Peripheral neuropathy (PN) is a common complaint among human immunodeficiency virus (HIV) –infected adults receiving antiretroviral therapy (ART) in resource-limited settings. We measured incidence of PN among Kenyan patients and found PN to be common during the first year of ART, particularly among women.
Abstract
Background. Peripheral neuropathy (PN) is common among patients receiving antiretroviral therapy (ART) in resource-limited settings. We report the incidence of and risk factors for PN among human immunodeficiency virus (HIV)–infected Kenyan adults initiating ART.
Methods. An inception cohort was formed of adults initiating ART. They were screened for PN at baseline and every 3 months for 1 year. We used the validated Brief Peripheral Neuropathy Screen (BPNS) that includes symptoms and signs (vibration perception and ankle reflexes) of PN.
Results. Twenty-two (11%) of 199 patients had PN at baseline screening. One hundred fifty patients without evidence of PN at baseline were followed for a median of 366 days (interquartile range, 351–399). The incidence of PN was 11.9 per 100 person-years (95% confidence interval [CI], 6.9–19.1) and was higher in women than men (17.7 vs 1.9 per 100 person-years; rate ratio, 9.6; 95% CI, 1.27–72, P = .03). In stratified analyses, female sex remained statistically significant after adjustment for each of the following variables: age, CD4 cell count, body mass index, ART regimen, and tuberculosis treatment. Stratifying hemoglobin levels decreased the hazard ratio from 9.6 to 7.40 (P = .05), with higher levels corresponding to a lower risk of PN.
Conclusions. HIV-infected Kenyan women were almost 10 times more likely than men to develop PN in the first year of ART. The risk decreased slightly at higher hemoglobin levels. Preventing or treating anemia in women before ART initiation and implementing BPNS during the first year of ART, the period of highest risk, could ameliorate the risk of PN.
Peripheral neuropathy (PN) is a common complaint among human immunodeficiency virus (HIV)–infected patients receiving antiretroviral therapy (ART) in resource-limited settings [1–7]. In sub-Saharan Africa it is the most common side effect leading to a change in regimen among patients receiving stavudine (D4T). Although D4T exposure is a known risk factor for PN among HIV-infected individuals receiving ART, other known risk factors may also contribute, including nutritional deficiencies; toxic effects of anti–tuberculosis (TB) medications, such as isoniazid and ethambutol; alcoholism; diabetes; and HIV infection itself [8]. Several studies have reported the risk of PN in patients receiving ART in resource-limited settings and show a wide range of estimates, from as low as 1.73% to as high as 38% [2–5, 9–11]. These differences probably reflect the use of varying definitions of PN and lack of use of a validated screening tool for PN. We conducted a prospective, longitudinal study using a validated PN screening tool to more accurately estimate prevalence, incidence, and risk factors for developing PN among HIV-infected patients beginning ART in Kenya [12].
METHODS
Study Site and Patient Population
Bomu Medical Centre is located in Mombasa, Kenya. It is a nongovernmental general outpatient clinic that serves the urban poor community. The HIV care and treatment program started in 2004 and is funded via the President's Emergency Plan for AIDS Relief through a cooperative agreement between the Centers for Disease Control and Prevention and New York University School of Medicine. As of 2011, the clinic cares for ∼7000 adult patients receiving ART and has provided care for 14000 patients since 2004. HIV testing, treatment and prophylactic medications, and laboratory monitoring are provided to patients free of charge. CD4 cell counts are conducted at baseline and every 6 months using a FacsCount instrument at Bomu Medical Centre. The laboratory is enrolled in an external quality control program through the National External Quality Assessment Service. Viral load monitoring and genotyping are not routinely conducted because of limited funding.
Patients attending the Bomu Medical Centre HIV clinic were eligible for inclusion in this study if they were ≥15 years of age and starting ART between November 2007 and May 2008. The ART initiation eligibility criteria at that time included a CD4 cell count of <200 cells/mm3 or World Health Organization (WHO) stage 3 or 4 HIV infection at any CD4 cell count. First-line therapy includes zidovudine (AZT) or weight-based dose of D4T with lamivudine and nevirapine or efavirenz. Patients were enrolled into the study consecutively as they were found to be eligible to start ART, and no patient refused to participate. Clinical officers or physicians performed PN screening, in English or Kiswahili, at baseline (the visit when ART was initiated) and every 3 months thereafter for 1 year. Screening data were collected concurrently for this study. The Institutional Review Board for New York University School of Medicine and the Ethics Review Committee of the Kenyatta National Hospital, Nairobi, Kenya, approved this study.
