindamycin-associated colitis: a prospective study. Ann Intern Med. 1974;81:429–33. doi: 10.7326/0003-4819-81-4-429. [DOI] [PubMed] [Google Scholar]
- 13.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29:44–50. doi: 10.1086/524320. [DOI] [PubMed] [Google Scholar]
- 14.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 16.Baines SD, O'Connor R, Saxton K, Freeman J, Wilcox MH. Activity of vancomycin against epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother. 2009;63:520–5. doi: 10.1093/jac/dkn502. [DOI] [PubMed] [Google Scholar]
- 17.Louie TJ, Emery J, Krulicki W, Byrne B, Mah M. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261–3. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354–9. doi: 10.1099/mic.0.042010-0. [DOI] [PubMed] [Google Scholar]
- 19.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–8. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 20.Young GP, Ward PB, Bayley N, et al. Antibiotic-associated colitis due to Clostridium difficile: double-blind comparison of vancomycin with bacitracin. Gastroenterology. 1985;89:1038–45. doi: 10.1016/0016-5085(85)90206-9. [DOI] [PubMed] [Google Scholar]
- 21.Dudley MN, McLaughlin JC, Carrington G, Frick J, Nightingale CH, Quintiliani R. Oral bacitracin vs vancomycin therapy for Clostridium difficile-induced diarrhea: a randomized double-blind trial. Arch Intern Med. 1986;146:1101–4. [PubMed] [Google Scholar]
- 22.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043–6. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 23.Boero M, Berti E, Morgando A, Verme G. Treatment for colitis caused by Clostridium difficile: results of a randomized open study of rifaximin vs. vancomycin. Microbiol Med. 1990;5:74–7. [Google Scholar]
- 24.de Lalla F, Nicolin R, Rinaldi E, et al. Prospective study of oral teicoplanin versus oral vancomycin for therapy of pseudomembranous colitis and Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 1992;36:2192–6. doi: 10.1128/aac.36.10.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86:15–9. doi: 10.1016/0002-9343(89)90223-4. [DOI] [PubMed] [Google Scholar]
- 26.Keighley MR, Burdon DW, Arabi Y, et al. Randomised controlled trial of vancomycin for pseudomembranous colitis and postoperative diarrhoea. Br Med J. 1978;2:1667–9. doi: 10.1136/bmj.2.6153.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagrotteria D, Holmes S, Smieja M, Smaill F, Lee C. Prospective, randomized inpatient study of oral metronidazole versus oral metronidazole and rifampin for treatment of primary episode of Clostridium difficile-associated diarrhea. Clin Infect Dis. 2006;43:547–52. doi: 10.1086/506354. [DOI] [PubMed] [Google Scholar]
- 28.Musher DM, Logan N, Hamill RJ, et al. Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis. 2006;43:421–7. doi: 10.1086/506351. [DOI] [PubMed] [Google Scholar]
- 29.Wullt M, Odenholt I. A double-blind randomized controlled trial of fusidic acid and metronidazole for treatment of an initial episode of Clostridium difficile-associated diarrhoea. J Antimicrob Chemother. 2004;54:211–6. doi: 10.1093/jac/dkh278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.