Measures
The Brief Peripheral Neuropathy Screen (BPNS) assesses subjective and objective findings consistent with PN and was developed and validated by the National Institutes of Health–funded AIDS Clinical Trials Group [12]. The BPNS was validated using physiologic (quantitative sensory threshold testing) and pathologic (epidermal nerve fiber density) testing as the reference standards [12]. In this screen, patients were asked to rate presence and severity of symptoms in feet and/or legs, using a scale of 1 (mild) to 10 (severe) for each leg separately. Symptoms included pain, aching, or burning; a sensation of “pins and needles”; and numbness. The highest of the 3 scores was converted to a subjective PN grade, as follows: symptoms absent, grade 0; score of 1–3, grade 1; score of 4–6, grade 2; and score of 7–10, grade 3. Objective findings included in the BPNS were loss of vibration perception and abnormal ankle deep tendon reflexes. Vibration perception was evaluated using a 128-Hz tuning fork, maximally struck and applied at the great toe distal interphalangeal joint of each foot. Patients were not blinded during the vibration perception test. Vibration sense was defined as normal for vibration felt for >10 seconds, as mild loss for vibration felt for 6–10 seconds, as moderate loss for vibration felt for ≤5 seconds, and as severe loss for no feeling of vibration. Ankle reflexes were defined as absent, hypoactive, normal, hyperactive, or clonus. Results of the screen were recorded onto a 1-page standardized form. We have provided elsewhere the details of provider training and BPNS implementation at our clinic [13].
Data on patient demographic, clinical, and laboratory risk factors were extracted from the medical chart. Clinical staging was performed as part of routine care by clinicians and based on WHO guidelines [14]. Based on the BPNS validation study [12], we defined PN as present when someone had both subjective neuropathy (grade, >0) and ≥1 abnormal finding bilaterally on physical examination. Body mass index (BMI) was calculated using the following formula: weight (kg)/[height (m)]2. Anti-TB medication regimens included both isoniazid and ethambutol for all patients in the study, according to national guidelines (National NLTP Guideline [2005], available at http://www.gfmer.ch/Guidelines/Tuberculosis/Tuberculosis.htm); therefore, we refer to isoniazid and ethambutol exposure as “TB treatment.”
Data Analysis
Data management and statistical analysis were performed using SPSS for Windows (version 12.0) and R (version 2.12.0) software [15]. The Mann–Whitney U test was used to compare continuous variables, and Pearson’s χ2 test was used to compare differences in categorical variables. Differences were considered statistically significant at P < .05. The R package, EPITools was used to compute rate ratios and confidence intervals (CIs) for incidence densities [16]. To examine risk factors for the prevalence of PN at baseline, we performed a series of univariate logistic regressions. Two variables, WHO stage and age, each had odds ratios for the presence of PN at baseline associated with P values of ≤.20. These variables were included in a multivariate logistic regression analysis to assess their interaction.
We compared incidence rates of PN using R’s EPITools package [16]. We assessed the influence of each of the following potential risk factors on the incidence of PN: sex, WHO clinical stage, tertile of age, tertile of baseline CD4 count, tertile of hemoglobin, tertile of BMI, initial nucleoside reverse-transcriptase inhibitor (NRTI) backbone, and prior exposure to anti-TB medications. After identifying sex as the only significant risk factor for incident PN, we performed a series of multivariate analyses, using Cox regression and stratified Kaplan–Meier analysis to assess the impact of each of the other factors listed above on hazard ratios and neuropathy-free survival times of men and women. We limited ourselves to models that adjusted the effect of sex on only 1 variable at a time, because with only 17 incident cases of PN, a Cox regression model with 2 independent variables would only have 8.5 events per predictor, less than the recommended cutoff of >10 events per predictor to ensure valid results in a proportional hazards regression model [17].
RESULTS
Prevalence of and Risk Factors for PN Among ART-Naive HIV-Infected Adults
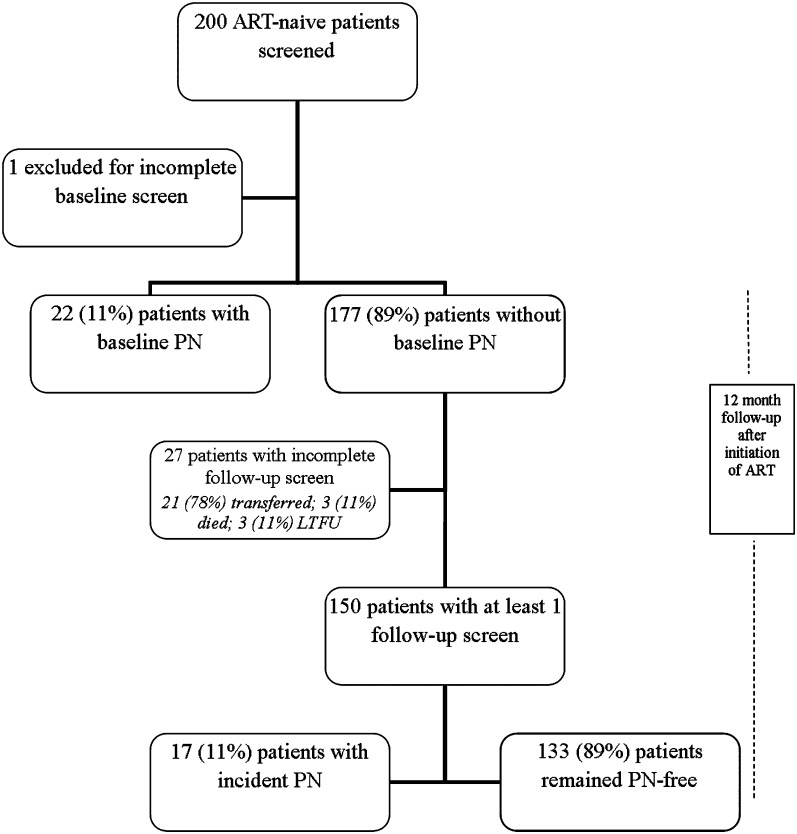
Initially, 200 consecutive patients eligible to initiate ART were enrolled; 1 patient was excluded from the study because of incomplete documentation of the baseline screen. At baseline, before ART initiation, 22 (11%) of 199 patients had PN. In univariate analyses, the presence of PN at baseline in this ART-naive cohort was not associated with age, sex, weight, height, BMI, CD4 count, hemoglobin, history of TB treatment, or pregnancy. There was a graded relationship between the presence of baseline PN and WHO stage of HIV infection. Relative to stage 4 disease, the odds ratios were 0.7 (95% CI,.1–1.3) for stage 3 disease, 0.3 (95% CI,.04–7.5) for stage 2 disease, and 0 for stage 1 disease (no cases of PN among 24 patients in stage 1). The omnibus test of the stage factor showed a trend toward statistical significance (P = .052). In multivariate analysis, the graded relationship between baseline PN and clinical stage remained of borderline significance after adjustment for age (P = .068). Patients with PN at baseline were significantly less likely to be started on a D4T-based regimen than those without PN (6% vs 17%; P = .016).
Incidence of and Risk Factors for PN of HIV-Infected Patients During First Year of ART
A total of 177 patients without evidence of PN at baseline were eligible for longitudinal study, but 27 patients did not undergo follow-up PN screening and therefore were excluded from the analysis. Among the 27 patients without a follow-up screen, 21 patients transferred to another facility, 3 died, and 3 were unavailable for follow-up before the second PN screen. There were no statistically significant differences in terms of sex, age, or WHO stage between the 27 excluded patients and the 150 patients who were followed up for a total of 52022 days (median, 366 days) and included in the final analysis (Figure 1). Among the 150 patients followed up for incident PN, 19 of 94 (20.2%) who initially received a D4T-based regimen changed to a different ART backbone within 1 year, in contrast to 6 of 53 (11.3%) who initially received an AZT-based regimen (P = .25).
Figure 1.
Study flow diagram. ART, antiretroviral therapy; LTFU, lost to follow-up; PN, peripheral neuropathy.
Among the 150 patients without PN at baseline, the median age was 35 years (range, 19–61 years; interquartile range [IQR], 31–41 years) and 98 patients (65.3%) were women (Table 1). This was similar to the age and sex characteristics of the entire HIV clinic population (median age 33 years, 60% women). At baseline, the median weight was 57 kg (IQR, 49.1–63.7 kg), and the median hemoglobin level was 9.8 g/dL (IQR, 8.5–11.5). The median CD4 count was 142 cells/μL (IQR, 64–208); the CD4 count was ≤50 cells/μL in 34 patients (23%); 51–100 cells/μL in 18 (12%), 101–200 cells/μL in 54 (36%), and >200 cells/μL in 44 (29%). Eighty-seven patients (58%) had WHO stage 3 or 4 HIV infection. Thirty-two patients (21.3%) had a history of treatment for TB; 6 patients completed TB treatment before study entry, 15 started TB treatment before study entry and continued it during the study period, and 11 started treatment after study entry. None of the patients had diabetes. Four patients were pregnant at the time of ART initiation. Ninety-four patients (63%) began ART with a D4T-based regimen, 53 (35%) with an AZT-based regimen, and 3 (2%) with a tenofovir- or abacavir-based regimen. Compared with men, women were younger (median 34 vs 39 years, P = .001), more anemic (median hemoglobin level, 9.3 vs 11 g/dL; P < .0001), and had slightly higher BMIs than men (median BMI, 21 vs 20 kg/m2; P = .05). These findings did not change after pregnant women were excluded from the analysis (data not shown).
Table 1.
Baseline Characteristics in Study Group
| Baseline characteristic | Total (n = 150) | Peripheral neuropathy |
|
| Present (n = 17; 11%) | Absent, (n = 133; 89%) | ||
| Female sex, no. (%) | 98 (65.3) | 16 (94.1) | 82 (61.7) |
| Age, median (range; IQR), years | 35 (19–61; 31–41) | 34 (32–42) | 35 (31–41) |
| Baseline CD4 count, median (IQR), cells/μL | 142 (64–208) | 170 (116–242) | 136 (55–204) |
| Baseline weight, median (IQR), kg | 57 (49.1–63.7) | 59.4 (53.7–69.1) | 57.0 (48.3–63.6) |
| Baseline height, median (IQR), cm | 164 (158–170) | 163.5 (158.5–167.5) | 164 (157.3–170) |
| Baseline BMI, median (IQR), kg/m2 | 20.7 (18.3–24.4) | 22.4 (20.3–25.3) | 20.4 (18.1–24) |
| WHO clinical stage of HIV infection, no. (%) | |||
| Stage 1 | 21 (14.0) | 3 (17.6) | 18 (13.5) |
| Stage 2 | 42 (28.0) | 4 (23.5) | 38 (28.6) |
| Stage 3 | 83 (55.3) | 10 (58.8) | 73 (54.9) |
| Stage 4 | 4 (2.6) | 0 (0) | 4 (3.0) |
| Initial NRTI backbone, no. (%) | |||
| Zidovudine | 53 (35) | 4 (23.5) | 49 (36.8) |
| Weight-based stavudine | 94 (63) | 13 (76.5) | 81 (60.9) |
| Tenofovir or abacavir | 3 (2) | 0 (0) | 3 (2.3) |
| Duration of observation during ART, median (IQR), days | 366 (351–399) | 293 (160–369) | 370 (355–402) |
| TB treatmenta completed before study entry, no. (%) | 6 (4.0) | 1 (6.8) | 5 (3.8) |
| TB treatmenta received during study period, no. (%) | 26 (17.3) | 3 (18.6) | 23 (17.3) |
| History of diabetes, no. (%) | 0 (0) | … | … |
| Baseline hemoglobin, median (IQR), g/dL | 9.8 (8.5–11.5) | 9.1 (8.4–9.8) | 10.0 (8.5–11.6) |
NOTE. ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; NRTI, nucleoside reverse-transcriptase inhibitor; TB, tuberculosis; WHO, World Health Organization.
All TB treatment regimens included both isoniazid and ethambutol.
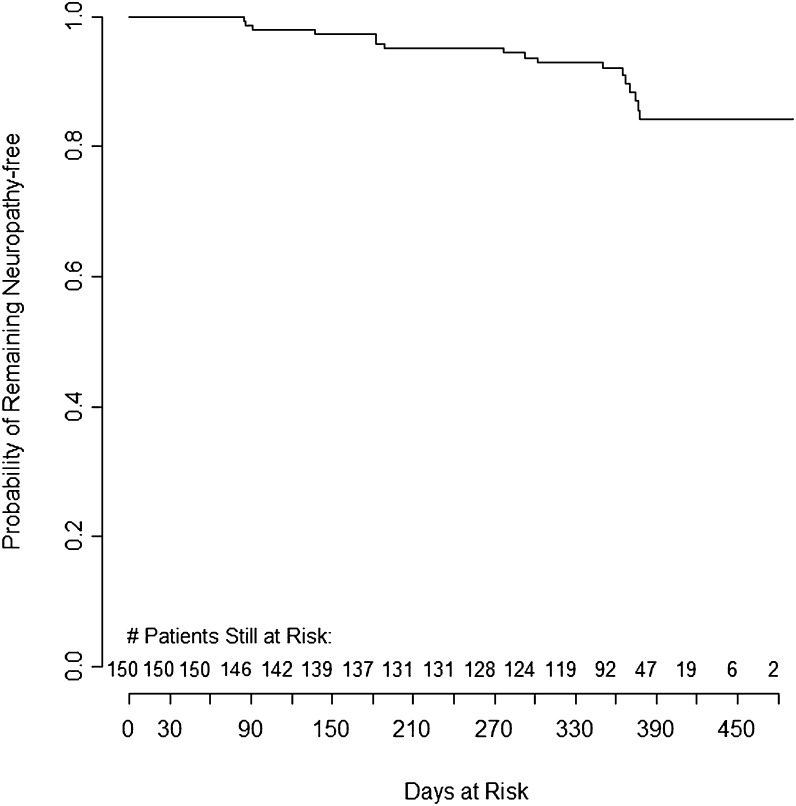
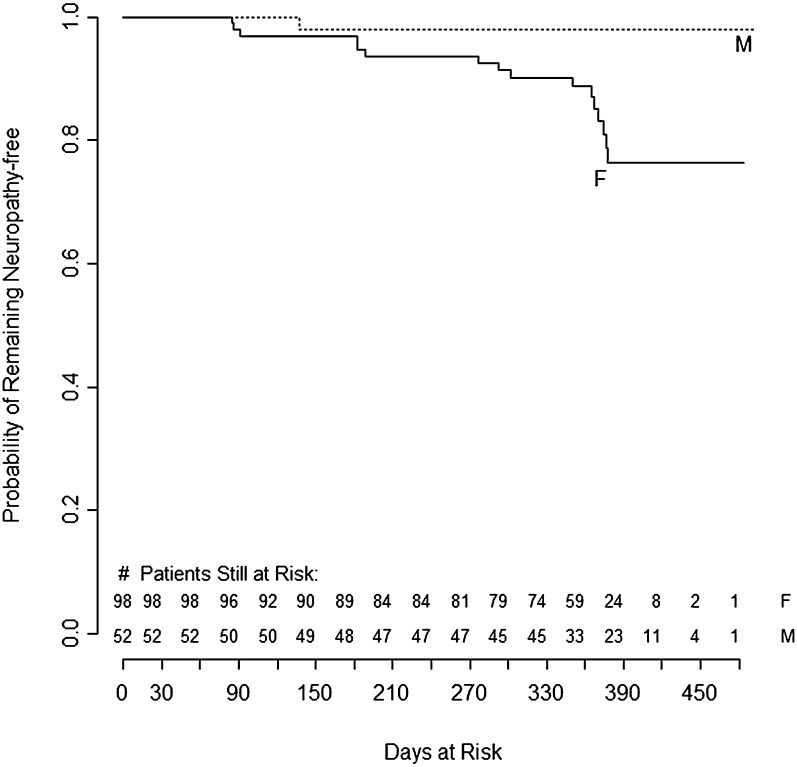
Seventeen (11.3%) of 150 patients developed PN during the study period. The crude incidence rate for PN was 11.9 per 100 person-years (95% CI, 6.9–19.1) (Figure 2). In univariate analysis, women were 9.6 times more likely to develop PN than men (95% CI, 1.27–72; P = .03) (Table 2) and neuropathy-free survival was significantly shorter in women (Figure 3). There were no significant associations between incidence of PN and age, initial NRTI backbone, exposure to isoniazid or ethambutol, or baseline values for CD4 count, weight, height, BMI, and WHO clinical stage. However, a nonsignificant increased risk for PN was observed at low hemoglobin levels (P = .07). Similar results were obtained when these potential risk factors were analyzed by comparing incidence rates rather than the overall proportion or patients with PN (data not shown). The number of follow-up visits for men and women were similar, and the rates of patients unavailable for follow-up were similar for men and women. In stratified analyses, female sex remained a significant risk factor for PN after adjustment for the aforementioned variables, although hemoglobin levels decreased the hazard ratio from 9.6 to 7.40 and the P value to .05 (Table 2).
Figure 2.
Neuropathy-free survival among 150 patients beginning antiretroviral therapy in Mombasa, Kenya.
Table 2.
Univariate and Multivariate Analysis of Risk Factors for Peripheral Neuropathy Among 150 Patients With Human Immunodeficiency Virus Infection Beginning Antiretroviral Therapy
| Factor | Univariate analysis, | P | Multivariate analysis,a | P |
| HR (95% CI) | aHR (95% CI) | |||
| Sex | ||||
| Male (n = 52) | 1 | NA | ||
| Female (n = 98) | 9.6 (1.27–72) | .03 | NA | |
| Age, years | ||||
| 19–32 (n = 58) | 1 | 1 | ||
| 33–39 (n = 44) | 1.40 (.45–4.34) | .56 | 1.99 (.64–6.22) | .23 |
| 40–61 (n = 48) | 1.08 (.33–3.55) | .90 | 1.72 (.51–5.72) | .38 |
| Female sex adjusted for age | … | 11.30 (1.47–86.76) | .02 | |
| CD4 cell count, cells/μL | ||||
| 2–98 (n = 50) | 1 | |||
| 99–196 (n = 51) | 2.20 (.57–8.52) | .25 | 2.23 (.58–8.63) | .25 |
| 197–338 (n = 49) | 2.21 (.57–8.55) | .25 | 2.00 (.52–7.73) | .32 |
| Female sex adjusted for CD4 cell count | 9.47 (1.25–71.61) | .03 | ||
| Hemoglobin, g/dL | ||||
| 5.5–8.9 (n = 53) | 1 | |||
| 9.0–10.6 (n = 48) | 0.86 (.31–2.38) | .77 | 0.86 (.31–2.39) | .77 |
| 10.7–16.9 (n = 49) | 0.24 (.05–1.14) | .07 | 0.41 (.08–1.99) | .26 |
| Female sex, adjusted for hemoglobin | … | 7.40 (.94–58.03) | .05 | |
| BMI, kg/m2 | ||||
| 13.4–19.3 (n = 50) | 1 | |||
| 19.4–22.7 (n = 50) | 2.10 (.53–8.41) | .29 | 1.69 (.42–6.78) | .36 |
| 22.8–35.3 (n = 50) | 2.52 (.67–9.50) | .17 | 1.85 (.49–7.03) | .37 |
| Female sex adjusted for BMI | … | 8.67 (1.14–66.06) | .04 | |
| Initial NRTI backbone | ||||
| Zidovudine (n = 53) | 1 | |||
| Weight-based stavudine (n = 94) | 1.91 (.62–5.88) | .25 | 1.39 (.45–4.33) | .56 |
| Tenofovir or abacavir (n = 3) | 0 (0 to infinity) | 0 (0 to infinity) | ||
| Female sex adjusted for NRTI | … | 8.41 (1.10–64.38) | .04 | |
| TB treatmentb | ||||
| No history of TB treatment (n = 118) | 1 | |||
| TB treatment completed before study entry (n = 6) | 3.04 (.38–24.1) | .29 | 2.70 (.34–21.4) | .34 |
| TB treatment received during study period (n = 26) | 1.17 (.33–4.11) | .80 | 1.43 (.40–5.04) | .58 |
| Female sex adjusted for any TB treatment | … | 9.73 (1.28–73.8) | .03 |
NOTE. aHR, adjusted hazard ratio; BMI, body mass index; CI, confidence interval; HR, hazard ratio; NA, not applicable; NRTI, nucleoside reverse-transcriptase inhibitor; TB, tuberculosis.
Each panel represents a Cox regression analysis including the variables of sex and the additional variable indicated.
All TB treatments included both isoniazid and ethambutol.
Figure 3.
Neuropathy-free survival stratified by sex among 150 patients beginning antiretroviral therapy in Mombasa, Kenya. F, female; M, male.
Among 17 patients with PN, 13 (76.5%) reported mild (grade 1) symptoms, and 4 were more severely affected. Sixteen (94.1 %) had abnormal bilateral vibratory sensation, and 5 (29.4%) had abnormal bilateral ankle reflexes. Among the 16 patients with abnormal bilateral vibratory sensation, 13 had grade 1 loss, 1 had grade 2 loss, and 2 had grade 3 loss. Among the 5 patients with abnormal bilateral ankle reflexes, 4 (80%) had hypoactive or absent ankle reflexes. Four patients had both abnormal bilateral vibratory sensation and ankle reflexes. BPNS results were abnormal in 4 patients (23.5%) at the month 3 visit, in 3 (17.6%) at month 6, in 5 (29.4%) at month 9, and in 5 (29.4%) at month 12.
DISCUSSION
This study is novel in its use of a validated PN screen to measure the incidence of PN in an incipient cohort of patients initiating ART in a resource-limited setting. Patients diagnosed with PN at baseline were significantly less likely to start on D4T-based regimens, suggesting that the screening tool helped clinicians make beneficial treatment decisions. Using the PN screening tool, we report for the first time that women were ∼10 times more likely than men to develop PN during the first year after ART was initiated.
Previous studies in HIV-infected patients receiving ART in sub-Saharan Africa have not found an increase in risk of PN among women, although none used an incipient cohort to study the incidence of PN, the BPNS tool, or the same definition of PN as we did in the current work [2–5, 9–11]. Furthermore, studies conducted in resource-rich countries on PN among HIV-infected patients receiving ART have low female enrollment, limiting their power to evaluate sex differences [18–20]. A recent cross-sectional study conducted in 2 Asian countries and in Australia that did use the BPNS for the diagnosis of PN in HIV-infected patients receiving ART did not find a sex difference [21]. In fact, they found that women were less likely to have PN in Indonesia and Malaysia and equally likely in Australia. Differences in study design (cross-sectional vs longitudinal) and racial characteristics of the cohort may account for this discrepancy. Studies from resource-rich settings have shown that women tolerate NRTI-containing regimens less well than men [22, 23]. A recent study found that women discontinued ART for PN more often than men [22–24]. The reason for greater adverse events in women has not been clearly shown; however, suggested mechanisms for sex differences have included differences in weight or BMI and hormonal changes that may affect drug distribution and metabolism [25]. In our study, women were more likely to be younger, more anemic and have slightly higher BMI than men. Although we did not identify anemia as an independent risk factor for PN overall, there was a trend toward a higher risk of PN in adults with hemoglobin levels of 5.5–8.9 g/dL than in those with levels of 10.7–16.9 g/dL. Furthermore, the hazard ratio for developing PN among women decreased from 9.6 to 7.4 when hemoglobin levels were controlled for. It is possible that anemia may be a marker for other risk factors unmeasured in this study, such as general micronutrient deficiencies. Larger studies using the BPNS should be conducted in resource-limited settings to better identify modifiable risk factors for PN in HIV-infected men and women, such as iron, vitamin B12, and other deficiencies.
We did not find the expected association between development of PN and initiation of ART with a D4T-based regimen. Furthermore, patients in our study developed PN despite our use of the recommended weight-based dosing for D4T. This result supports continued vigilance in routine monitoring for the development of PN among ART-treated patients, even with the revised 2010 WHO guidelines for ART, which no longer recommend D4T as first-line therapy [26].
The use of the BPNS in routine care may help to identify PN earlier and prevent disability. As we have reported elsewhere, clinical and medical officers in our busy urban clinic in Kenya easily integrated the BPNS into patient visits [13]. Use of the screening tool by trained clinicians added 5 min to patient visits. Our experience suggests that the BPNS can be a useful tool for routine care of HIV-infected patients in resource-limited settings, although the optimal frequency of testing is unclear. However, previous work has shown that if PN does occur in HIV-infected patients, it does so within the first year after the start of ART, and if PN did not develop during the first year, it was less likely to occur in subsequent years [27]. Therefore, in resource-limited settings, it may be sufficient to limit screening for PN to the first year of ART.
Limitations of our study include a small sample size that reduced our statistical power to detect weaker associations. In addition, several known risk factors for PN were not assessed, including alcohol use, micronutrient status, hepatitis C status, syphilis, thyroid disorders, and renal dysfunction. Because we did not have data on adherence to ART, we cannot determine the impact of higher rates of PN among women on nonadherence to a medication regimen. Selection bias is a concern and may partially explain our finding that women developed PN more frequently than men. However, it is unlikely to explain this finding completely. Because enrollment occurred among consecutive eligible patients beginning ART, the demographic makeup of the study cohort was similar to the HIV cohort of patients at the clinic as a whole (60% female), and the 27 patients excluded from analysis due to lack of follow-up screens were similar in sex and age to the analyzed population.
CONCLUSIONS
Using a validated neuropathy screen, we found that 11% of Kenyan patients initiating ART developed PN during the first year of treatment. Women were ∼10 times more likely to develop PN, and this risk increased in women who were anemic. The risk of PN could be reduced by implementing the BPNS during the first year of ART to ensure early diagnosis during the period of highest risk and implementing measures to prevent or treat anemia, especially in women, before ART initiation.
Acknowledgments
We would like to thank the patients and staff from Bomu Medical Centre, particularly Hayati Anjarwalla, Farhad Abdulaziz, Fanuel Omasete, Swaleh Said, Mohammed Mwakazi, and Saada Mahmood for their assistance with the study. We also thank Megan Mendillo and Donald Chen from NYU School of Medicine for their assistance in data management and study oversight.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Financial support. This research was supported in part by the National Center for Research Resources, National Institutes of Health (grant 1UL1RR029893), the New York University Center for AIDS Research (grant P30 AI027742), the Gilead Foundation, and the Centers for Disease Control and Prevention (CDC) (cooperative agreement 5U62PS224509-04).
Potential conflicts of interest All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Boulle A, Orrel C, Kaplan R, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12:753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 2.Forna F, Liechty CA, Solberg P, et al. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:456–62. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45:304–10. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann CJ, Fielding KL, Charalambous S, et al. Antiretroviral therapy using zidovudine, lamivudine, and efavirenz in South Africa: tolerability and clinical events. AIDS. 2008;22:67–74. doi: 10.1097/QAD.0b013e3282f2306e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millogo A, Lankoande D, Yameogo I, Yameogo AA, Sawadogo AB. Polyneuropathies in patients treated with HAART in Bobo-Dioulasso hospital, Burkina Faso [in French] Bull Soc Pathol Exot. 2008;101:11–3. [PubMed] [Google Scholar]
- 6.van Oosterhout JJ, Bodasing N, Kumwenda JJ, et al. Evaluation of antiretroviral therapy results in a resource-poor setting in Blantyre, Malawi. Trop Med Int Health. 2005;10:464–70. doi: 10.1111/j.1365-3156.2005.01409.x. [DOI] [PubMed] [Google Scholar]
- 7.Wester CW, Kim S, Bussmann H, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–43. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clin Proc. 2006;81:213–9. doi: 10.4065/81.2.213. [DOI] [PubMed] [Google Scholar]
- 9.Njoroge J, Reidy W, John-Stewart G, Attwa M, et al. In Abstracts of the Fifth IAS Conference on HIV Pathogenesis, Treatment and Prevention. Cape Town, South Africa: IAS: 2009. Incidence of peripheral neuropathy among patients receiving HAART regimens containing stavudine vs. zidovudine in Kenya. [Google Scholar]
- 10.Sacktor N, Nakasujja N, Skolasky RL, et al. Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda. Neurology. 2009;72:165–70. doi: 10.1212/01.wnl.0000339042.96109.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Griensven J, Zachariah R, Rasschaert F, Mugabo J, Att E, Reid T. Stavudine- and nevirapine-related drug toxicity while on generic fixed-dose antiretroviral treatment: incidence, timing and risk factors in a three-year cohort in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010;104:148–53. doi: 10.1016/j.trstmh.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65:1778–81. doi: 10.1212/01.wnl.0000187119.33075.41. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SA, Ahmed A, Kariuki BW, et al. Implementation of a validated peripheral neuropathy screening tool in patients receiving antiretroviral therapy in Mombasa, Kenya. Am J Trop Med Hyg. 2010;83:565–70. doi: 10.4269/ajtmh.2010.09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO case definitions of HIV for surveillance and revised clinical staging and immunologic classification of HIV-related disease in adults and children. Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. Accessed 25 April 2010. [Google Scholar]
- 15.Peduzzi P, Guo Z, Marottoli RA, Gill TM, Araujo K, Allore HG. Improved self-confidence was a mechanism of action in two geriatric trials evaluating physical interventions. J Clin Epidemiol. 2007;60:94–102. doi: 10.1016/j.jclinepi.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Guarino P, Lamping DL, Elbourne D, Carpenter J, Peduzzi P. A brief measure of perceived understanding of informed consent in a clinical trial was validated. J Clin Epidemiol. 2006;59:608–14. doi: 10.1016/j.jclinepi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto B, McMurtray A, Davis J, et al. Incident neuropathy in HIV-infected patients on HAART. AIDS Res Hum Retroviruses. 2010;26:759–65. doi: 10.1089/aid.2009.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis R, Rosario D, Clifford D, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–8. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson DM, Kitch D, Evans SR, et al. HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology. 2006;66:1679–87. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 21.Cherry CL, Affandi JS, Imran D, et al. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology. 2009;73:315–20. doi: 10.1212/WNL.0b013e3181af7a22. [DOI] [PubMed] [Google Scholar]
- 22.Currier JS, Spino C, Grimes J, et al. Differences between women and men in adverse events and CD4+ responses to nucleoside analogue therapy for HIV infection. AIDS Clinical Trials Group 175 Team. J Acquir Immune Defic Syndr. 2000;24:316–24. doi: 10.1097/00126334-200008010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Moore RD, Fortgang I, Keruly J, Chaisson RE. Adverse events from drug therapy for human immunodeficiency virus disease. Am J Med. 1996;101:34–40. doi: 10.1016/s0002-9343(96)00077-0. [DOI] [PubMed] [Google Scholar]
- 24.Kempf MC, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 2009;52:336–41. doi: 10.1097/QAI.0b013e3181b628be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofotokun I, Pomeroy C. Sex differences in adverse reactions to antiretroviral drugs. Top HIV Med. 2003;11:55–9. [PubMed] [Google Scholar]
- 26. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 31 August 2010. [Google Scholar]
- 27.Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148–57. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]